Abstract
After COVID-19 infection, some patients develop a post-COVID condition (PCC) that is popularly referred to as long COVID. Among its symptoms is persistent cognitive dysfunction that is potentially linked to altered brain functional connectivity (FC). While research has explored functional reorganization in patients with PCC, the intra- and inter- network connectivity and its relationship with cognitive status and clinical outcomes remain unclear. In this study, we recruited 121 individuals with PCC (67 with, and 54 without, cognitive impairment), 20 months after infection, along with 37 non-infected healthy controls from the NAUTILUS Project (ClinicalTrials.gov IDs: NCT05307549 and NCT05307575). Participants underwent resting-state functional magnetic resonance imaging and comprehensive neuropsychological assessment. Resting-state networks were characterized using independent component analyses, dual regression and network modelling for individual FC characterization. Group differences in intra- and inter-network FC, and their associations with clinical and neuropsychological data, were studied. Significance was set at a corrected p-value of < 0.05. Patients with PCC showed increased intra-network FC in 10 cognitively relevant networks, including the default mode, salience, executive control, auditory and basal ganglia networks, correlating positively with general cognition (Montreal Cognitive Assessment scores), time since infection, fatigue and subjective memory failures. Increased inter-network FC between default mode and sensorimotor networks was also observed. Increases in FC may reflect an inefficient compensatory mechanism in patients with PCC, associated with fatigue, subjective memory complaints and persistence of PCC.
Similar content being viewed by others
Introduction
The repercussions of COVID-19 extend beyond the acute infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Many people experience a collection of persistent symptoms, collectively referred to as the post-COVID condition (PCC), that affect multiple systems and organs1,2,3,4. The World Health Organization (WHO) defines PCC as “the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation”5.
Cognitive dysfunction is common in people with PCC, often manifesting as impairments in attention, concentration, memory, information processing speed, and executive function4. However, PCC may represent distinct phenotypes6, and cognitive symptoms vary among patients7. Emerging evidence also suggests that cognitive deficits may be linked to underlying brain changes4. Therefore, identifying structural and functional imaging correlates is critical to the characterization of PCC, and could serve as markers of progression. Although structural changes identified with brain magnetic resonance imaging (MRI) have been linked to cognitive decline in people with PCC8,9,10,11,12, the study of functional alterations remains limited and encompasses diverse approaches.
Functional connectivity (FC) is commonly evaluated using techniques based on resting-state functional MRI (rs-fMRI) imaging, with region of interest (ROI)-based analyses revealing patterns of increased and decreased FC in people with PCC12,13,14. Hypoconnectivity in PCC groups compared with control groups may indicate reduced functional integration of local and long-range connections8,15. Other studies based on graph analysis have reported altered graph connectivity metrics in PCC groups at the whole brain level, along with regional differences in the olfactory gyrus, cingulate cortex, subcortical structures or the cerebellum16,17,18. Moreover, the relationship between FC and different clinical symptoms in PCC remains unresolved. Research on the correlation between FC and neuropsychological scores has produced mixed results, with both positive8,29 and negative18 correlations observed between FC of different regions and memory performances. There are also inconsistent findings when assessing the correlation between FC measures and blood biomarkers14,30, physiological measures12 and clinical assessment scores16,31,32, although this most likely reflects methodological differences. Few studies have explored FC by comparing PCC subgroups by the presence or severity of neurological and psychiatric symptoms33,34, and none has divided them according to the definition cognitive impairment based on a comprehensive neuropsychological assessment.
Independent component analysis (ICA) assesses functional integration by identifying well-documented resting-state networks (RSNs)19,20. The synchronous activity of these networks during rest plays a key role in a wide range of sensory, motor, and cognitive tasks20,21. Consequently, disruptions in these networks have been associated to neurological and psychiatric disorders22,23,24. In addition to examining intrinsic FC within the identified networks, this technique facilitates post-hoc analysis on the temporal relationship between ICA-derived RSNs, enabling the study of inter-network connectivity. In this sense, alterations in between-network interactions may contribute to cognitive impairment, as evidenced by studies linking such changes to cognitive decline25,26,27,28. Nevertheless, despite the advantages of ICA-based analyses, no studies have assessed the integration of ICA-based RSNs and inter-network connectivity in patients with PCC.
The objectives of the current work are three-fold: (1) to study connectivity within brain networks (intra-network) and between brain networks (inter-network) in a sample properly characterized as PCC according to WHO criteria; (2) to investigate the relationship between FC, cognitive symptoms and clinical measures; and (3) to focus on the potential differences between individuals with PCC with and without significant cognitive impairment.
Results
Sociodemographic and clinical characteristics
The PCC group showed no significant differences in age, education, sex and estimated intelligence quotient (IQ) compared with the healthy controls (HC) (Table 1). During acute infection, 38 patients with PCC (31.4%) required hospitalization, of which 18 (14.9%) were admitted to intensive care. Of note, only 14.9% of the PCC group were not vaccinated, but all in the HC group were vaccinated. The PCC group showed higher scores on the Patient Health Questionnaire-9 (PHQ-9), Generalized Anxiety Disorder 7-item scale (GAD-7), Memory Failure of Everyday (MFE), and Chalder Fatigue Scale (CFQ) questionnaires (Supplementary Table 1), suggesting greater levels of depression, anxiety, memory failure and fatigue.
Recruited patients with PCC were infected between February 2020 and June 2023. For a detail on the predominant COVID variant over this period, see (Supplementary Fig. 1).
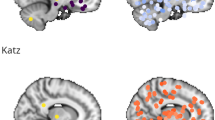
Resting state networks (RSNs) presenting between-groups significant clusters of increased functional connectivity in PCC respect HC, obtained from dual regression analyses (p < 0.05, corrected for multiple comparisons). Maximum coordinates based on Montreal Neurological Institute (MNI) space are indicated. The right hemisphere is displayed on the left side of axial and coronal views. Abbreviations: HC, healthy controls; IC, independent components; PCC, post-COVID condition.
Neuropsychological assessment
The PCC group showed statistically significant lower performance than the HC group in the Montreal Cognitive Assessment (MoCA), the Spanish version of Rey’s Auditory verbal learning test (RAVLT) total score, delayed recall and recognition, Digit Span forward and Backwards, Digit Symbol Coding (DSC), Trail Making test (TMT) part A, Stroop words, Stroop Colors, Semantic and Phonetic Fluency, as well as in the Spanish version of the 40-item University of Pennsylvania Smell Identification Test (UPSIT-40) (Table 2).
Brain functional connectivity analysis
Intra-network connectivity differences
We identified 47 meaningful RSNs among the 50 components extracted by ICA from the whole sample. The PCC group showed increased intra-network FC compared with the control group in 10 RSNs (Fig. 1 and Supplementary Table 5), including the default mode network (DMN), executive control network (ECN) and salience network (SN), auditory network (AUN), superior parietal lobule, anterior cingulate cortex, and basal ganglia network (BGN) (p < 0.05, corrected for multiple comparisons).
Inter-network analysis
Correlation matrices derived from FSL Nets indicated a significant increase in inter-network connectivity between the sensorimotor network (SMN), notated as independent component (IC) 18, and the DMN (IC26) in the PCC group compared with the HC group (p = 0.049, corrected for multiple comparisons). As shown in Fig. 2A-C, the PCC group (mean = 0.10, SD = 0.73) exhibits a loss of the anticorrelation observed in the control group (mean = -0.48, SD = 0.90) between these two networks.
(A) Full correlation between IC18 (SMN) and IC26 (DMN) of the groups HC and PCC obtained by FSL Nets matrices. (B) IC18 SMN resting state network. (C) IC26 DMN resting state network. Coordinates are expressed in Montreal Neurological Institute (MNI) space. Abbreviations: DMN, default mode network; HC, healthy controls; IC, independent components; PCC, post-COVID condition; SMN, sensorimotor network.
Correlation with neuropsychological and clinical data
Correlation analysis between the mean connectivity strength of significant components and significant neuropsychological tests revealed the following within the PCC group: positive correlations with the MoCA, RAVLT Total, RAVLT recognition, and DSC, and negative correlations with the TMT A (Supplementary Table 6). Amongst these, only correlations with the MoCA remained significant after correction for false discovery rate (FDR). The MoCA showed a positive correlation with the mean connectivity strength of seven RSNs (Fig. 3), listed in descending order: anterior ECN (IC29, R = 0.264, pFDR = 0.005; IC42, R = 0.222, pFDR = 0.018), superior parietal (IC44, R = 0.257, pFDR = 0.006), AUN (IC07, R = 0.249, pFDR = 0.008; IC15, R = 0.240, pFDR = 0.011), SN (IC13, R = 0.222, pFDR = 0.018) and anterior cingulate (IC09, R = 0.221, pFDR = 0.019).
Partial regression plots regressed by age showing the significant correlations of the mean connectivity strengths with the MoCA scores. Abbreviations: AUN, auditory network, BGN, basal ganglia network; IC, independent component; DMN, default mode network; ECN, executive control network; SN, salience network.
Correlation analysis revealed that the mean connectivity strength of all components presenting significant clusters correlated positively with the interval between COVID infection and MRI acquisition in the PCC group (Supplementary Fig. 3, Supplementary Table 7). Specifically, this interval positively correlated, in descending order by R value, with the BGN (IC48, R = 0.483, pFDR < 0.001), anterior cingulate (IC0009, R = 0.430, pFDR < 0.001), superior parietal (IC44, R = 0.417, pFDR < 0.001), anterior ECN (IC0042, R = 0.424, pFDR < 0.001; IC29, R = 0.339, pFDR < 0.001), SN (IC0013, R = 0.409, pFDR < 0.001; IC0017, R = 0.392, pFDR < 0.001), DMN (IC0003, R = 0.384, pFDR < 0.001) and AUN (IC0007, R = 0.295, pFDR = 0.001; IC0015, R = 0.276, pFDR = 0.002).
Correlation analysis between the mean connectivity strength of components presenting significant clusters and the MFE and CFQ revealed positive correlations within the PCC group (Supplementary Figs. 4 and 5, Supplementary Table 8). MFE demonstrated positive correlations with the mean connectivity strength in the anterior cingulate (IC09, R = 0.304, pFDR = 0.010), DMN (IC03, R = 0.258, pFDR = 0.025), anterior ECN (IC42, R = 0.249, pFDR = 0.025), BGN (IC48, R = 0.247, pFDR = 0.025) and SN (IC17, R = 0.234, pFDR = 0.030). CFQ showed positive correlations with connectivity strength in the anterior ECN (IC42, R = 0.384, pFDR < 0.001; IC29, R = 0.249, pFDR = 0.014), anterior cingulate (IC09, R = 0.356, pFDR < 0.001), SN (IC13, R = 0.318, pFDR = 0.003; IC17, R = 0.274, pFDR = 0.008), DMN (IC03, R = 0.306, pFDR = 0.003), AUN (IC15, R = 0.251, pFDR = 0.014), superior parietal (IC44, R = 0.206, pFDR = 0.040) and BGN (IC48, R = 0.203, pFDR = 0.040).
No statistically significant correlations were observed for the inter-network connectivity of IC18 and IC26 with clinical or neuropsychological variables of interest. Moreover, no significant correlations were found with the PHQ-9 and GAD-7 scores.
Cognitive impaired versus non-cognitively impaired patients
Sixty-seven patients with PCC who had lower-than-normal performance on two or more cognitive tests were identified as cognitively impaired. The frequency of impairment by test, including any between-group differences in neuropsychological performance are reported in Supplementary Table 4 and Supplementary Fig. 2. Upon categorizing patients with PCC into cohorts based on the presence of cognitive impairment, significant differences in age, education, and estimated IQ appeared between groups (Supplementary Table 2). However, there were no differences between cognitively impaired and non-cognitively impaired PCC groups for the PHQ-9, GAD-7, MFE, and CFQ questionnaires (Supplementary Table 3).
Regarding FC, no statistically significant differences appeared between the non-impaired and impaired groups when considering age and education as covariates. The inter-network analysis also showed no differences between groups with full correlation of the different networks.
Hospitalized versus non-hospitalized patients
As a complementary analysis, differences among patients with PCC based on infection severity (defined by hospitalization status during the infection) were explored. Significant differences were found in sex, education and PCC duration between non-hospitalized and hospitalized patients with PCC during the infection, as well as in sex between non-hospitalized patients with PCC and HC (Supplementary Table 9). Accounting for the demographic differences and movement parameters (Supplementary Table 10), however, we observed no differences in intra-network FC between non-hospitalized and hospitalized PCC groups.
Discussion
This work explores intra- and inter-network brain FC alterations in participants with PCC, together with how these relate to cognition and clinical variables. We observed increased intra-network connectivity in the anterior DMN, ECN and SN, AUN, superior parietal lobule, anterior cingulate cortex, and BGN, alongside increased inter-network connectivity between the DMN and the SMN. Our findings also showed a correlation between intra-network connectivity changes and PCC sequelae, including global cognition, subjective memory complaints, fatigue, and the time interval since the infection.
Previous literature has reported both increases12,17,35,36,37 and decreases8,12,13,14,15,32 in FC among patients with PCC. These heterogeneous results reflect the scarceness of studies assessing FC in individuals with properly defined PCC and the use of disparate methods to study FC. Although we noted an increase in connectivity among patients with PCC, these issues make it difficult to compare our findings with previous literature.
Our findings align with reports of increased intra-network FC in the DMN in a sample of 29 patients with acute infection36, though differences in location within the network suggested different manifestations of the same process. The increased FC involving the DMN seems to persist months after COVID-19 infection, as evidenced by another study that found increased connectivity both within the DMN, and between the DMN and the olfactory network, in patients experiencing persistent olfactory dysfunction37.
In other brain systems, our results differ from previous findings. The present study evidenced increased FC in several frontal regions, including the middle (IC13) and superior (IC29) frontal lobe, the anterior cingulate cortex (IC3, IC9, IC17) and the frontal pole (IC42). By contrast, other studies have reported FC decreases in similar regions among individuals with PCC8,14. Declining in FC has been speculated to reflect hypometabolism, as identified through positron emission tomography imaging during the acute phase of the COVID-19 infection38,39. However, this reduction in metabolic activity tends to resolve over time, with minimal or absent residual effects after 6 months6,41, suggesting that factors other than cortical hypometabolism may cause the neurocognitive symptoms observed in PCC40. Indeed, hypermetabolism has been observed in COVID-19 without significant changes from acute phase to PCC. Although, these results must be interpreted with caution and need replication in bigger samples, they suggest that persistent hypermetabolism in the brainstem, cerebellum, hippocampus and amygdala might represent a neural substrate of the neurological sequelae in PCC41. Therefore, the potential relationship between increases in FC and hypermetabolism in PCC, including its trajectories, is worth further research.
In our study, we assessed a sample of post-COVID patients an average of 20 months after infection. It is noteworthy that most research to date has included patients with shorter times since infection, ranging from 3 months32 to 11 months8, with many studies also showing significant improvement in symptomatology and low percentages of ongoing symptoms14,15. Research has shown that 91% of patients with PCC gradually recover over 2-year periods, with 4% continuing to suffer from persistent symptoms beyond that period42. This proportion is more pronounced in our sample, with 36.36% of participants with PCC (44 of 121) remaining symptomatic beyond 2 years after infection onset. Different mechanisms may underlie long-term sequelae and PCC compared with the acute or post-acute infection periods. In this context, the increase in FC among RSNs evidenced in our results may reflect an attempt to compensatory upregulation in blood-oxygen-level-dependent (BOLD) activity, as suggested by previous studies on the PCC12,14,17,37,43. For example, enhanced BOLD levels may be associated with a neuroprotective response to viral damage12,17, which in turn, could be linked to the neuropathological mechanisms of COVID-19, such as reactive gliosis and microglial activation44,45. We observed a positive correlation between FC in all significant networks and the time interval from infection, suggesting that increased FC is associated with a longer duration of PCC symptoms.
We observed a positive correlation between increased intra-network connectivity strength in seven cognitively relevant networks (i.e., ECN, frontoparietal network, SN and AUN) and global cognitive function, as measured by MoCA test scores, in patients with PCC. Similar findings on ROI-based studies have described increased FC in association with better memory performance8,29.
We also observed positive correlations between increased FC and scores on the MFE, indicating that greater FC is associated with more reported subjective memory failures. These results agree with a previous study, in which increased FC was identified in the SN, SMN, dorsal attention network and visual networks in patients with general complaints of post-COVID-19 cognitive issues compared with those who had no cognitive issues46.
Moreover, our results showed a positive correlation between fatigue scores and FC strength in RSNs critically involved in cognitively demanding tasks, such as the DMN (IC3), SN (IC13 and IC17), and BGN (IC48), which are key brain regions in a fatigue network47. This may indicate that FC could be a useful marker for monitoring interventions to modify the progression of fatigue, which is highly prevalent in PCC48.
Overall, the increased intra-network FC and the positive correlation between mean FC strength and MoCA scores in PCC may indicate a suboptimal compensatory mechanism. Although patients with PCC exhibit higher FC, which correlates positively with MoCA scores, significant cognitive differences remained between the PCC and HC groups. This indicates that the extra effort that the brain of a patient with PCC makes to compensate for deficiencies is not enough to restore normal brain function in all cases. Patients suffering from the condition for longer, as well as those with more fatigue and memory complaints, present increased FC. Interestingly, those reporting greater fatigue and memory issues also had a longer condition duration, suggesting that a prolonged, albeit suboptimal attempt at compensation leads to increased malaise and discomfort.
An increased correlation between the SMN and DMN was also observed in patients with PCC, which is intriguing, given that both networks are typically anti-correlated49. Studies in other pathologies have pointed to hyperconnectivity being a common response to neurological disruptions associated with cognitive decline50,51,52,53. In PCC, patients with olfactory dysfunction who underwent hyperbaric oxygen therapy were shown to experience a significant decrease in inter-network FC between the SMN and SN compared with non-treated patients, as well as a trend-level reduction in FC between the DMN, frontoparietal network and SN13. These findings suggest that therapy aids cognitive recovery by reorganizing and restoring brain FC from a PCC-induced hyperconnected state, with reduced FC correlating with improved cognitive function. FC between DMN and SMN nodes has also been positively correlated with post-traumatic distress30, suggesting that the presence of clinical symptoms in COVID-19 survivors is associated with inter-network FC disruption. However, we could not corroborate these previous findings, with no significant correlations observed between inter-network FC strength and cognitive or clinical measurements.
Intra-network connectivity changes did not depend on whether the patient was hospitalized during the acute COVID-19 infection, as no differences were observed between non-hospitalized and hospitalized patients with PCC. These findings highlight the relevance of studying subjects with PCC suffering mild symptoms during the acute infection.
No statistically significant differences in either intra-network or inter-network connectivity appeared between cognitively impaired and non-impaired patient groups. Despite our methodological rigor in objectively assessing cognitive status, our results suggest that the clinical criteria for distinguishing these patient groups is insufficient for identifying differences in functional cerebral alterations. More restrictive criteria might be needed to identify those with a worse cognitive profile. Both longitudinal observational and interventional studies are needed to elucidate if the observed increase in FC could be associated with different patient subtypes (i.e., specific progression profiles) and trends to chronicity.
The main strength of the current work is the use of a data-driven ICA approach to compare intra- and inter-network resting-state FC between an HC group and a large PCC group, as defined by WHO criteria, and subsequently characterized by the presence of clinical and cognitive impairment. Moreover, in our study participants completed a comprehensive battery of neuropsychological tests, allowing us to study different cognitive domains and identify clinically meaningful cognitive impairment. However, some limitations must also be noted. First, our control group did not test positive for SARS-CoV-2 and did not exhibit any related symptoms; it is possible that some asymptomatic, previously infected individuals were included. Due to vaccination protocols, we were unable to exclude participants based on antibody presence. Furthermore, our findings do not provide insight into how different variants may affect brain changes related to COVID-19 sequelae. As a cross-sectional study, we were limited to comparing PCC and HC groups, and we did not assess changes that occurred prior to infection with COVID-19. Longitudinal studies are needed to explore how brain and cognitive changes evolve over time, including whether they are temporary or permanent.
Conclusions
This study provides evidence of increased intra- and inter-network FC across multiple brain networks in patients with PCC almost 2 years after the acute infection, particularly in cognitively relevant networks (e.g., DMN, ECN and SN). The positive correlations between increased connectivity and cognitive performance, together with the presence of fatigue and memory complaints, suggest a possible compensatory mechanism in response to COVID-19-induced brain dysfunction. However, this mechanism appears insufficient to fully restore normal brain function.
Methods
Participants
The sample included 158 participants from the DIANA and NAUTILUS project (ClinicalTrials.gov IDs: NCT05307549 and NCT05307575): 121 in the PCC group and 37 in the HC group. Participants with PCC met the following criteria: (a) age between 18 and 65 years; (b) confirmed diagnosis of COVID-19, according to WHO criteria, with symptoms and signs of disease during the acute phase; and (c) at least 12 weeks after the infection. The HC group comprised people with no history of symptoms compatible with Sars-CoV-2 infection and who did not have a positive test for Sars-CoV-2 infection prior to the study. The same exclusion criteria were used for both the PCC and HC groups: (a) no established diagnoses, before COVID-19, of neurological, psychiatric, neurodevelopmental or systemic pathologies known to cause cognitive deficits; and (b) no motor or sensory alterations that would obstruct neuropsychological evaluation. All participants were Spanish native speakers.
Recruitment occurred between June 2021 and December 2023. The study was approved by the Drug Research Ethics Committee of Consorci Sanitaria de Terrassa (reference: 02–20-107–070) and the Ethics Committee of the University of Barcelona (reference: IRB00003099). All participants provided written informed consent to participate in the study and all methods were performed in accordance with relevant national and international guidelines and regulations.
Neuropsychological assessment
Participants completed questionnaires in the following domains: demographics (e.g., age, sex, years of education, ethnicity, citizenship, profession, income); medical history and health-related behavior details and comorbidities; COVID-19 symptoms, treatment, hospitalization, and time since diagnosis; and post-COVID symptoms.
Cognitive assessment comprised a previously established comprehensive neuropsychological battery7. MoCA was used for general cognitive screening. The Matrix subtest from the Wechsler Adult Intelligence Scale (WAIS) III was used to assess abstract reasoning. RAVLT was used to assess verbal learning (total learning), delayed recall and recognition. The immediate and the 30-min delayed recall test from the Rey-Osterrieth Complex Figure Test (ROCF) was used for visual memory, with the copy trial of the ROCF used to evaluate visuo-constructive abilities. The WAIS-III Digit Span subtest was used to measure working memory (digit span backwards) and verbal attention (digit span forward). Parts A and B of the TMT were administered to measure visual scanning, motor speed and attention, and mental flexibility. Semantic fluency was assessed using the category “animals” by considering the correct number of animals recalled in one minute; also, the number of words beginning with P, M and R recalled in one minute was used to measure phonemic fluency. The three subtests of the Stroop were used (words, colors, and color words that conflict with the color in which they are presented) to measure cognitive control and selective attention. The Digit Symbol test from the WAIS-III was used to assess visual scanning, tracking and motor speed. The Boston Naming Test (BNT) was used to evaluate language.
The neuropsychological test scores have been validated using normative data available for our country, and they have been adjusted by age and education levels. Direct scores from these tests were transformed into Z-scores based on normative scales recommended previously, including the Digit Symbol, Matrix, Digit Span54, RAVLT55, TMT, Stroop, phonemic fluency, semantic fluency, BNT and ROCF tests56. Z-scores lower than -1.5 were considered indicative of lower expected cognitive performance in the test. Patients with lower-than-expected performance on two or more cognitive measurements from the previously specified tests, were categorized as cognitively impaired. Patients with lower-than-expected performance on fewer than two tests were considered non-cognitively impaired.
The Reading the Mind in the Eye Test was used to evaluate emotion recognition. The UPSIT-40 was conducted to assess odor identification performance. Additionally, the Word Accentuation Test (TAP) was included as an estimate of premorbid IQ.
Furthermore, the CFQ57 was used to assess fatigue, the GAD-758 to assess anxiety, the PHQ-959,60 to assess depression and the MFE test61 to assess memory complaints.
Evaluations were conducted by trained neuropsychologists. The time intervals between acute infection and evaluation were also registered.
MRI acquisition
MRIs were acquired with a 3 T scanner (MAGNETOM Prisma, Siemens, Germany). The scanning protocol used high-resolution 3-dimensional T1-weighted images acquired in the sagittal plane (repetition time [TR] = 2400 ms, time to echo [TE] = 2.22 ms, inversion time [TI] = 1000 ms, 208 slices, field of view [FOV] = 256 mm, 0.8 mm isotropic voxel), an axial fluid attenuated inversion recovery (FLAIR) sequence (TR = 6000 ms, TE = 397 ms). A resting-state (rs) sequence, 10-min-long functional gradient-echo echo-planar was acquired (480 T2*-weighted volumes, TR = 800 ms, TE = 37 ms, flip angle = 52º, slice thickness = 2 mm, FOV = 208 mm, in which subjects were instructed to keep their eyes closed and not to think of anything in particular and not fall asleep).
Preprocessing of rs-fMRI
The rs-fMRI images were preprocessed using in-house developed scripts that combined AFNI (http://afni.nimh.nih.gov/afni) and FSL (https://www.fmrib.ox.ac.uk/fsl) tools. To allow for magnetic stabilization, the first five volumes were discarded. In brief, our preprocessing workflow included brain extraction, despiking, motion correction, slice-timing correction and deobliquing to standardize anatomical alignment. Grand-mean scaling was applied to normalize signal intensity, followed by linear detrending and temporal filtering to retain frequencies above 0.01 Hz62. Data quality was ensured through visual inspection throughout the pre-processing stages.
Concerning head motion, an exclusion cut-off was established based on preprocessing summary parameters: ≥ 0.3 mm translation or 0.3° rotation for mean interframe motion; and ≥ 1 mm translation or 1° rotation for maximum interframe motion. Based on these criteria, 7 participants were excluded from the PCC group, leaving a sample of 121 participants.
To mitigate the effects of head motion further, we used an ICA based strategy for automatic removal of motion artifacts (ICA-AROMA)63. This decomposed data via ICA and automatically identified which components were related to head motion, based on four robust standardized features. Nuisance regression was also used to remove non-neural sources of signal variation. Average white-matter and cerebrospinal fluid signals extracted from the binary masks of segmented structural images were regressed out from the functional data, along with the six motion parameters obtained by motion correction64. Finally, data were registered to the Montreal Neurological Institute (MNI) space, using a two-stage registration procedure that included linear registration (from the functional to the anatomical individual spaces) and non-linear registration (from the subject to the MNI space).
ICA and dual regression
To characterize whole brain FC, preprocessed images were analyzed using MELODIC 3.0 from FSL (Analysis Group, FMRIB, Oxford, UK)65. A predetermined dimensionality of 50 was chosen, resulting in the extraction of 50 temporally and spatially coherent independent component networks (ICNs). The extracted functional patterns were classified as RSNs or noise/artifact via spatial cross-correlation with a template comprising 10 RSNs21. Additional verification was conducted through visual inspection based on described RSNs66.
Dual regression analysis67 was applied to the extracted ICNs, regardless of their identification. Voxel-wise one-sample t-tests were performed to determine the significant ICN maps per group, using non-parametric permutation testing with 5000 permutations. For intergroup connectivity analyses, subject maps per ICN were compared using a voxel-wise general linear model (GLM) with 5000 permutations for robust statistical evaluation.
Statistical maps were assessed at a statistical significance threshold of 0.05. Of the obtained significant clusters, only those within the RSN and of size greater than 40 voxels were considered. Unidentifiable ICNs, or those associated with noise or movement artefacts, were also excluded from further analysis.
Finally, we created binary masks per selected component, determined by Z ≥ 3, and the mean of each participant’s map was computed within these masks to measure mean connectivity strength.
Assessing inter-network connectivity
The FSL Nets toolbox (FSL, Analysis Group, FMRIB, Oxford, UK) was utilized to analyze inter-network connectivity. This toolbox allows for a comprehensive analysis of the first stage outputs of the dual regression (i.e., the component- and subject- specific time series). ICs labelled as noise were removed and regressed from the other valid time series. We derived connectivity matrices using full and partial correlation, as well as measures such as network amplitude and inter-network correlation.
FSL Nets also provided its own GLM function to perform statistical analyses and correction for multiple comparisons when looking for significant connections and differences between groups.
Other statistical analysis
Demographic, neuropsychological and clinical data were statistically analyzed using IBM SPSS version 27.0.1 (IBM Corp., Armonk, NY; 2020) and MATLAB (The MathWorks Inc., 2022). Between-group differences were assessed by applying t-tests or non-parametric analysis (Kruskal–Wallis H and Mann–Whitney U) as needed. Pearsons’s chi-square test was used for categorical variables. A GLM non-parametric F test with 1000 permutations was used to determine differences in cognitive performance and psychiatric questionnaires between groups; p-values were then corrected for FDR.
The mean connectivity strength of significant ICNs and inter-network correlations were correlated with neuropsychological and clinical by Pearson’s correlation or Spearman’s correlation, as needed, using IBM, SPSS and RStudio (https://www.R-project.org). FDR correction was used for multiple comparisons (p < 0.05).
Data availability
The datasets generated and analysed during the current study are not publicly available to protect participant’s privacy, but are available from the corresponding author upon reasonable request.
References
Lechner-Scott, J., Levy, M., Hawkes, C., Yeh, A., & Giovannoni, G. Long COVID or post COVID19 syndrome. Mult. Scler Relat. Disord. https://doi.org/10.1016/j.msard.2021.103268 (2021).
Perumal, R., et al. Long COVID: a review and proposed visualization of the complexity of long COVID. Front Immunol. https://doi.org/10.3389/fimmu.2023.1117464 (2023).
Mohammadi, S. & Ghaderi, S. Post-COVID-19 conditions: a systematic review on advanced magnetic resonance neuroimaging findings. Neurol. Sci. 45(5), 1815–1833 (2024).
Monje, M. & Iwasaki, A. The neurobiology of long COVID. Neuron 110(21), 3484–3496 (2022).
Soriano, J. B. et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 22(4), e102–e107 (2022).
Zhao, S., Toniolo, S., Hampshire, A. & Husain, M. Effects of COVID-19 on cognition and brain health. Trends Cogn Sci. 27(11), 1053–1067 (2023).
Ariza, M., et al. Neuropsychological impairment in post-COVID condition individuals with and without cognitive complaints. Front Aging Neurosci. 14, 1029842, https://doi.org/10.3389/fnagi.2022.1029842 (2022).
Díez-Cirarda, M. et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 146(5), 2142–2152 (2023).
Heine, J., et al. Structural brain changes in patients with post-COVID fatigue a prospective observational study. EClinicalMedicine. 58, 101874, https://doi.org/10.1016/j.eclinm.2023.101874 (2023).
Serrano del Pueblo, V. M., et al. Brain and cognitive changes in patients with long COVID compared with infection-recovered control subjects. Sci Rep. 14(1), 4997 (2024).
Pacheco-Jaime, L., et al. Structural brain changes in post-COVID condition and its relationship with cognitive impairment, Brain Communications, 7(1), fcaf070, https://doi.org/10.1093/braincomms/fcaf070 (2025).
Barnden, L., Thapaliya, K., Eaton-Fitch, N., Barth, M., & Marshall-Gradisnik, S. Altered brain connectivity in Long Covid during cognitive exertion: a pilot study. Front Neurosci. 17, 1182607; https://doi.org/10.3389/fnins.2023.1182607 (2023).
Catalonga, M., et al. Effects of hyperbaric oxygen therapy on functional and structural connectivity in post-COVID-19 condition patients: A randomized, sham-controlled trial. Neuroimage Clin. 36, 103218; https://doi.org/10.1016/j.nicl.2022.103218 (2022).
Li, R. et al. Altered intrinsic brain activity and functional connectivity in COVID-19 hospitalized patients at 6-month follow-up. BMC Infect Dis. 23(1), 521. https://doi.org/10.1186/s12879-023-08331-8 (2023).
Churchill, N. W., et al. Effects of post-acute COVID-19 syndrome on the functional brain networks of non-hospitalized individuals. Front Neurol. 14, 1136408; https://doi.org/10.3389/fneur.2023.1136408 (2023).
Bungenberg, J. et al. Characteristic functional connectome related to Post-COVID-19 syndrome. Sci Rep. 14(1), 4997. https://doi.org/10.1038/s41598-024-54554-3 (2024).
Esposito, F. et al. Olfactory loss and brain connectivity after COVID-19. Hum Brain Mapp. 43(5), 1548–1560 (2022).
Muccioli, L., et al. Cognitive and functional connectivity impairment in post-COVID-19 olfactory dysfunction. Neuroimage Clin. 38, 103410; https://doi.org/10.1016/j.nicl.2023.103410 (2023).
Lv, H. et al. Resting-State Functional MRI: Everything That Nonexperts Have Always Wanted to Know. Neuroimage Clin. 39(8), 1390–1399 (2018).
Seitzman, B. A., Snyder, A. Z., Leuthardt, E. C. & Shimony, J. S. The State of Resting State Networks. Top. Magn. Reson. Imaging 28(4), 189–196 (2019).
Smith, S. M. et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 106(31), 13040–13045 (2009).
Dennis, E. L. & Thompson, P. M. Functional Brain Connectivity using fMRI in Aging and Alzheimer’s Disease. Neuropsychol Rev. 24(1), 49–62 (2014).
Li, K., Su, W., Li, S.-H., Jin, Y., & Chen, H.-B. Resting State fMRI: A Valuable Tool for Studying Cognitive Dysfunction in PD. Parkinson Dis. 23, 6278649; https://doi.org/10.1155/2018/6278649 (2018).
Mulders, P. C., van Eijndhoven, P. F., Schene, A. H., Beckmann, F. C. & Tendolkar, I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev. 56, 330–344 (2015).
Nong, H., et al. Alterations in intra- and inter-network connectivity associated with cognition impairment in insulinoma patients. Front Endocrinol (Lausanne). 14, 1234921; https://doi.org/10.3389/fendo.2023.1234921 (2023).
Peraza, L. R. et al. Intra- and inter-network functional alterations in Parkinson’s disease with mild cognitive impairment. Hum Brain Mapp. 38(3), 1702–1715 (2017).
Xing, C., et al. Disrupted Functional Network Connectivity Predicts Cognitive Impairment in Presbycusis Patients. Front Aging Neurosci. 12, 246; https://doi.org/10.3389/fnagi.2020.00246 (2020).
La, C., et al. Age-related changes in inter-network connectivity by component analysis. Front Aging Neurosci. 7, 237; https://doi.org/10.3389/fnagi.2015.00237 (2015).
Voruz, P. et al. Brain functional connectivity alterations associated with neuropsychological performance 6–9 months following SARS-CoV-2 infection. Hum Brain Mapp. 44(4), 1629–1646 (2023).
Benedetti, F., et al. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: A multimodal magnetic resonance imaging study. Brain Behav Immun Health. 18, 100387; https://doi.org/10.1016/j.bbih.2021.100387 (2021).
Cattarinussi, G. et al. Altered brain regional homogeneity is associated with depressive symptoms in COVID-19. J Affect Disord. 313, 36–42 (2022).
Scardua-Silva, L. et al. Microstructural brain abnormalities, fatigue, and cognitive dysfunction after mild COVID-19. Sci Rep. 14(1), 1758. https://doi.org/10.1038/s41598-024-52005-7 (2024).
Chen, Z., et al. Neural connectome prospectively encodes the risk of post-traumatic stress disorder (PTSD) symptom during the COVID-19 pandemic. Neurobiol Stress. 15, 100378; https://doi.org/10.1016/j.ynstr.2021.100378 (2021).
Churchill, N. et al. Persistent post-COVID headache is associated with suppression of scale-free functional brain dynamics in non-hospitalized individuals. Brain Behav. 13(11), e3212. https://doi.org/10.1002/brb3.3212 (2023).
Fischer, D. et al. Disorders of Consciousness Associated with COVID-19: A Prospective Multimodal Study of Recovery and Brain Connectivity. Neurology 98(3), E315–E325 (2022).
Niroumand Sarvandani, M. et al. COVID-19 and the Brain: A Psychological and Resting-state Functional Magnetic Resonance Imaging (fMRI) Study of Whole-brain Functional Connectivity. Basic Clin Neurosci. 14(6), 753–772 (2023).
Zhang, H., Chung, T. W. H., Wong, F. K. C., Hung, I. F. N. & Mak, H. K. F. Changes in the Intranetwork and Internetwork Connectivity of the Default Mode Network and Olfactory Network in Patients with COVID-19 and Olfactory Dysfunction. Brain Sci. 12(4), 511. https://doi.org/10.3390/brainsci12040511 (2022).
Blazhenets, G. et al. Slow but Evident Recovery from Neocortical Dysfunction and Cognitive Impairment in a Series of Chronic COVID-19 Patients. J. Nucl. Med. 62(7), 910–915 (2021).
Kas, A. et al. The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur J Nucl Med Mol Imaging. 48(8), 2543–2557 (2021).
Dressing, A. et al. Neuropsychologic Profiles and Cerebral Glucose Metabolism in Neurocognitive Long COVID Syndrome. J Nucl Med. 63(7), 1058–1063 (2022).
Martini, A. L. Time-dependent recovery of brain hypometabolism in neuro-COVID-19 patients. Eur J Nucl Med Mol Imaging. 50(1), 90–102 (2022).
Servier, C., Porcher, R., Pane, I., Ravaud, P. & Tran, V. T. Trajectories of the evolution of post-COVID-19 condition, up to two years after symptoms onset. Int J Infect Dis. 133, 67–74 (2023).
Tu, Y. et al. Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol Psychiatry. 26(12), 7475–7480 (2021).
Leng, A. et al. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 12(5), 816. https://doi.org/10.3390/cells12050816 (2023).
Pajo, A. T., Espiritu, A. I., Apor, A. D. A. A. O. & Jamora, R. D. G. Neuropathologic findings of patients with COVID-19: a systematic review. Neurol. Sci. 42(4), 1255–1266 (2021).
Paolini, M. et al. Brain correlates of subjective cognitive complaints in COVID-19 survivors: A multimodal magnetic resonance imaging study. Eur. Neuropsychopharmacol. 68, 1–10 (2023).
Wylie, G. R., Yao, B., Genova, H. M., Chen. M. H., & DeLuca, J. Using functional connectivity changes associated with cognitive fatigue to delineate a fatigue network. Sci Rep. 10(1), 21927; https://doi.org/10.1038/s41598-020-78768-3 (2020).
Ceban, F. et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 101, 93–135 (2022).
Uddin, L. Q., Kelly, A. M. C., Biswal, B. B., Castellanos, F. X. & Milham, M. P. Functional Connectivity of Default Mode Network Components: Correlation, Anticorrelation, and Causality. Hum Brain Mapp. 30(2), 625–637 (2009).
Eyler, L. T. et al. Resting State Abnormalities of the Default Mode Network in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 70(1), 107–120 (2019).
Hampson, M., Driesen, N., Roth, J. K., Fore, J. C. & Constable, R. T. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 28(8), 1051–1057 (2010).
Hillary, F. G. et al. Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology 29(1), 59–75 (2015).
Krishnadas, R., et al. Resting state functional hyperconnectivity within a triple network model in paranoid schizophrenia. The Lancet. 383, S65; https://doi.org/10.1016/s0140-6736(14)60328-7 (2014).
Wechsler, D. WAIS-III: Escala de Inteligencia de Wechsler para Adultos III (ed. TEA Ediciones) (TEA Ediciones, Madrid, 1999).
Schmidt, M. Rey Auditory Verbal Learning Test: A Handbook (Western Psychological Services, Los Angeles, 1996).
Peña-Casanova, J. et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for Verbal Fluency Tests. Arch. Clin. Neuropsychol. 24(4), 395–411 (2009).
Jackson, C. The Chalder Fatigue Scale (CFQ 11). Occup Med (Chic Ill). 65(1), 86 (2015).
Spitzer, R. L., Kroenke, K., Williams, J. B. W. & Löwe, B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 166(10), 1092–1097 (2006).
Diez-Quevedo, C., Rangil, T., Sanchez-Planell, L., Kroenke, K. & Spitzer, R. L. Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom. Med. 63(4), 679–686 (2001).
Kroenke, K., Spitzer, R. L. & Williams, J. B. W. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16(9), 606–613 (2001).
Sunderland, A., Harris, J. & Baddeley, A. Do laboratory tests predict everyday memory? A neuropsychological study. J. Verbal Learn. Verbal Behav. 22, 341–357 (1983).
Campabadal, A., et al. Disruption of posterior brain functional connectivity and its relation to cognitive impairment in idiopathic REM sleep behavior disorder. Neuroimage Clin. 25, 102138; https://doi.org/10.1016/j.nicl.2019.102138 (2020).
Pruim, R. H. R. et al. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277 (2015).
Baggio, H. C., et al. Cerebellar resting-state functional connectivity in Parkinson’s disease and multiple system atrophy: Characterization of abnormalities and potential for differential diagnosis at the single-patient level. Neuroimage Clin. 22, 101720; https://doi.org/10.1016/j.nicl.2019.101720 (2019).
Beckmann, C. F., & Smith, S. M. Probabilistic independent component analysis for functional magnetic resonance imaging. FMRIB Technical Report TR02CB1. (2004).
Sala-Llonch, R., Bartrés-Faz, D., Junqué, C. Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol. 6, 663, https://doi.org/10.3389/fpsyg.2015.00663 (2015).
Beckmann, C., Mackay, C., Filippini, N. & Smith, S. Group comparison of resting-state fMRI data using multi-subject ICA and dual regression. Neuroimage 47, S39–S41 (2009).
Funding
This work was sponsored by the Generalitat de Catalunya (SGR 2021SGR00801), Agency for Management of University and Research Grants (AGAUR) from the Generalitat de Catalunya (Pandemies, 2020PANDE00053), La Marató de TV3 Foundation (202111–30-31–32), TED2021-130409B-C51 and TED2021-130409A-C52 by MCIU/AEI/https://doi.org/10.13039/501100011033 and Unión Europea NextGenerationEU/PRTR and supported by María de Maeztu Unit of Excellence (Institute of Neurosciences, University of Barcelona) (CEX2021-001159-M), Ministry of Science and Innovation. JP was supported by a fellowship from Spanish Ministry of Economy and Competitiveness (PRE2021-099674). JO was supported by a fellowship from Spanish Ministry of Science, Innovation and Universities (PRE2018-086675). IR was supported by a fellowship from La Caixa Fundation (LCF/BQ/DR22/11950012). CGV was supported by a fellowship from Spanish Ministry of Science, Innovation and Universities (PREP2023-001119)
Author information
Authors and Affiliations
Consortia
Contributions
MA, CJ, RSL, and BS contributed to the conceptualization and design of the study. LPJ, CGV, NC, IR, CCL, JP, LCV, CMB and the NAUTILUS Project Collaborative Group contributed to the acquisition of data. LCV, LPJ, CGV, and CMB contributed to the pre-process and analysis of the data. LCV, BS, RSL, LPJ, and CGV contributed to the interpretation of the data. LCV, BS, LPJ, and CGV contributed to the preparation of the first draft of the article. LCV, LPJ, MA, NC, MG, CGV, IR, CCL, JO, JP, CMB, AC, RSL, NB, CB, JB, UC, CJ, and BS revised the manuscript critically for important intellectual content and approved the final version of the manuscript. MG, UC, CJ and BS contributed to the acquisition of funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Carreras-Vidal, L., Pacheco-Jaime, L., Ariza, M. et al. Functional brain abnormalities in post COVID-19 condition and their relationship with cognition. Sci Rep 15, 22259 (2025). https://doi.org/10.1038/s41598-025-00739-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00739-3