Abstract
Gastric cancer (GC) is a prevalent digestive tract malignancy, and Huangqi Fuling decoction (HF) has shown potential in enhancing immune function and exhibiting anti-GC activity. However, its mechanisms remain unclear. This study utilized network pharmacology, molecular docking, and in vitro experiments to preliminarily explore the mechanisms by which HF inhibits gastric cancer invasion and metastasis while promoting apoptosis. Public databases identified differentially expressed genes (DEGs), HF targets, and GC-related genes. GO and KEGG analyses revealed signaling pathways. Clinical relevance, immune infiltration, immunotherapy, and molecular docking of hub genes were analyzed. Eight hub genes—PTGS2, MMP9, SELE, CCL2, VCAM1, ICAM1, CXCL2, and CXCL10—associated with the TNF signaling pathway were identified. HF inhibits the invasion and metastasis of GC cells by down-regulating MMP9 and PTGS2 expression, while inducing apoptosis by suppressing BCL-2 expression and promoting BAX expression. Additionally, HF can arrest the cell cycle, blocking AGS cells in the S phase and HGC-27 cells in the G0/G1 phase. This study confirms that HF promotes apoptosis and inhibits metastasis and invasion in GC cells, primarily by modulating the TNF signaling pathway. Additionally, the anti-tumor effects of HF on GC may involve immune regulatory mechanisms, but the mechanism require further experimental verification.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is a tumor of the digestive tract, with its incidence ranking fifth and mortality ranking third among cancers1,2. In China, GC is the second leading cause of cancer-related deaths, following lung cancer3,4. The current treatment options for GC primarily include surgical resection, radiotherapy, chemotherapy, and targeted therapy5,6. However, for patients with metastatic GC, surgical eradication remains difficult, and chemotherapy agents, while effective, often come with significant toxicity and side effects. Additionally, these treatments can lead to drug resistance in some patients7,8. Recent studies have highlighted the potential of certain traditional Chinese medicines in cancer treatment, such as reducing chemotherapy-related side effects and drug resistance, while also exhibiting anti-tumor properties by boosting the patient’s immune response and alleviating inflammation.
Huangqi Fuling Decoction (HF) is a traditional Chinese herbal formula originating from the classical Chinese medical text Qianjin Fang. Its ingredients include Astragalus membranaceus Fisch. ex Bunge. (huangqi, HQ), Poria cocos (Schw.) Wolf. (Fu Ling, FL), Cinnamomum cassia (L.) D.Don. (guixin, GX), Ophiopogon japonicus (Thunb.) Ker Gawl. (maidong, MD), Ligusticum wallichii Franch. (chuanxiong, CX), Zingiber officinale Roscoe. (shengjiang, SJ), Ziziphus jujuba Mill. (dazao, DZ), and Kadsura longepedunculata Finet & Gagnep. (nanwuweizi. NWWZ), the ratio of them is 3:2:2:3:2:4:4:1. The names of the aforementioned plants have been checked with http://www.worldfloraonline.orgas of December 1, 2024. There are limited English publications on HF, but existing studies indicate that HF has significant therapeutic effects on diabetic nephropathy9and proliferative diabetic retinopathy10. As an adjuvant to chemotherapy drugs, HF can notably alleviate oxaliplatin-induced side effects in colorectal cancer patients and reduce the severity of post-chemotherapy nausea and vomiting11,12. Additionally, HF exhibits potent anti-inflammatory13, hepatoprotective14,15, and immunomodulatory effects16. Chinese literature reports that HF is also effective in treating postpartum urinary retention, renal edema, and chronic heart failure. HQ and FL are the two primary herbal ingredients in HF. HQ and its extracts are commonly used in combination with chemotherapy drugs to enhance tumor sensitivity while reducing the toxic side effects associated with chemotherapy17,18. Additionally, they have also been proven to exhibit significant anti-inflammatory19,20and anti-tumor activities21,22. These compounds can boost immune function by promoting the proliferation and maturation of immune cells, thereby enhancing the immune system’s ability to recognize and eliminate cancer cells23,24. FL is an edible saprophytic fungus known for its various therapeutic effects. FL and its extracts have been shown to regulate immunity25,26, anti-tumor27,28, anti-inflammation29, anti-oxidation30,31, liver protection32,33 and so on.
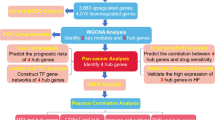
In this study, we found that HF may inhibit GC invasion and metastasis through the TNF signaling pathway. In addition, based on the analysis of the database, HF may also be related to immune regulation and has the potential to enhance the immunity of patients. However, further experimental verification is still needed. Cell experiments showed that HF regulates the expression of MMP9, PTGS2, BCL-2, and BAX, promoting apoptosis while inhibiting invasion and metastasis in GC cells. Moreover, HF may influence immune function by regulating chemokines such as CXCL2, CXCL10, VCAM1, and ICAM1. The study design is illustrated in Fig. 1.
Materials and methods
Acquisition of HF target genes and construction of PPI network
The HF comprised of eight components: HQ, FL, DZ, MD, SJ, GX, CX, and NWWZ. We utilized the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://old.tcmsp-e.com/tcmsp.php) to apply the inclusion criteria of drug-likeness (DL) ≥ 0.1 and oral bioavailability (OB) ≥ 30%. The target genes for the aforementioned traditional Chinese medicine components were identified, and the protein-protein interaction (PPI) network for each component and its corresponding targets was constructed using Cytoscape 3.7 software. The active components of HF and their target genes are provided in Supplementary Materials 1 and 2.
Acquisition of GC-related genes and DEGs
We used “gastric cancer” as the search term and obtained GC-related genes from the GeneCards database (https://www.genecards.org/), and GC microarray data were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/). Based on the sample information, we selected the GSE118916 dataset as the research subject, which contains gene expression data from 15 pairs of GC and adjacent tissues. Genes that were up-regulated or down-regulated were screened using the criteria of |LogFC|>1 and p-value < 0.05. A volcano plot was generated using Prism 6.0 software. The list of GC-related genes and GC microarrays can be found in Supplementary Materials 3 and 4.
Acquisition of the intersection genes
The intersection genes were input into the “Multiple proteins” module of STRING database (https://cn.string-db.org/). Select “Homo sapiens” in the Organisms option, submit it, and then download the corresponding PPI network as a TSV file. Then, we constructed a PPI network of the intersecting genes using Cytoscape v3.7.2 software, based on degree values. Prism 6.0 software was used to visualize the intersection genes with a degree value greater than 10. Gene Set Enrichment Analysis (GSEA) of the DEGs was performed using the online tool (http://www.bioinformatics.com.cn/). The intersection genes were subsequently imported into the Metascape database (https://metascape.org/) to conduct Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. Select “H. sapiens” for both the “Input as species” and “Analysis as species” options. Set the Min Overlap to 3, the p-Value Cutoff to 0.01, the Min Enrichment to 1.5, and the Min Network Size to 3 respectively. Finally, use the online tool (http://www.bioinformatics.com.cn/) to visualize the pathway information. Lastly, choose the TNF signaling pathway as the research focus, and the intersection genes involved in this pathway were identified as hub genes. The molecular functions of these hub genes in the TNF signaling pathway were then annotated.
Clinical relevance analysis of hub genes
Firstly, we utilized the “Gene Expression Differential Analysis” module of the Sangerbox 3.0 database (http://sangerbox.com/home.html) to analyze the expression differences of hub genes between GC and normal tissues. The “Clinical Stage and Gene Expression” module was then used to examine the expression of hub genes across different stages and grades. Next, we assessed the copy number variations of hub genes in GC and normal tissues using the “Expression DIY” module of the GEPIA database (http://gepia.cancer-pku.cn/). Finally, the correlation between the expression of hub genes and survival was analyzed using Kaplan-Meier website (https://kmplot.com/).
Correlation analysis of hub genes with immune infiltration and immunotherapy
Associations between hub genes and immune infiltration were analyzed using the “Immune Infiltration” module of the Sangerbox 3.0 database. The correlation between hub genes and immune cells was assessed using the “Dataset” module of the TISCH2 database (http://tisch.comp-genomics.org/) and the “Immune Cell Analysis” module of the Sangerbox 3.0 database. Finally, the “Immunotherapy” module of the ROC Plotter database (https://www.rocplot.org/) was used to evaluate the expression changes of hub genes following immunotherapy (PD-1 treatment) in GC.
Molecular docking of hub genes with quercetin
We used Cytoscape 3.7 to construct a PPI network of hub genes and active ingredients, identifying quercetin as a key ingredient of HF. Next, we retrieved the 2D and 3D structures of quercetin from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). High-resolution crystal structures with relatively complete configurations were selected as receptors from the RCSB PDB database (https://www.rcsb.org/). The PYMOL software was used to remove water molecules and heteroatoms from the receptors. Finally, AutoDock Tools software (http://autodock.scripps.edu/) and AutoDock Vina (https://scripps.edu) were employed for molecular docking, using quercetin as the ligand and the protein crystal structures of the hub proteins as the receptors. The docking results with the lowest binding energy were visualized using PYMOL software.
Determination of the optimal drug concentration
GES-1, AGS, and HGC-27 cells were seeded into 96-well plates and allowed to attach. After cell adhesion, different concentrations of HF (Cat. No. bencaowu 999, CR SANJIU, China) were added for 24 and 48 h, respectively. Following drug treatment, Cell counting kit-8 (CCK-8) reagent was added, and optical density (OD) was measured. Cell viability was calculated using the formula: cell viability (%) = [(drug group OD – blank OD)/(no drug group OD – blank OD)] × 100%.
GES-1 cells (Cat. No. 337969, BNCC, China) were cultured in DMEM medium (Cat. No. 10566016, Gibco, USA); AGS cells (Cat. No. CL-0022, Procell, China) were cultured in DME/F12 medium (Cat. No. 11320033, Gibco, USA); and HGC-27 cells (Cat. No. CL-0107, Procell, China) were cultured in RPMI 1640 medium (Cat. No. 11875119, Gibco, USA). All culture media were supplemented with 10% fetal bovine serum (Cat. No. 12483020, Gibco, USA) and 1% penicillin-streptomycin (Cat. No. PB180120, Procell, China). AGS and HGC-27 cells were obtained from Wuhan Pricella Biotechnology Co.,Ltd. All cell lines were authenticated by STR profiling.
Wound scratch and transwell
HGC-27 and AGS cells were seeded into 6-well plates. Once cell confluence reached 80%, different concentrations of HF were added, and straight lines were drawn using a 10 µL pipette tip. After cleaning with PBS, cell migration was recorded at 0 h, 24 h, and 48 h, respectively. Cell mobility was then calculated.
Transwell chambers (Cat. No. Costar3422, Corning, USA) and Matrigel (Cat. No. B-P-00002-2, B-P-00002–4, B-P-00002–10, Biozellen, China) were used for the assay. The experimental procedure followed the manufacturer’s instructions for Matrigel.
Cell cycle and cell apoptosis
AGS and HGC-27 cells were seeded into 6-well plates, and when cell confluence reached 30%, different concentrations of HF were added. After 24 h, cells were stained according to the instructions of the cell cycle detection kit (Cat. No. KGA512, KeyGEN BioTECH, China) and the apoptosis detection kit (Cat. No. KGA107, KeyGEN BioTECH, China). After staining, cell cycle progression and apoptosis were analyzed by flow cytometry. Notably, for cell cycle analysis, cells were starved for 12 h prior to treatment with HF to synchronize the cell cycle.
qRT-PCR
Total RNA was extracted from GC cells using Trizol reagent. Both reverse transcription polymerase chain reaction (RT-PCR) (Cat. No. RR047 A, TaKaRa, Japan) and qRT-PCR (Cat. No. RR820 A, TaKaRa, Japan) kits were purchased from TaKaRa Corporation. The experimental procedures were performed according to the manufacturer’s instructions. The primer sequences are shown in Table 1.
Western blotting
GC cells were treated with HF for 24 and 48 h. Total protein was extracted using RIPA lysis buffer (Cat. No. P0013 C, China), and protein concentration was determined using the BCA kit (Cat. No. P0012, China). The proteins were then separated by 7.5% SDS-PAGE (Cat. No. PG211, Epizyme Biomedical Technology, China). PVDF membranes were used for protein transfer, and 5% skim milk was used for blocking. The proteins were subsequently incubated with primary and secondary antibodies. Protein signals were visualized using ECL chemiluminescence solution (Cat. No. 34075, Thermo Fisher, USA). The primary antibodies used were: anti-actin (Cat. No. 81115-1-RR, Proteintech, China), anti-MMP9 (Cat. No. 10375-2-AP, Proteintech, China), anti-PTGS2 (Cat. No. 66351-1-Ig, Proteintech, China), anti-BAX (Cat. No. 50599-2-Ig, Proteintech, China), and anti-BCL-2 (Cat. No. 68103-1-Ig, Proteintech, China).
Statistical analysis
All statistical analyses and plots were generated using GraphPad Prism 6.0. Data are expressed as the mean ± standard deviation (SD). T-tests or one-way ANOVA were used for statistical comparison. Asterisks denote statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Acquisition of HF ingredient target genes and construction of PPI network
From the TCMSP database, we obtained 68 target genes for SJ, 29 target genes for FL, 23 target genes for NWWZ, 292 target genes for MD, 211 target genes for HQ, 65 target genes for GX, 229 target genes for DZ, and 81 target genes for CX. The results are shown in Fig. 2A. We screened the top 10 key active components of HF decoction based on the number of target genes contained in each active component. The results are shown in Table 2.The PPI network analysis of HF active ingredients and their target genes revealed that PTGS2, PTGS1, CHRM1, CHRM2, CHRM3, and NCOA2 were common target genes shared by multiple ingredients (Fig. 2B). From the GC microarray, we identified 1817 DEGs, as shown in Fig. 2C. Additionally, 11,366 GC-related genes were retrieved from the GeneCards database, and 45 intersection genes were found in DEGs, HF target genes, and GC-related genes (HF-GC-DEGs) (Fig. 2D). The heatmap of intersection genes revealed that approximately 3/4 of the genes were up-regulated in GC, while 1/4 showed significant down-regulation (Fig. 2E). Among the top 20 intersection genes with the most significant expression differences, 14 genes were up-regulated, and 6 genes were down-regulated (Fig. 2F).
Construction of the PPI network for HF and identification of HF-GC-DEGs. (A) Number of active components in HF and their associated target genes. (B) PPI networks between the active components of HF and their corresponding target genes. (C) Volcano plot of DEGs in the GC microarray. (D) Venn diagram illustrating HF-GC-DEGs. (E) Heatmap of HF-GC-DEG expression in the GC microarray. (F) Top 20 differentially expressed genes among HF-GC-DEGs in the GC microarray. HF-GC-DEGs: The intersection genes of DEGs, HF target genes, and GC-related genes.
The intersection genes are related to cell adhesion and TNF signaling pathway
The PPI network of intersection genes revealed that the top six intersection genes with the highest degree value were IL6, PTGS2, MYC, MMP9, MMP2, and CCL2 (Fig. 3A), with 20 genes showing degree values greater than 10 (Fig. 3B). GSEA analysis indicated that DEGs were primarily associated with extracellular matrix receptor interactions, adhesion plaques, and cancer signaling pathways (Fig. 3C). Gene Ontology (GO) analysis of the intersection genes showed that in biological processes, intersection genes are mainly related to positive regulation of cell migration, cellular response to lipids, response to nutrients, etc. In cellular components, the genes were associated with caveola, collagen-containing extracellular matrix, focal adhesion, etc. In molecular function, they are mainly related to CXCR-chemokine receptor binding, oxidoreductase activity, protein kinase C binding, etc. (Fig. 3D). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis demonstrated that intersection genes were involved in pathways such as the AGE-RACE signaling pathway in diabetic complications and the TNF signaling pathway, etc. (Fig. 3E). The Sankey diagram results illustrated that MMP9, PTGS2, CXCL2, CCL2, CXCL8, SELE, ICAM1, and VCAM1 in the intersection genes were involved in the TNF signaling pathway (Fig. 3F). Within this pathway, CCL2, CXCL2, and CXCL10 are associated with leukocyte recruitment; MMP9 with cell migration; and VCAM1, ICAM1, and SELE with cell adhesion and immune regulation. PTGS2 is involved in the synthesis of inflammatory mediators (Fig. 3G).
Construction of the PPI network and pathway enrichment analysis of HF-GC-DEGs. (A) PPI network of HF-GC-DEGs. (B) Top 20 HF-GC-DEGs with the highest degree centrality. (C) Gene set enrichment analysis (GSEA) of HF-GC-DEGs. (D) Gene Ontology (GO) enrichment analysis of HF-GC-DEGs. (E) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of HF-GC-DEGs. (F) Sankey diagram illustrating the associations between HF-GC-DEGs and signaling pathways. (G) Molecular functions of hub genes within the TNF signaling pathway.
These findings suggest that HF may modulate the expression of intersection genes within the TNF signaling pathway, potentially inhibiting the inflammatory response, regulating immune function, and suppressing the invasion and metastasis GC.
Relationship between hub gene expression and patient survival
The analysis showed that hub gene expression levels were significantly higher in GC tissues compared to normal gastric tissues at the mRNA level (Fig. 4A). Similar results were observed at the copy number and protein levels (Fig. 4B-C). In examining hub gene expression across clinical stages, VCAM1, SELE, and CCL2 displayed significant variations across stages, while CXCL2, CXCL10, and ICAM1 showed significant differences across grade stages (Fig. 4D). Furthermore, the correlation between hub gene expression and overall survival (OS) revealed that, except for SELE (ELAM1), VCAM1, and CCL2, other genes had a strong association with OS. Notably, higher expression levels of MMP9, CXCL2 (GRO2), CXCL10, and PTGS2 correlated positively with OS, whereas ICAM1 expression was negatively associated with patient OS (Fig. 4E).
Clinical relevance analysis of hub genes. (A) Differential expression of hub genes between GC and adjacent tissues, with data sourced from the SangerBox database. (B) Copy number variations of hub genes in GC and adjacent tissues, with data retrieved from the GEPIA database. (C) Differences in protein expression levels of hub genes between GC and normal gastric tissues, with data from The Human Protein Atlas database. (D) Expression differences of hub genes across various GC stages, with data from the GEPIA database. (E) Correlation between hub gene expression and the survival rates of GC patients, using data from the Kaplan-Meier Plotter database.
The above findings suggest that the expression of hub genes in GC is generally upregulated and closely associated with clinical stages and patient survival. These genes may serve as potential targets through which HF exerts its inhibitory effects on the progression of GC.
The hub genes are associated with immune infiltration and immunotherapy
Tumor immune infiltration is a critical factor in cancer development, and immunotherapy has emerged as one of the most effective treatments for cancer. Correlation analysis between the hub genes and immune infiltration revealed that, except for PTGS2 and CXCL2, all other hub genes showed a significant positive correlation with immune infiltration (Fig. 5A). Additionally, all hub genes exhibited strong associations with immune cells, whereas most demonstrated significant correlations with stromal cells, with the exception of CXCL2 (Fig. 5B).
Correlation analysis between hub genes, immune infiltration, and immunotherapy. (A) Immunoscore analysis of hub genes in GC, using data from the SangerBox database. (B) Correlation between hub genes and immune cells in GC, based on data from the TISCH2 database. (C) Correlation analysis between hub genes and six major immune cells in GC, with data from the SangerBox database. (D) Correlation analysis of hub genes with PD1 immunotherapy response in GC, using data obtained from the ROC Plotter database.
We further examined the correlation between the hub genes and six major immune cell types, including B cells, CD4 T cells, CD8 T cells, neutrophils, macrophages, and dendritic cells. Most hub genes displayed strong positive correlations with these immune cell types, with the exceptions of CXCL2 and PTGS2 (Fig. 5C).Lastly, we assessed the relationship between hub gene expression and response to PD1 immunotherapy. The results showed that in the response group, the expression levels of MMP9, ICAM1, CXCL10, and CXCL2 were significantly elevated following PD1 treatment. Conversely, the expression levels of ICAM1, SELE, and CCL2 were significantly reduced, while PTGS2 expression remained unchanged (Fig. 5D).
The above analysis results suggest that HF may participate in and regulate the immune function of gastric cancer patients through such pathways as activating immune cells. However, the specific mechanism still needs to be further verified.
Molecular docking of hub genes with quercetin
The PPI network results of hub genes and the active ingredients of HF revealed that PTGS2 was a common target of multiple ingredients, suggesting that PTGS2 could be one of the main target genes of HF in its anti-gastric cancer effect. Among all the active ingredients, all the hub genes were associated with quercetin, which was the only active ingredient that contained all the hub genes (Fig. 6A). We obtained the 2D and 3D structures of quercetin (Fig. 6B-C) and performed molecular docking with hub proteins, and the docking results were shown in Fig. 6D. We visualized the result with the lowest docking energy and marked the name of amino acid residues at the binding site and the size of hydrogen bonds (Fig. 6E).
Molecular docking of hub genes with quercetin. (A) PPI network of major active ingredients and hub genes. (B) 2D structure of quercetin. (C) 3D structure of quercetin. (D) Heatmap of molecular docking scores between hub genes and quercetin. (E) Visualization of the molecular docking result with the lowest binding energy using PyMOL. In the image: proteins are shown in bright blue, quercetin in green, interacting amino acid residues in red, amino acid stick structures in orange, and hydrogen bonds as yellow dashed lines, with bond lengths indicated by numeric labels.
PTGS2 exhibited the best docking energy with quercetin (−9.2 kCal/mol), followed by MMP9 and CXCL2, with docking energies of −7.7 kCal/mol and − 7.6 kCal/mol, respectively. In contrast, CXCL10 and SELE showed weaker docking interactions with quercetin, with docking energies of −6.4 kCal/mol and − 6.7 kCal/mol, respectively. It is generally believed that the smaller the docking energy, the better the docking effect will be. A docking energy less than 0 kCal/mol suggests that the drug and protein can bind under natural conditions, while a docking energy lower than − 5 kCal/mol indicates a strong affinity. In our study, the docking energies of all target proteins with quercetin were below − 5 kCal/mol, implying that quercetin can bind closely with these targets in the natural state. As the main active ingredient of HF, quercetin may exert its anti-gastric cancer effects by regulating the expression of these target proteins.
HF can inhibit the metastasis and invasion ability of GC cells
HF treatment significantly inhibited the migration of AGS cells in a dose-dependent manner (Fig. 7A), and similar effects were observed in HGC-27 cells (Fig. 7B). Additionally, AGS and HGC-27 cells were treated with different concentrations of HF for 24 h to evaluate its impact on GC cell invasion. As shown in Figs. 7C-D, HF treatment markedly reduced the invasion ability of both AGS and HGC-27 cells, with higher HF concentrations resulting in more pronounced inhibition of GC cell invasion.
Impact of HF at varying concentrations on the migration and invasion of gastric cancer cells. (A) Effect of HF on the migration of AGS cells, evaluated using a wound-scratch assay and corresponding statistical analysis. (B) Effect of HF on the migration of HGC-27 cells, assessed using a wound-scratch assay and corresponding statistical analysis. (C) Effect of HF on the invasion of AGS cells, evaluated using a transwell assay with Matrigel and corresponding statistical analysis. (D) Effect of HF on the invasion of HGC-27 cells, assessed using a transwell assay with Matrigel and corresponding statistical analysis. Data are shown as mean ± SD, with statistical significance denoted by * p < 0.05, ** p < 0.01, *** p < 0.001.
HF can arrest the cell cycle and promote apoptosis of GC cells
GC cells were treated with varying concentrations of HF for 24 h, and cell cycle were analyzed. The results revealed that HF treatment induced cell cycle arrest in the S phase of AGS cells, accompanied by a reduction in the G2/M and G0/G1 phases (Fig. 8A). Conversely, in HGC-27 cells, HF treatment resulted in cell cycle arrest in the G0/G1 phase, with a decrease in the S and G2/M phases (Fig. 8B).
Impact of HF at varying concentrations on the cell cycle and apoptosis in gastric cancer cells. (A) Cell cycle distribution of AGS cells treated with varying concentrations of HF and corresponding statistical analysis. (B) Cell cycle distribution of HGC-27 cells treated with varying concentrations of HF and corresponding statistical analysis. (C) Ratio of apoptosis of AGS cells treated with various concentrations of HF, with corresponding statistical analysis. (D) Ratio of apoptosis of HGC-27 cells treated with various concentrations of HF, with corresponding statistical analysis. Data are presented as mean ± SD, with significance levels indicated as * p < 0.05, ** p < 0.01, *** p < 0.001.
To further explore the effect of HF on apoptosis in GC cells, AGS and HGC-27 cells were treated with different concentrations of HF for 24 h. The findings demonstrated a significant increase in the number of apoptotic cells following HF treatment. Moreover, the proportion of apoptotic cells was positively correlated with the concentration of HF (Fig. 8C-D).
HF can regulate the expression of metastasis-related and apoptosis-related proteins
The sensitivity of GES-1, AGS, and HGC-27 cells to HF treatment was evaluated using the CCK-8 assay. The results indicated that GES-1 cells exhibited the lowest sensitivity to HF, while HGC-27 cells showed the highest sensitivity after 24 h and 48 h of treatment (Fig. 9A). The 50% inhibitory concentration (IC50) of HF for GC cells was determined to be 4.5 mg/mL. Based on this, 4 mg/mL was selected as the low concentration, 6 mg/mL as the medium concentration, and 8 mg/mL as the high concentration for subsequent experiments. We investigated the impact of HF on the expression of hub genes, and the results showed that HF treatment promoted the expression of CXCL2 while decreasing the expression of MMP9, PTGS2 and CCL2 in both AGS and HGC-27 cells (Fig. 9B). To further investigate, we selected the metastasis-related proteins MMP9 and PTGS2 for validation. In addition, to explore the mechanism underlying HF-induced apoptosis in GC cells, we also assessed the expression of BAX and BCL-2. The results demonstrated that, after 24 h and 48 h of HF treatment, the protein expression levels and mRNA level of BCL-2, PTGS2, and MMP9 were significantly reduced in AGS cells, while BAX expression was increased (Fig. 9C-D). In HGC-27 cell, after 24 h of HF treatment, BCL-2, PTGS2, and MMP9 expression were decreased, but BAX expression did not change significantly (Fig. 9E). After 48 h of HF treatment, BCL-2 and PTGS2 expression were decreased, The expression of Bax was up-regulated, while MMP9 expression showed a downward trend but without statistical significance. (Fig. 9F).
Impact of HF at varying concentrations on the expression of metastasis- and apoptosis-related proteins in gastric cancer cells. (A) Cell viability after treatment with varying concentrations of HF for 24 h and 48 h. (B) Differential expression of mRNA for hub genes in AGS and HGC-27 cells after 48 h of treatment with various concentrations of HF. (C-D) Changes in the expression levels of BCL-2, BAX, PTGS2, and MMP9 at the protein and mRNA levels in AGS cells after 24 h and 48 h of HF treatment. (E-F) Changes in the expression levels of BCL-2, BAX, PTGS2, and MMP9 at the protein and mRNA levels in HGC-27 cells after 24 h and 48 h of HF treatment. Data are shown as mean ± SD, with significance levels indicated by * p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
GC is a tumor of the digestive tract that has the characteristics of strong concealment, high mortality, poor prognosis, frequent recurrence, and a tendency for metastasis. In this study, we identified the hub genes and signaling pathways involved in HF’s inhibition of GC progression using network pharmacology and molecular docking approaches. We also analyzed the correlation between hub genes and immune infiltration, and immunotherapy. Finally, we preliminarily explored the molecular mechanisms by which HF inhibits the invasion and metastasis of gastric cancer cells and promotes the apoptosis of gastric cancer cells at the cellular level. However, the drawback is that the verification through animal experiments has not been carried out yet. Further research is required for the in-depth molecular mechanisms and the regulatory effects on the immune system.
Firstly, we identified the hub genes of HF active ingredients, GC-related genes, and DEGs. A total of 45 intersection genes were screened. Subsequently, we constructed the PPI network of intersection genes and performed GO and KEGG analyses on intersection genes. The TNF signaling pathway was selected as the research object, and the intersection genes within this pathway were identified as the key targets of HF, and the clinical correlation and immune infiltration were analyzed. The clinical correlation analysis revealed that the expression of the hub genes in GC was significantly higher than in normal gastric tissues. Moreover, these genes were associated with the clinical stage of GC and the prognosis of GC patients. Additionally, we explored the correlation between hub genes and immune infiltration, immune cells, and immunotherapy in GC. The results indicated that all hub genes were significantly correlated with immune cells, with the exception of PTGS2 and CXCL2. Most of the other target genes showed a significant positive correlation with immune infiltration and major immune cells. In the correlation analysis between immunotherapy and gene expression, there were also differences in the expression of hub genes between the response and non-response groups to PD1 treatment.
Then, by constructing PPI networks of hub genes and active components, we identified that PTGS2 as a common target gene for several components, this suggests that PTGS2 may be an important target gene for HF to inhibit GC invasion and metastasis. Furthermore, we found that quercetin was the only component containing all the hub genes, which might be an important active ingredient of HF in the anti-gastric cancer effect. Molecular docking results showed that the eight hub genes had a significant affinity for quercetin, with docking energies all below − 6 kcal/mol. which further indicated that quercetin was the main active ingredient of HF. It may exert its anti-metastatic and pro-apoptotic effects on GC cells by regulating the expression of these eight hub genes.
Finally, we tested the drug sensitivity of GSE-1, AGS, and HGC-27 cells to HF and verified its effects on promoting apoptosis and inhibiting invasion and metastasis in GC cells. The results from the wound scratch assay and the Transwell assay demonstrated that HF could significantly inhibit the invasion and metastasis of GC cells. Cell cycle analysis showed that HF could block AGS cell in S phase and HGC-27 cell in G0/G1 phase. The results of the apoptosis assay revealed that HF could promote apoptosis of AGS and HGC-27 in a concentration-dependent manner. Given that high concentrations of HF killed more than half of the GES-1 cells, we selected low and medium concentrations of HF for subsequent mechanistic studies. We found that HF could down-regulate the expression of MMP9 and PTGS2 through the TNF signaling pathway, thereby inhibiting the invasion and metastasis of GC cells. Additionally, we detected the expression of the apoptosis-related proteins BAX and BCL2 observed that HF could down-regulate the expression of BCL2 and up-regulate the expression of BAX, which may explain why HF can promote the apoptosis of GC cells.
Quercetin is a flavonol containing o-diphenol hydroxyl and m-diphenol hydroxyl groups. It is widely present in vegetables, fruits, seeds, and other natural herbal medicines. Previous studies have shown that quercetin has significant anti-inflammatory34,35,36, antiviral37,38, anti-tumor39,40,41, immune regulation42, liver protection43,44,45, and other drug activities. In GC, quercetin can induce morphological changes and promote apoptosis in AGS cells46. As an adjuvant to chemotherapy drugs, quercetin can enhance the chemosensitivity of GC cells to irinotecan47. It can also improve the multidrug resistance of GC patients to 5-fluorouracil (5-FU), adriamycin, tamoxifen, paclitaxel, and other chemotherapy drugs48.
PTGS2, also known as COX2, is a key inducer of prostaglandin biosynthesis and participates in multiple biological processes, including the inflammatory response. In GC, PTGS2 serves as a biomarker for guide treatment and predict the prognosis49,50. When combined with chemotherapy drugs, PTGS2 can alleviate the resistance of GC patients to cisplatin and 5-FU and promote the apoptosis of GC cells51,52. It can also inhibit the invasion and metastasis of GC by regulating mTOR signaling pathways53,54. In addition, PTGS2 mutations can also increase the risk of inflammation and GC55. In this study, as a common target gene of multiple active components in HF, PTGS2 is a potentially important target gene for promoting apoptosis and inhibiting invasion and metastasis of GC. In addition to PTGS2, other hub genes were also associated with the poor progression of GC. The expression levels of VCAM1 and ICAM1 were correlated positively with the progression and poor prognosis of GC56,57. CXCL2 and CXCL10 are down-regulated in GC, which can significantly reduce the migration ability of GC58. CXCL2 can regulate the peritoneal metastasis of GC59. MMP9 can be used as a marker for the diagnosis and prognosis of aggressive GC60and can regulate the invasion and metastasis of GC through the EMT pathway61,62. CCL2 is associated with the infiltration of tumor-associated macrophages and trastuzumab resistance in GC63. Down-regulation expression of SELE can reduce the inflammation caused by surgical stress, thereby reducing the risk of hematogenous metastasis of GC64.
These results suggest that HF can inhibit the invasion and metastasis of GC cells by down-regulating the expressions of MMP9 and PTGS2, and promote the apoptosis by down-regulating the expression of BCL2 and up-regulating the expression of BAX. In addition, the results of network pharmacology and clinical correlation analysis showed that HF inhibited the progression of GC, which may also be related to regulating the expression of chemokines such as CXCL2, CXCL10, VCAMI, ICAM1, and so on, and achieved the purpose of anti-metastasis and pro-apoptosis of GC cells by improving immune function. We will prove this ratiocination in subsequent studies.
Conclusions
In summary, HF can inhibit gastric cancer invasion and metastasis by regulating the expression of MMP9 and PTGS2 within the TNF signaling pathway, and promote cancer cell apoptosis by modulating BCL2 and BAX levels. In addition, HF may enhance the immune function of patients by regulating the expression of chemokines in target proteins and the activation of immune cells, thereby exhibiting anti-cancer activity. However, this is merely a reasonable speculation based on literature reports and database analysis, and further experimental verification is still needed. A possible mechanism is illustrated in Fig. 10.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- GC:
-
Gastric cancer
- HF :
-
Huangqi Fuling decoction
- DEGs:
-
Differentially expressed genes in gastric cancer microarray.
- HF-GC-DEGs:
-
The intersection genes of DEGs, HF target genes, and GC-related genes.
- GO:
-
Gene Ontology.
- BP:
-
Biological process.
- CC:
-
Cellular component.
- MM:
-
Molecular function
- KEGG:
-
The Kyoto encyclopedia of genes and genomes.
- TCMSP:
-
Traditional chinese medicine systems pharmacology database and analysis platform.
- MW:
-
Molecular weight.
- DL:
-
Drug-likeness.
- OB:
-
Oral bioavailability.
- PPI:
-
Protein-protein Interaction.
- GSEA:
-
Gene set enrichment analysis.
- OD:
-
Optical density.
- CCK-8:
-
Cell counting kit-8.
- RT-PCR:
-
Reverse transcription polymerase chain reaction.
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction.
- IC50:
-
50% Inhibitory concentration.
References
Gullo, I. et al. Precancerous lesions of the stomach, gastric cancer and hereditary gastric cancer syndromes. Pathologica 112, 166–185. https://doi.org/10.32074/1591-951X-166 (2020).
Van Cutsem, E., Sagaert, X., Topal, B., Haustermans, K. & Prenen, H. Gastric cancer. Lancet 388, 2654–2664. https://doi.org/10.1016/S0140-6736(16)30354-3 (2016).
Feng, R. M., Zong, Y. N., Cao, S. M. & Xu, R. H. Current cancer situation in China: good or bad news from the 2018 global Cancer statistics?? Cancer Commun. (Lond). 39, 22. https://doi.org/10.1186/s40880-019-0368-6 (2019).
Cao, M., Li, H., Sun, D. & Chen, W. Cancer burden of major cancers in China: A need for sustainable actions. Cancer Commun. (Lond). 40, 205–210. https://doi.org/10.1002/cac2.12025 (2020).
Cats, A. et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 19, 616–628. https://doi.org/10.1016/S1470-2045(18)30132-3 (2018).
Zhao, X. et al. Deep Learning-Based protein features predict overall survival and chemotherapy benefit in gastric Cancer. Front. Oncol. 12, 847706. https://doi.org/10.3389/fonc.2022.847706 (2022).
Wei, L. et al. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol. Cancer. 19, 62. https://doi.org/10.1186/s12943-020-01185-7 (2020).
Zhou, Y. et al. FAM117B promotes gastric cancer growth and drug resistance by targeting the KEAP1/NRF2 signaling pathway. J. Clin. Invest. 133, https://doi.org/10.1172/JCI158705 (2023).
Chen, X. et al. Huangqi (astragalus) Decoction ameliorates diabetic nephropathy via IRS1-PI3K-GLUT signaling pathway. Am. J. Transl Res. 10, 2491–2501 (2018).
Huai, B. et al. Systematic evaluation of combined herbal adjuvant therapy for proliferative diabetic retinopathy. Front. Endocrinol. 14, 1157189. https://doi.org/10.3389/fendo.2023.1157189 (2023).
Han, J. et al. Efficacy and safety of traditional plant-based medicines for preventing chronic oxaliplatin-induced peripheral neurotoxicity in patients with colorectal cancer: A systematic review and meta-analysis with core herb contribution. J. Ethnopharmacol. 326, 117735. https://doi.org/10.1016/j.jep.2024.117735 (2024).
Chen, M. H., May, B. H., Zhou, I. W., Zhang, A. L. & Xue, C. C. Integrative medicine for relief of nausea and vomiting in the treatment of colorectal Cancer using Oxaliplatin-Based chemotherapy: A systematic review and Meta-Analysis. Phytother Res. 30, 741–753. https://doi.org/10.1002/ptr.5586 (2016).
Ma, L. et al. The therapeutic effects of traditional Chinese medicine on insulin resistance in obese mice by modulating intestinal functions. Heliyon 10, e30379. https://doi.org/10.1016/j.heliyon.2024.e30379 (2024).
Yimam, M., Jiao, P., Hong, M. & Jia, Q. Hepatoprotective activity of an herbal composition, MAP, a standardized blend comprising Myristica Fragrans, Astragalus membranaceus, and Poria cocos. J. Med. Food. 19, 952–960. https://doi.org/10.1089/jmf.2016.0048 (2016).
Li, J. et al. Polysaccharides from Chinese herbal medicine: a review on the hepatoprotective and molecular mechanism. Chin. J. Nat. Med. 22, 4–14. https://doi.org/10.1016/s1875-5364(24)60558-3 (2024).
Bai, Y. et al. Explore the mechanism of Astragalus Membranaceus and Poria cocos drug pair in improving immunity based on network Pharmacology. Medicine 103, e38531. https://doi.org/10.1097/md.0000000000038531 (2024).
Li, C. et al. Astragalus polysaccharides increase the sensitivity of SKOV3 cells to cisplatin. Arch. Gynecol. Obstet. 297, 381–386. https://doi.org/10.1007/s00404-017-4580-9 (2018).
Li, H. et al. Astragalus IV undermines Multi-Drug resistance and Glycolysis of MDA-MB-231/ADR cell line by depressing hsa_circ_0001982-miR-206/miR-613 Axis. Cancer Manag Res. 13, 5821–5833. https://doi.org/10.2147/CMAR.S297008 (2021).
Fan, J., Jia, F., Liu, Y. & Zhou, X. Astragalus polysaccharides and Astragaloside IV alleviate inflammation in bovine mammary epithelial cells by regulating Wnt/beta-catenin signaling pathway. PLoS One. 17, e0271598. https://doi.org/10.1371/journal.pone.0271598 (2022).
Xu, J. et al. Astragalus polysaccharides attenuate Ovalbumin-Induced allergic rhinitis in rats by inhibiting NLRP3 inflammasome activation and NOD2-Mediated NF-kappaB activation. J. Med. Food. 24, 1–9. https://doi.org/10.1089/jmf.2020.4750 (2021).
Wang, D. et al. Application of dendritic cells in tumor immunotherapy and progress in the mechanism of anti-tumor effect of Astragalus polysaccharide (APS) modulating dendritic cells: a review. Biomed. Pharmacother. 155, 113541. https://doi.org/10.1016/j.biopha.2022.113541 (2022).
Jiao, J., Yu, J., Ji, H. & Liu, A. Synthesis of macromolecular Astragalus polysaccharide-nano selenium complex and the inhibitory effects on HepG2 cells. Int. J. Biol. Macromol. 211, 481–489. https://doi.org/10.1016/j.ijbiomac.2022.05.095 (2022).
Xu, S. et al. pH-responsive Astragalus polysaccharide-loaded PLGA nanoparticles as an adjuvant system to improve immune responses. Int. J. Biol. Macromol. 222, 1936–1947. https://doi.org/10.1016/j.ijbiomac.2022.09.283 (2022).
Liu, J. et al. Evaluation of the effects of Astragalus polysaccharides as immunostimulants on the immune response of crucian carp and against SVCV in vitro and in vivo. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 253, 109249. https://doi.org/10.1016/j.cbpc.2021.109249 (2022).
Tian, H., Liu, Z., Pu, Y. & Bao, Y. Immunomodulatory effects exerted by Poria cocos polysaccharides via TLR4/TRAF6/NF-kappaB signaling in vitro and in vivo. Biomed. Pharmacother. 112, 108709. https://doi.org/10.1016/j.biopha.2019.108709 (2019).
Dong, X. et al. Poria cocos polysaccharide induced Th1-type immune responses to ovalbumin in mice. PLoS One. 16, e0245207. https://doi.org/10.1371/journal.pone.0245207 (2021).
Lee, C. Y. et al. Poria cocos Regulates Cell Migration and Actin Filament Aggregation in B35 and C6 Cells by Modulating the RhoA, CDC42, and Rho Signaling Pathways. Evid Based Complement Alternat Med https://doi.org/10.1155/2021/6854860 (2021).
Qin, L., Huang, D., Huang, J., Qin, F. & Huang, H. Integrated analysis and finding reveal Anti-Liver Cancer targets and mechanisms of Pachyman (Poria cocos Polysaccharides). Front. Pharmacol. 12, 742349. https://doi.org/10.3389/fphar.2021.742349 (2021).
Wu, Y., Li, D., Wang, H. & Wan, X. Protective effect of Poria cocos polysaccharides on fecal Peritonitis-Induced Sepsis in mice through Inhibition of oxidative stress, inflammation, apoptosis, and reduction of Treg cells. Front. Microbiol. 13, 887949. https://doi.org/10.3389/fmicb.2022.887949 (2022).
Fang, C. L. et al. Poria cocos (Fuling) targets TGFbeta/Smad7 associated collagen accumulation and enhances Nrf2-antioxidant mechanism to exert anti-skin aging effects in human dermal fibroblasts. Environ. Toxicol. 36, 729–736. https://doi.org/10.1002/tox.23075 (2021).
Zhao, J. et al. Poria cocos polysaccharides attenuated ox-LDL-induced inflammation and oxidative stress via ERK activated Nrf2/HO-1 signaling pathway and inhibited foam cell formation in VSMCs. Int. Immunopharmacol. 80, 106173. https://doi.org/10.1016/j.intimp.2019.106173 (2020).
He, J. et al. Effects of Poria cocos extract on metabolic dysfunction-associated fatty liver disease via the FXR/PPARalpha-SREBPs pathway. Front. Pharmacol. 13, 1007274. https://doi.org/10.3389/fphar.2022.1007274 (2022).
Cheng, Y. et al. Structural characterization and hepatoprotective activity of a Galactoglucan from Poria cocos. Carbohydr. Polym. 263, 117979. https://doi.org/10.1016/j.carbpol.2021.117979 (2021).
Aleebrahim-Dehkordi, E. et al. Quercetin and its role in reducing the expression of Pro-inflammatory cytokines in osteoarthritis. Antiinflamm Antiallergy Agents Med. Chem. 21, 153–165. https://doi.org/10.2174/1871523022666221213155905 (2023).
Foudah, A. I. et al. Quercetin Attenuates Nitroglycerin-Induced Migraine Headaches by Inhibiting Oxidative Stress and Inflammatory Mediators. Nutrients 14, https://doi.org/10.3390/nu14224871 (2022).
Singh, S., Sahu, K., Kapil, L., Singh, C. & Singh, A. Quercetin ameliorates lipopolysaccharide-induced neuroinflammation and oxidative stress in adult zebrafish. Mol. Biol. Rep. 49, 3247–3258. https://doi.org/10.1007/s11033-022-07161-2 (2022).
F, D. I. P. et al. Quercetin Phytosome(R) as a potential candidate for managing COVID-19. Minerva Gastroenterol. (Torino). 67, 190–195. https://doi.org/10.23736/S2724-5985.20.02771-3 (2021).
Chen, N. et al. Quercetin Inhibits Hsp70 Blocking of Bovine Viral Diarrhea Virus Infection and Replication in the Early Stage of Virus Infection. Viruses 14, https://doi.org/10.3390/v14112365 (2022).
Davoodvandi, A., Shabani Varkani, M., Clark, C. C. T. & Jafarnejad, S. Quercetin as an anticancer agent: focus on esophageal cancer. J. Food Biochem. 44, e13374. https://doi.org/10.1111/jfbc.13374 (2020).
Reyes-Farias, M. & Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20133177 (2019).
Vinayak, M. & Maurya, A. K. Quercetin loaded nanoparticles in targeting cancer: recent development. Anticancer Agents Med. Chem. 19, 1560–1576. https://doi.org/10.2174/1871520619666190705150214 (2019).
Shen, P. et al. Potential implications of Quercetin in autoimmune diseases. Front. Immunol. 12, 689044. https://doi.org/10.3389/fimmu.2021.689044 (2021).
Yang, H. et al. Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in Db/db mice. Phytother Res. 33, 3140–3152. https://doi.org/10.1002/ptr.6486 (2019).
Sotiropoulou, M. et al. Nonalcoholic fatty liver disease: the role of Quercetin and its therapeutic implications. Saudi J. Gastroenterol. 27, 319–330. https://doi.org/10.4103/sjg.sjg_249_21 (2021).
Jafari-Garageshlaghi, F., Hashtarkhani, F., Soraya, H. & Malekinejad, H. Quercetin protected from aluminum Phosphide-induced acute and subacute Cardio- and hepatotoxicity in rats. Curr. Pharm. Des. 28, 3513–3524. https://doi.org/10.2174/1381612829666221130123706 (2022).
Shang, H. S. et al. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ. Toxicol. 33, 1168–1181. https://doi.org/10.1002/tox.22623 (2018).
Lei, C. S., Hou, Y. C., Pai, M. H., Lin, M. T. & Yeh, S. L. Effects of Quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J. Nutr. Biochem. 51, 105–113. https://doi.org/10.1016/j.jnutbio.2017.09.011 (2018).
Hyun, H. B., Moon, J. Y. & Cho, S. K. Quercetin Suppresses CYR61-Mediated Multidrug Resistance in Human Gastric Adenocarcinoma AGS Cells. Molecules 23, https://doi.org/10.3390/molecules23020209 (2018).
Yoo, H. J., Kim, T. J., Kim, D. J. & Kim, W. Role of COX2 as a biomarker for estimating survival of patients with clinical stage I gastric Cancer. Anticancer Res. 40, 341–347. https://doi.org/10.21873/anticanres.13958 (2020).
Ren, J., Liu, J. & Sui, X. Correlation of COX-2 and MMP-13 expressions with gastric cancer and their effects on prognosis. J. BUON. 24, 187–193 (2019).
Lin, X. M. et al. Cisplatin induces chemoresistance through the PTGS2-mediated anti-apoptosis in gastric cancer. Int. J. Biochem. Cell. Biol. 116, 105610. https://doi.org/10.1016/j.biocel.2019.105610 (2019).
Choi, S. M., Cho, Y. S., Park, G., Lee, S. K. & Chun, K. S. Celecoxib induces apoptosis through Akt Inhibition in 5-fluorouracil-resistant gastric cancer cells. Toxicol. Res. 37, 25–33. https://doi.org/10.1007/s43188-020-00044-3 (2021).
Xu, J. et al. Tumor-associated macrophages induce invasion and poor prognosis in human gastric cancer in a cyclooxygenase-2/MMP9-dependent manner. Am. J. Transl Res. 11, 6040–6054 (2019).
Zhang, Y. H. et al. gamma-tocotrienol inhibits the invasion and migration of human gastric cancer cells through downregulation of cyclooxygenase-2 expression. Oncol. Rep. 40, 999–1007. https://doi.org/10.3892/or.2018.6497 (2018).
Ji, X. K. et al. Genetic variant of cyclooxygenase-2 in gastric cancer: more inflammation and susceptibility. World J. Gastroenterol. 27, 4653–4666. https://doi.org/10.3748/wjg.v27.i28.4653 (2021).
Shen, J. et al. Cancer-associated fibroblasts-derived VCAM1 induced by H. pylori infection facilitates tumor invasion in gastric cancer. Oncogene 39, 2961–2974. https://doi.org/10.1038/s41388-020-1197-4 (2020).
Jung, W. C. et al. Expression of intercellular adhesion molecule-1 and e-selectin in gastric cancer and their clinical significance. J. Gastric Cancer. 12, 140–148. https://doi.org/10.5230/jgc.2012.12.3.140 (2012).
Chen, X., Chen, R., Jin, R. & Huang, Z. The role of CXCL chemokine family in the development and progression of gastric cancer. Int. J. Clin. Exp. Pathol. 13, 484–492 (2020).
Natsume, M. et al. Omental adipocytes promote peritoneal metastasis of gastric cancer through the CXCL2-VEGFA axis. Br. J. Cancer. 123, 459–470. https://doi.org/10.1038/s41416-020-0898-3 (2020).
R, P., J, T. & N, P. & Expression of matrix Metalloproteinase-9 in gastric Cancer. Cureus 13, e18195. https://doi.org/10.7759/cureus.18195 (2021).
Zhao, C. et al. Capn4 contributes to tumor invasion and metastasis in gastric cancer via activation of the Wnt/beta-catenin/MMP9 signalling pathways. Exp. Cell. Res. 395, 112220. https://doi.org/10.1016/j.yexcr.2020.112220 (2020).
Liang, R. et al. Dihydroartemisinin inhibits the tumorigenesis and invasion of gastric cancer by regulating STAT1/KDR/MMP9 and P53/BCL2L1/CASP3/7 pathways. Pathol. Res. Pract. 218, 153318. https://doi.org/10.1016/j.prp.2020.153318 (2021).
Sun, W. et al. CD40xHER2 bispecific antibody overcomes the CCL2-induced trastuzumab resistance in HER2-positive gastric cancer. J. Immunother Cancer 10, https://doi.org/10.1136/jitc-2022-005063 (2022).
Hagi, T. et al. Anti-metastatic effect of Methylprednisolone targeting vascular endothelial cells under surgical stress. Sci. Rep. 11, 6268. https://doi.org/10.1038/s41598-021-85241-2 (2021).
Acknowledgements
We sincerely thank the public databases mentioned in this article for generously sharing extensive data.
Funding
This research was supported by Natural Science Foundation of Ningxia Province (No.2024 AAC03608) and Ningxia Medical University Foundation (No.XM2023021).
Author information
Authors and Affiliations
Contributions
Y.L. performed most of the experimental and analysis; J.W. and J.Z. designed the study; C.M. and S.Z. performed the data analysis and drew the fgures; M.Z., J.W., and J.R. carried out cell experiments; Y.T. And W.W. carried out molecular docking. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, Y., Ruan, J., Zhang, J. et al. Huangqi fuling decoction inhibits the invasion and metastasis of gastric cancer via the TNF signaling pathway. Sci Rep 15, 19628 (2025). https://doi.org/10.1038/s41598-025-00920-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00920-8