Abstract
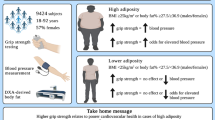
Grip strength, a surrogate for quantifying strength, correlates with function and longevity. However, this relationship is less clear in women with breast cancer. 138 women undergoing oncologic treatment for breast cancer were enrolled across three institutional review board-approved protocols with three months of resistance training with pre and post assessment of body composition, phase angle, functional movement screen (FMS), balance, weight lifted (load), quality of life, activity levels, and hand grip strength. Significant increases in maximum, minimum, and mean grip strength were seen. Mean grip strength increased by 12.6% and 3.5 kg. Right/left (R/L) mismatch significantly decreased from baseline to post-intervention (13.3 to 8.7%). On univariable analysis, greater baseline mean grip strength was associated with younger age, greater baseline FMS, composite load lifted, activity levels, and decreased R/L balance mismatch. On multivariable analysis, greater mean grip strength was independently associated with greater baseline mobility and composite load lifted. Baseline mean grip strength was associated with baseline lower bodyfat percentage and greater muscle mass, whole body phase angle, and resting metabolic rate (all significant on multivariable analysis, R2 = 0.247). Grip strength may be a valuable surrogate biomarker within breast cancer survivorship care, particularly in resource-limited settings.
Similar content being viewed by others
Introduction
Handgrip strength is a practical and readily available biomarker associated with strength, functional status, prognosis, and overall health across a range of patient populations1. Among older adults, each standard deviation increase in grip strength is associated with a 5.8% reduction in all-cause mortality2. However, it is rarely used in oncologic clinical practice to assess performance status or tolerance of treatment, despite the high risks of fragility and sarcopenia from both cancer diagnoses and treatment.
As the relative mortality rates for patients with early-stage breast cancer now approximate those for population-matched controls, functional status and overall health are increasingly relevant to breast cancer survivorship care3. A safe, easy, and cost-effective method to measure functional status in the clinic during and after treatment is needed to help monitor patients and minimize morbidity and mortality. Among breast cancer survivors, greater grip strength is significantly associated with superior overall mortality and health-related quality of life, while absolute grip strength is inversely related to risk of developing breast cancer (HR 0.93, HR 0.91–0.96, p= 0.03)4,5,6,7. Such findings are of particular concern given the associations of surgery, radiation therapy, chemotherapy, and hormone therapy to sarcopenia and decreased quality of life8,9,10.
The emerging field of exercise oncology seeks to optimize oncologic outcomes and quality of life through exercise regimens designed to optimize strength, metabolic, and functional parameters. A growing body of data suggest that principles of hypertrophy established in non-oncologic populations are both safe and effective in breast cancer survivors, including high-intensity dose escalated resistance training that utilizes compound movements across a range of functional movement patterns11,12,13 However, noninvasive and cost-effective methods to track physical improvement remain limited, particularly in the breast cancer setting. We hypothesize that, among breast cancer survivors, grip strength is a useful surrogate for quantifying improvements in strength and functional status that may correlate to metabolic parameters. Particularly in resource-limited settings, grip strength may serve as a valuable surrogate biomarker for longitudinal assessment within emerging exercise oncology programs. Thus, this work analyzes the results of three clinical trials assessing grip strength changes from intense resistance training in a population of women undergoing treatment for breast cancer.
Methods
Participants
Women undergoing oncologic treatment for breast cancer were enrolled across three institutional review board-approved (Allegheny Health Network Institutional Review Board) protocols registered at cliicaltrials.gov (NCT05747209, NCT05978960, and NCT06083324. Informed consent was obtained from all subjects, and all research was performed in accordance with relevant guidelines/regulations, and in accordance with the Declaration of Helsinki. While inclusion criteria varied slightly across these trials, all participants were women > 18 years old receiving some combination of oncologic surgery (mastectomy or lumpectomy) with or without axillary sentinel lymph node biopsy, axillary lymph node dissection, anti-estrogen therapy, cytotoxic chemotherapy, and/or radiation therapy. Inclusion criteria included women aged 20–95 years old diagnosed with breast cancer and able to get up and down from the floor and squat their body weight. Exclusion criteria included the inability to engage in group exercise, pregnancy, and severe arthritic or cardiovascular conditions deemed unsafe to engage in resistance training. Individuals on chemotherapy were excluded from two of the studies.
Exercise regimen
All participants completed a three-month resistance training exercise regimen under the direct supervision of dually certified MD and Certified Strength and Conditioning Specialist personnel, previously described in detail11,14. In brief, all participants were screened for safety with respect to medical comorbidities and baseline functional mobility including the ability to perform basic functional mobility patterns. The resistance training program, derived from evidence-based principles for optimal induction of hypertrophy15,16,17, emphasized dose escalation of high-intensity compound exercises across four primary movement patterns: split squat, trap bar deadlifts, incline dumbbell bench press, and bird dog row. To avoid overestimating increases in load lifted from initial neuromuscular adaptation to novel stimuli, baseline measurements in load lifted (pounds x repetitions x sets) were performed following the first month of the exercise regimen. Total load lifted was then remeasured at completion of month 3, with compound load lifted calculated as the sum of load across all four compound exercises.
Anthropometric, metabolic, and functional parameters
Baseline and post-regimen assessment included current exercise adherence, body composition, quality of life, balance, and mobility. Body composition parameters (i.e., percent body fat and muscle mass; bone mineral content [g/cm]), whole body phase angle (degrees), and resting metabolic rate (calories/day) were measured with bioimpedance analysis. Balance was measured via the Y-balance test, functional mobility via the Functional Movement Screen (FMS), and patient-reported quality of life via the Godin Leisure-Time Exercise Questionnaires. Further details on these methods are previously reported11.
Grip strength
Grip strength was measured using a Jamar Hand Dynamometer device. Participants were instructed to sit comfortably while holding the forearm in a neutral position and elbow bent at a 90-degree angle consistent with the American Society of Hand Therapists guidelines18. In this position, participants were encouraged to squeeze the dynamometer as hard as possible in each hand. The highest of 3 measurements was recorded at each timepoint for each hand. Statistical analysis included the following grip strength parameters: maximum right/left (R/L) value across both left and right measurements (kg), minimum R/L value (kg), mean R/L value (kg), and percent R/L mismatch, calculated as the absolute difference in R/L values divided by the R/L mean.
Statistical analysis
All anthropometric, metabolic, functional, and quality of life parameters were analyzed as continuous variables. Pairwise comparison was assessed via the paired t test. Multivariable linear regression was performed for all non-collinear parameters demonstrating significant correlation on univariable linear regression with α = 0.05. No data were missing for hand grip strength, demographic, anthropometric, functional or metabolic parameters. Given the rarity of missing quality of life data (2 instances), participants with missing data were excluded from analysis. All statistical analyses were performed using R version 4.1.2 (R Project for Statistical Computing).
Results
138 participants completed a three-month resistance training regimen under the direct supervision of dually certified MD, CSCS personnel. Patient demographics, pre-intervention body composition, and cancer treatments are provided in Table 1. Median age at enrollment was 54.5 years (interquartile range [IQR], 46.3–64.0 years) with a median BMI of 28.9 kg/m2 (IQR 24.4–33.1 kg/m2). A majority of participants (84%) had stage 0–2 breast cancer, with 56.5% of participants undergoing lumpectomy and 82.6% completing sentinel lymph node biopsy alone (82.6%) rather than axillary lymph node dissection. Receipt of anti-estrogen therapy was documented in 70.3%, cytotoxic chemotherapy in 16.7%, and radiation therapy in 80.4%.
Regarding Jamar dynamometer grip strength (Table 2), significant baseline to post-intervention pair-wise increases were observed for R/L maximum ([median 24 kg, IQR 20–28 kg] to [27 kg, IQR 24–30 kg]), R/L minimum ([20 kg, IQR 16–24 kg] to [24 kg, IQR 20–27 kg]), and R/L mean ([22.5 kg, IQR 17.6–26.0 kg] to [26.0 kg, IQR 22.1–28.4 kg) values (all p < 0.001). Across all participants, R/L mean grip strength increased by a mean of 12.6% (IQR 1.8–31.5% increase), resulting in an absolute mean improvement of 3.5 kg (IQR 2.4–4.5 kg). R/L percent mismatch in grip strength significantly decreased from baseline (13.3%, IQR 6.5–25.8%) to post-intervention (8.7%, IQR 3.9–15.4%; p < 0.001). As seen in Supplemental Table 1, pair-wise comparison of pre- and post-intervention metabolic parameters demonstrated significant decreases in BMI and body fat percentage, as well as significant increases in muscle mass percentage, whole body phase angle, and resting metabolic rate. As seen in Supplemental Table 2, while the limited distribution of pre- and post-intervention values precluded formal statistical analysis of parameters scored on a Likert scale, Godin Leisure-Time Exercise and Euro-QoL Group EQ-5D responses assessed on a continuous scale uniformly showed significant increases in patient-reported quality of life.
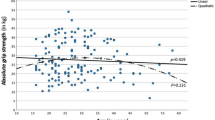
Table 3 summarizes linear regression models for parameters associated with baseline R/L mean grip strength. On univariable analysis, greater baseline R/L mean grip strength was significantly associated with younger age (R2 = 0.116, p < 0.001), greater baseline Y-balance (R2 = 0.116, p < 0.001), decreased baseline Y-balance R/L mismatch (R2 = 0.060, p = 0.004), greater FMS baseline (R2 = 0.227, p < 0.001), greater baseline composite load lifted across compound exercises (R2 = 0.316, p < 0.001), and baseline Godin questionnaire scores (R2 = 0.083, p < 0.001). On multivariable analysis, greater R/L mean grip strength was independently associated with greater baseline FMS (p = 0.032) and greater baseline composite load lifted (p < 0.001; model R2 = 0.360).
Table 4 reports grip strength with respect to metabolic parameters. Baseline R/L mean grip strength was associated with lower baseline bodyfat percentage (R2 = 0.057, p = 0.005), greater baseline muscle mass percentage (R2 = 0.065, p = 0.002), greater whole body phase angle (R2 = 0.183, p < 0.001), and greater resting metabolic rate (R2 = 0.057, p = 0.003). These correlations all remained statistically significant on multivariable analysis (model R2 = 0.247), while excluding bodyfat percentage due to collinearity with muscle mass percentage. Baseline R/L grip strength mismatch demonstrated no association with metabolic parameters.
Table 5 provides univariable and multivariable linear regression models exploring parameters associated with pre- to post-intervention percent improvement in R/L mean grip strength. On univariable analysis, significant correlation to greater R/L mean grip strength improvement was observed across the following parameters: lower baseline FMS (R2 = 0.151, p < 0.001), lower baseline composite load lifted (R2 = 0.086, p < 0.001), and lower baseline Godin score, without significant correlation to pre/post-regimen improvements across the same parameters. Absolute improvement in pre- to post-regimen Godin scores were not significantly associated with improvements in mean R/L grip strength. Multivariable analysis of treatment and functional parameters demonstrated no significant correlation to percent improvement in R/L mean grip strength with a trend toward greater baseline composite load lifted (p = 0.06).
Discussion
Significant increases in grip strength were observed across breast cancer survivors completing a three-month, dose-escalated resistance training regimen employing high-intensity compound exercises. In line with the well-established utilization of grip strength as a simple and practical surrogate for strength and functional status, baseline grip strength was independently associated with greater baseline FMS and composite load lifted across compound exercises. As presently seen and previously reported, these increases in grip strength were observed alongside corresponding improvements in body composition, functional mobility, and quality of life11. These data may lend support for utilization of grip strength as a simple and practical surrogate parameter within breast cancer survivorship care.
Among breast cancer patients at baseline, the present data demonstrate that greater baseline grip strength correlates not only to generalized muscular strength and functional parameters, but also to a range of favorable baseline metabolic parameters (muscle mass, phase angle, and resting metabolic rate)19,20,21,22. In the present data, such correlations in functional and metabolic parameters appear to be stronger than those of grip strength to oncologic treatment parameters. Aside from cytotoxic chemotherapy, oncologic treatments including surgical management of the breast and axilla, anti-estrogen therapy, and radiation therapy did not significantly correlate with baseline grip strength. Independent of exercise and strength goals, these data inform the use and interpretation of grip strength as a biomarker among breast cancer survivors.
Given the well-defined association of grip strength to long-term mobility and quality of life, related improvements in grip strength, general muscular strength, and functional mobility are of prime importance23. Moreover, while relative survival rates for women with early stage disease approximate those of the general population3, breast cancer patients are at high risk for obesity, sarcopenia, and decreased quality of life from systemic, surgical, and radiation therapies8,9. In this high-risk population, the present data, while not randomized against a control group, may suggest that a high intensity exercise regimen may lead to significant improvements in strength and functional status which are quantifiable through the surrogate biomarker of grip strength. Notably, improvement in grip strength showed stronger association to baseline values rather than the magnitude of pre- to post-regimen improvement across strength, balance, and functional mobility parameters. Regarding quality of life, greater baseline grip strength was significantly associated with higher Godin scores, while improvements in hand grip strength were significantly associated with higher baseline Godin scores though not with greater improvements in pre- to post-regimen Godin scores. These findings may support the importance of strength training as a prophylactic measure in the general population, particularly among those of young and middle age. Although meta-analysis suggests 5.0 kg as a clinically meaningful difference in grip strength22, the present mean pre- to post-regimen improvement in grip strength of 3.5 kg (IQR 2.4–4.5 kg) is better interpreted as a surrogate measure in their direct clinical context of related improvements across strength, functional, metabolic, and quality of life parameters.
The present study has several limitations. While the reported median BMI of 29 is slightly above the median value for American women 50–70 years old, participants who engage in exercise studies may nevertheless be more motivated to exercise than the general population24. The small number of patients across a number of prospective trial protocols may limit the ability to detect small but clinically meaningful associations in grip strength across oncologic treatment parameters as seen in other reports10 Limited longitudinal data address the utilization of grip strength across breast cancer survivors, regardless of adherence to an exercise regimen. Future studies should analyze correlation of grip strength to clinical and oncologic outcomes in the presence versus absence of an accompanying exercise regimen. Additionally, the majority of patients had stage I or II breast cancer, so the generalizability across more advanced disease may be limited. That being said, 44% of patients did undergo mastectomy as part of their treatment. Lastly, though changes were similar across the studies and the exercise regimen was the same, varying nutritional intakes of participants during the protocols may have impacted the results and is a confounding factor.
In conclusion, among breast cancer survivors, grip strength correlates significantly with favorable increases across strength, functional, and metabolic parameters. Improvements in grip strength were associated with baseline values rather than pre- to post-regimen improvements across strength, balance, and functional mobility parameters. While the present authors would advocate for direct and longitudinal measurement of functional, metabolic, and strength parameters as a gold standard within the emerging field of exercise oncology, grip strength may be a valuable surrogate biomarker within breast cancer survivorship care, particularly in resource-limited settings.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to ongoing analysis and manuscript creation but are available from the corresponding author on reasonable request.
References
López-Bueno, R. et al. Associations of handgrip strength with all-cause and cancer mortality in older adults: a prospective cohort study in 28 countries. Age Ageing. 51, 1–11 (2022).
Wang, Y. et al. Association of grip strength and comorbidities with all-cause mortality in the older hypertensive adults. Front. Public. Heal. 11, 1162425 (2023).
Marcadis, A. R., Morris, L. G. T. & Marti, J. L. Relative survival with Early-Stage breast Cancer in screened and unscreened populations. Mayo Clin. Proc. 97, 2316–2323 (2022).
Parra-Soto, S., Pell, J. P., Celis-Morales, C. & Ho, F. K. Absolute and relative grip strength as predictors of cancer: prospective cohort study of 445 552 participants in UK biobank. J. Cachexia Sarcopenia Muscle. 13, 325–332 (2022).
Cantarero-Villanueva, I. et al. The handgrip strength test as a measure of function in breast cancer survivors: relationship to cancer-related symptoms and physical and physiologic parameters. Am. J. Phys. Med. Rehabil. 91, 774–782 (2012).
Paek, J. & Choi, Y. J. Association between hand grip strength and impaired health-related quality of life in Korean cancer survivors: a cross-sectional study. BMJ Open. 9 (2019).
Zhuang, C. et al. Associations of low handgrip strength with cancer mortality: a multicentre observational study. J. Cachexia Sarcopenia Muscle. 11, 1476 (2020).
Guigni, B. A. et al. Skeletal muscle atrophy and dysfunction in breast cancer patients: role for chemotherapy-derived oxidant stress. Am. J. Physiol. Cell. Physiol. 315, C744–C756 (2018).
Klassen, O. et al. Muscle strength in breast cancer patients receiving different treatment regimes. J. Cachexia Sarcopenia Muscle. 8, 305–316 (2017).
Van der Weijden-Van Doornik, E. M., Slot, D. E., Burtin, C. & van der Weijden, G. A. Grip strength in women being treated for breast Cancer and receiving adjuvant endocrine therapy: systematic review. Phys. Ther. 97, 904–914 (2017).
Carpenter, D. J. et al. EXERT-BC: A pilot study of an exercise regimen designed to improve functional mobility, body composition, and strength after the treatment for breast cancer. Cancer Med. 13, e7001 (2024).
Shaibi, G. Q. et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med. Sci. Sport Exerc. 38, 1208–1215 (2006).
Schoenfeld, B. J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength. Cond Res. 24, 2857–2872 (2010).
Champ, C. E. et al. EXERT-BC: prospective study of an exercise regimen after treatment for breast cancer. Sport Med. Int. Open. https://doi.org/10.1055/a-2193-0922 (2023).
Schoenfeld, B. & Grgic, J. Evidence-based guidelines for resistance training volume to maximize muscle hypertrophy. Strength. Cond J. 40, 107–112 (2018).
Schoenfeld, B. J., Ogborn, D. & Krieger, J. W. Dose-response relationship between weekly resistance training volume and increases in muscle mass: A systematic review and meta-analysis. J. Sports Sci. 35, 1073–1082 (2017).
Champ, C. E. et al. Resistance training for patients with cancer: A conceptual framework for maximizing strength, power, functional mobility, and body composition to optimize health and outcomes. Sport Med. https://doi.org/10.1007/s40279-022-01759-z (2022).
Sousa-Santos, A. R. & Amaral, T. F. Differences in handgrip strength protocols to identify sarcopenia and frailty - a systematic review. BMC Geriatr. 17, 238 (2017).
Bohannon, R. W., Magasi, S. R., Bubela, D. J., Wang, Y. C. & Gershon, R. C. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve. 46, 555–558 (2012).
Bohanon, R. W. Are hand-grip and knee extension strength reflective of a common construct? Percept. Mot Skills. 114, 514–518 (2012).
Soyuer, F., Cankurtaran, F., Menevşe, Ö. & Zararsiz, G. E. Examination of the correlation between hand grip strength and muscle mass, balance, mobility, and daily life activities in elderly individuals living in nursing homes. 74, 1371–1378. https://doi.org/10.3233/WOR-205075 (2022).
Bohannon, R. W. Minimal clinically important difference for grip strength: a systematic review. J. Phys. Ther. Sci. 31, 75 (2019).
Rantanen, T. et al. Midlife hand grip strength as a predictor of old age disability. JAMA 281, 558–560 (1999).
Chen, Z. et al. Body mass index, waist circumference, and mortality in a large multiethnic postmenopausal Cohort-Results from the women’s health initiative. J. Am. Geriatr. Soc. 65, 1907–1915 (2017).
Funding
None.
Author information
Authors and Affiliations
Contributions
Author contributions: DJC, CP, JR, AKD, CEC: Design, data analysis, manuscript drafting, revision, and final approval. CH and RK: Data analysis, manuscript drafting, revision, and final approval.
Corresponding author
Ethics declarations
Competing interests
CEC receives income from books and lectures pertaining to nutrition and exercise and is on the scientific advisory board for Simply Good Foods. DJC, CP, JR, AKD, RK, and CH declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Champ, C.E., Peluso, C., Hilton, C. et al. Grip strength as a surrogate measure of strength, functional, and metabolic parameter increases in breast cancer survivors undergoing an exercise regimen. Sci Rep 15, 15782 (2025). https://doi.org/10.1038/s41598-025-00867-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00867-w