Abstract
Porous ZnFe2O4 microspheres consisting of interwoven nanosheets doped with different concentrations of cobalt (ZC) with a diameter of approximately 1.5 μm were synthesized by a simple hydrothermal method. The synthesized samples were comprehensively characterized using XRD, FESEM, FTIR, BET, and UV-Vis analyses. The synthesized ZCs were studied to investigate the effect of doping on the gas sensing properties. Interestingly, the prepared ZCs showed enhanced gas sensing performance towards ethylene glycol, with the response value increasing from 107.5 to 119.6 for 500 ppm ethylene glycol compared to the pure ZC sample under the same conditions. The rough surface morphology that creates a high surface area and the appropriate doping effect that enhances the surface chemical oxygen accumulation by creating some new energy levels, provide a fast response (less than 3.6 s) with excellent response, allowing us to perform sensing experiments at concentrations of 20–500 ppm ethylene glycol in a short time. The ZCs-based sensors also showed significant selectivity over ethylene glycol at a low operating temperature of 210 °C through a comparison of the sensing properties with ethanol, acetone, isopropanol, and dimethylamine. The results indicate that the ZC material has special potential for ethylene glycol sensors.
Similar content being viewed by others
Introduction
Ethylene glycol is a widely used substance in various industries due to its advantageous physicochemical properties, despite being highly toxic and lethal. Its odorless and colorless nature, combined with its sweet taste, makes it particularly hazardous, as it can easily be ingested accidentally by both humans and animals1,2,3,4. While ethylene glycol has relatively low toxicity, its metabolites—glycolic and oxalic acids—can inflict significant damage to kidneys and other vital organs, potentially resulting in renal failure and serious metabolic disturbances. Additionally, spills of this hazardous substance pose a considerable threat to environmental safety through contamination of water sources and soil. Therefore, the urgent need for developing sensitive and reliable sensors for detecting ethylene glycol in instances of leaks and spills cannot be overstated5,6,7.
Single-component metal oxide semiconductors (MOS) are often limited in selectivity for gas sensing applications due to their tendency to react similarly to a wide range of gases, complicating the differentiation between target gases through comparable chemical pathways. This similarity can lead to false positives or inaccuracies in assay outcomes, exacerbated by their high degree of surface activity, which fosters nonspecific interactions with multiple analytes8,9. In contrast, spinel metal oxides exhibit enhanced selectivity owing to their unique structural characteristics; the presence of multiple metal ions within the spinel framework enables diverse interaction mechanisms with target gases, improving both separation and detection capabilities. Furthermore, oxide semiconductors, including spinel structures have gained considerable attention for gas sensing applications due to beneficial characteristics, including high sensitivity, affordability, controllable synthesis methods, non-toxicity, and excellent electrical conductivity.
Consequently, ternary oxide semiconductors, particularly spinel ferrites, emerge as vital materials for various applications, addressing the selectivity challenges faced by single-component MOS10,11,12,13,14,15. Among these materials, ZnFe2O4 (ZF) is an n-type semiconductor that has attracted much attention as a gas sensing material with excellent selectivity and high response. This high sensing response is due to the ZF crystal structure in which Zn2+ and Fe3+ions are located at tetrahedral and octahedral sites, respectively, and cause defects in the structure16,17,18,19. Considering the fact that the gas sensing capability can be enhanced by doping, it is reasonable to expect that doping ZF with cobalt would be an efficient way to enhance the ZF sensing performance20,21,22,23. In addition, 3D sensing materials with porous surfaces provide numerous active sites and fast electron transport properties for potential use in gas sensing due to their high specific surface area24. Here, we report a facile method to fabricate cobalt-doped ZF porous spheres. FESEM, XRD, UV, FTIR and BET analyses of the samples were performed to investigate the morphological and structural characteristics of the samples. Gas sensing tests of the films against various gas vapors were performed at 210 °C. Since the layers were selective for ethylene glycol, their sensing properties against different concentrations of ethylene glycol were evaluated. It was indicated that sensing layers featuring porous structures and substantial specific surface areas hold considerable promise for enhancing the performance of highly sensitive gas sensors. In detail, the ZC-gas sensor doped with 15% cobalt showed a short response/recovery time of 1.8/18 s and an excellent response of 106.9 against 100 ppm ethylene glycol at 210 °C. Finally, the sensing mechanism was studied in detail.
Experimental section
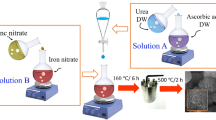
To synthesize ZF-doped porous microspheres, ascorbic acid (3.17 g) and urea (1.3 g) were combined with 20 cc ionized water and added dropwise to an aqueous solution of Fe(NO3)2.9H2O and Zn(NO3)2.6H2O with 0, 15 and 20 wt% Co(NO3)2.6H2O. After 0.5 h of magnetic stirring, the solution was heated in a 50 cc stainless steel autoclave for 6 h at 160 °C. The obtained precipitate was dried after washing with deionized water and ethanol at 60 °C. Then, they annealed at 500 °C. The samples were designated ZC0, ZC15, and ZC20, respectively, according to the cobalt percentage. In Fig. S1 (a) the steps of sample synthesis are schematically shown.
X-ray powder diffraction (XRD) were collected using a Philips diffractometer (\(\:{\uplambda\:}=1.5406\:\text{\AA\:}\)). Scanning electron microscopy (SEM) images and EDS elemental analysis were performed using a Mira 3-XMU. FT-IR spectra were collected using an AVATAR 360 spectrophotometer. The specific surface area was estimated via Brunauer-Emmett-Teller (BET) technique with a Belsorp II instrument. The optical properties of the samples were analyzed using a Varian Cary 100 spectrophotometer.
To perform the sensing tests, a glass setup with a volume of approximately 6 L was designed, allowing the target gas to enter the chamber via the inlet and after saturating the sample, it was exposed to the environment by opening the chamber inlet (Fig. S1 (b)). The voltage of the sample was measured using a digital multimeter. The test was performed at a relative humidity of 35% at different temperatures. The volume of liquid introduced into the chamber was measured and documented with precision. To ascertain the suitable concentration of the vapor (ppm), the following formula is utilized:
Here, R represents the universal gas constant. valued at 8.3145 \(\:\frac{Pa.\:\:L}{mol.\:\:K}\), \(\:{\updelta\:}\)represents the density in g/cm3, \(\:{\text{V}}_{\text{r}}\) indicates the volume, M refers to the molecular weight associated with the liquid analyte in g/mol, T denotes the absolute temperature measured in K, \(\:{\text{V}}_{\text{b}}\) is the volume of the chamber in L, and \(\:{\text{P}}_{\text{b}}\) is the pressure within the chamber expressed in Pa37. The value of R was determined with the equation R = Ra/Rg, where Rg and Rarefer to the respective resistances.in the presence of ethylene glycol and air, respectively, and are defined by the following equation25:
VRg and VRa represent the voltages across the samples in the presence of the target gas and air, respectively, while VC is the active circuit voltage (4 V)37. In general, the response and recovery times (tres and trec) are defined as reaching 90% of their maximum and a decrease to 10% of their maximum, respectively26.
Results
SEM images were analyzed to investigate the morphology of ZCs, which is shown in Fig. 1 (a-c). According to the presented images, the morphology of the samples is in the form of porous microspheres consisting of intertwined nanosheets. The average diameter of the spheres for sample ZC0 is approximately 1.72 μm, which decreases to 0.89 μm with 15% cobalt doping, while with an increase cobalt to 20%, the diameter of the spheres increases to 1.81 μm. Also, the thickness of the sheets was estimated to be the highest value (almost 35 nm) for sample ZC15 and the lowest value (16 nm) for sample ZC20.
According to the EDS analysis of the synthesized samples in Fig. 1 (d-f), peaks related to zinc, iron, and oxygen elements are observed in the ZC0 spectrum, while peaks related to cobalt appear in the ZC15 and ZC20 spectra depending on the doping concentration, confirming the successful doping of the samples.
The XRD analysis of ZC spheres was performed to investigate the samples’ chemical components and crystal structure are illustrated in Fig. 2. The diffraction peaks of the samples exhibit high intensity, suggesting a high level of crystallinity. Also, no other peaks were seen, indicating high purity. The samples have distinct peaks at 2θ = 30.0°, 35.3°, 37.1°, 43.0°, 53.3°, 56.7°, 62.3°, 70.8°, and 73.9°, which are assigned to (220), (311), (222), (400), (422), (511), (440), and (533). In addition, by the Scherrer equation (D = Kλ/βcosθ) on the FWHM of the main peaks, the crystallite size can be estimated27,28. The nanocrystallite sizes for the three samples ZC (0–15-20) were obtained as 6.63, 8.61, and 7.7 nm, respectively. The crystallite size of cobalt-doped samples was found to be larger than that of ZC0 because increasing cobalt content leads to agglomeration as the preference for Zn2+, Co2+, and Fe3+to be located at tetrahedral and octahedral sites of the spinel structure changes29. In this work, when the cobalt concentration increased from 0 to 15%, the lattice constant value increased from a = 0.8326 nm to a = 0.8369 nm, and when the cobalt concentration increased to 20%, this value reached a = 0.8339 nm.
FTIR spectra were used to confirm the new spinel structure in the samples. Figure 3 (a) displays the FTIR spectra of the synthesized ZCs. The bands observed at wavelengths below 600 cm−1 are characteristic of spinel ferrites. By adding cobalt ions to ZF, the band observed below 500 cm−1shifted slightly and their intensity changed, which could be due to the redistribution of Zn, Co and Fe in tetrahedral and octahedral sites. Other bands observed in the figure are due to the vibrations of the carbon bond, the nitrate group, the presence of moisture and carbon dioxide30,31.
To examine the pore characteristics of the synthesized samples, the results of N2 adsorption and desorption were examined. The hysteresis diagram and analysis results are plotted in Fig. 3 (b1-b3) and the pore size distribution is plotted in Fig. S2 (a-c). The results show that sample ZC15 with a specific surface area of 79.899 m2g−1 has the largest effective surface area, which is almost twice the specific surface area of ZC0. Although the volume of pores in samples ZC15 and ZC20 is larger than ZC0, ZC20 has pores with larger diameters than the other two samples. Although the large specific surface area can increase the surface reaction with gas molecules, porosity allows target gases to reach suitable areas of the sample surface. The surface area, porosity, and appropriate pore size are beneficial for the adsorption/desorption of gas and affect the sensor response and response/recovery time.
Figure S3 (a) shows the absorption spectra of the microspheres. The samples display a significant peak between 200 and 300 nm. The Egof the samples is determined by Tauc equation32:
The Kubelka-Munk function was evaluated from the reflectance data with the following equation:
The obtained value for the band gap is consistent with previous papers. Also, the band gap changes with the addition of cobalt to ZF and decreases from 1.73 eV for ZC0 to 1.69 eV for the doped samples (Fig. S3 (b-d)). Although factors such as grain size, lattice strain, structural parameter, and the presence of impurities affect the optical band gap values, the smaller band gap of the doped sample can be attributed to the increase in the lattice constant, size of the nanocrystals and lattice defects obtained from the XRD patterns33.
Since the temperature is an important parameter affecting the response of metal oxide gas sensors, we tested the sensor response of the fabricated layers at different temperatures, against 500 ppm ethylene glycol. As indciated in Fig. 4a, the layers have the highest response at 210 °C and the response decreases with increasing temperature up to 265 °C. Therefore, the optimum temperature was considered to be 210 °C and all gas sensing experiments were performed at this temperature. This tendency to increase the response at the appropriate operating temperature is due to the high surface reactivity for adsorption of oxygen species, but higher temperatures disrupt the gas adsorption and the sensor response decreases. Figure 4 (b-d) shows the sensor measurements toward different concentrations of ethylene glycol. It can be seen that the response increases as the concentration of ethylene glycol rises from 20 to 500 ppm. This increase in response is greater for ZC15 than for the other two layers. Figure 4e compares the dynamic response plots of the sensors against various concentrations of gas at 210 °C. The response of the sensing layers against 500 ppm ethylene glycol was calculated to be 107, 119 and 78 for the three layers ZC0, ZC15, and ZC20, respectively. The high response of the ZC15-based gas sensor may be due to the large surface area and suitable pore structure34. The response results and response times of all three sensing layers against concentrations of ethylene glycol are shown in Table 1. Having a short tres (less than 3.6 s) and trec (3.6–38.7 s) is one of the prominent advantages of these sensing layers. This excellent response of the ZC15 sample is the result of factors such as the high porous surface area according to BET analysis, this can enhance the oxygen adsorbed on the surface and offer more active sites for gas adsorption, leading to increased sensor sensitivity. Also, the small crystallite size for ZC15 obtained by the Debye-Scherer equation contributed to the better response of the ZC15 sample. The different trec of samples can be attributed to various factors. While optimal doping levels can increase response, over- or under-doping may negatively affect charge carrier dynamics and cause slower gas molecule desorption kinetics. In addition, material properties, including structural and morphological features, strongly influence the gas adsorption and desorption processes. Variations in porosity, surface area, and crystallinity among different compositions can lead to such discrepancies. In addition, the binding energy between the target gas and the sensing material can differ. If this binding energy is higher, the dissociation time of the gas molecules will be longer, resulting in a longer recovery period. Finally, environmental conditions such as temperature and humidity may also play a role. According to the data in Table 1, the response time for the ZC0 and ZC15 sensing layers is almost constant and about 1.8 to 2.8 s, which indicates the good stability of the layers in response to different gas concentrations. However, the recovery time of ZC15 is longer, which indicates the high adsorption capability. For ZC20, the response time increases significantly at higher concentrations (about 3.6 to 3.9 s), which may be due to the structure of the layer or the specific interactions between the sensing molecules and ethylene glycol. In general, it should be noted that the different behavior of the layers can be due to changes in their compositions, microscopic structure, and chemical interactions.
(a) Variation of response with temperature of the ZCs. (b)- (d) Response of ZC sensing layers after exposure to 20–500 ppm ethylene glycol for ZC0, ZC15, and ZC20 at 210 °C, respectively. (e) Comparison of the response of layers to different concentrations of gas. (f) Sensor response to 100 ppm vapor of various gases at 210 °C.
Selectivity, as an important characteristic of gas sensors, was investigated for sensors fabricated at the optimum temperature toward EG, ethanol (ET), acetone (AC), isopropanol (IP), methanol (ME), and dimethylformamide (DMF) with a concentration of 100 ppm. The response of all three sensor layers was significantly higher towards ethylene glycol than towards other gases. The response of ZC15 towards ethylene glycol was approximately 5.5 times that towards acetone and 5 times that towards ethanol. In Fig. 4f, the selectivity diagram of the samples was drawn. It is quite clear that these sensor layers have good selectivity towards ethylene glycol.
Based on the responses obtained for sample ZC15, we can calculate the selectivity coefficients for ethylene glycol (EG) compared to other gases using formula35,36:
where \(\:{R}_{EG}\) is the response to ethylene glycol and \(\:{R}_{inter/fering}\) denotes the layer’s response to the interfering gas. The selectivity coefficients for ethanol, acetone, isopropanol, and DMF were determined to be 4.91, 5.44, 2.46, and 2.20, respectively. These values indicate that ZC15 exhibits higher sensitivity to ethylene glycol compared to each of the other interfering gases. In Table 2, some previous reports for ethylene glycol sensors are compared with the data obtained in this study. In addition to the optimal temperature reduction, excellent response and very short tres/trec were obtained in this study. The cobalt-doped ZF sensor, demonstrated in our work, shows the highest response value of 106.9 at 100 ppm ethylene glycol and 210 oC, indicating superior sensitivity compared to the other mentioned materials. While our sensor shows a tres= 1.8 s and a trec= 18 s, it is comparable to the fast response of the ZnO, despite the fact that this material shows a much lower response at high temperatures, indicating a limitation in its overall performance.
The sensing capability of n-type semiconductors is linked to variations in electrical conductivity resulting from the chemical interactions between gas molecules and the surfaces of the samples. When the sensing layer is exposed to air, the oxygen molecules that are adsorbed onto the ZC microspheres can capture electrons from the sample surfaces, leading to the conversion of oxygen into oxygen ions (\(\:{\text{O}}_{2}^{-}\),O−, and O2−)41. These oxygen ions create an electron-deficient layer on the surface of the ZC material, resulting in a reduction of the Fermi level and an increase in the potential barrier42. Co-doping in semiconductor materials significantly enhances gas sensing performance by altering the electronic structure to introduce new energy levels within the bandgap. These levels act as electron traps, improving electrical conductivity and increasing the number of active sites for oxygen adsorption, which is crucial for optimized gas sensing. Additionally, Co boosts the density of hydroxyl groups on the surface, facilitating stronger hydrogen bonds with ethylene glycol molecules. This enhances electron transfer and surface reactivity, leading to a more robust sensor response. When ethylene glycol is introduced into the chamber, its molecules are adsorbed by the oxygen ions. The electrons captured by the O2are transferred into the conduction band of the ZC material, thereby reducing the thickness of the empty layer. O₂ molecules are adsorbed onto the surfaces of the sensing materials and become activated through surface interactions, leading to the breaking of the O = O bond and the generation of superoxide ions (O₂⁻). These ions can further react to generate peroxide ions (O₂²⁻) and ultimately oxide ions (O⁻). The processes are significantly influenced by factors such as temperature, the chemical composition of the sensing material, and the presence of other atmospheric species43,44. The excellent response of the ZC15 gas sensor is the result of several factors, including the large porous surface area (BET analysis). The extensive specific surface area can enhance the concentration of oxygen adsorbed on the surface, which provides a greater number of active sites for gas adsorption on the surface, and helps to higher sensor response. On the other hand, the small crystallite size obtained by the Debye-Scherer Equation is the reason for the better response of the ZC15 sample. However, Co doping probably produces a new energy level that can assist the adsorbed oxygen in capturing electrons from the valence band45. Thus, oxygen from the air is continuously taken up by the surface of the ZC and accumulate on the surface in large quantities after capturing electrons. The gas sensing process is schematically shown in Fig. 5.
Conclusion
In this work, porous ZnFe2O4 microspheres consisting of intercalated nanosheets with different concentrations of cobalt (ZC) with a diameter of approximately 1.5 μm were synthesized by a simple hydrothermal method. The synthesized ZCs were studied to investigate the effect of doping on the sensing properties of ethylene glycol. Interestingly, the prepared ZCs showed excellent gas sensing performance towards ethylene glycol with concentrations of 20–500 ppm. The rough surface morphology that creates a high surface area and the appropriate doping effect that enhances the accumulation of surface chemical oxygen provide fast response (less than 3.6 s) with excellent target gas response. The ZC doped with 15% cobalt showed a higher response than pure ZC due to the higher specific surface area, such that the response increased from 107.5 to 119.6 for 500 ppm ethylene glycol under the same conditions. Also, the selectivity of the samples for ethylene glycol compared to other gas vapors was 5.5 times higher than acetone and 5 times higher than ethanol at an operating temperature of 210 °C. The obtained results introduce ZC material as an attractive candidate for ethylene glycol sensors.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Liu, M. M. et al. ZnO/ZnCo2O4 composite prepared by one-step hydrothermal method for high-performance ethylene glycol sensor. Ceram. Int. 48, 22346 (2022).
Ding, J. et al. Highly sensitive ethylene glycol gas sensor based on ZnO/rGO nanosheets. Sens. Actuators B. 372, 132655 (2022).
Ibrahim, R. K. et al. Physical properties of ethylene glycol-based deep eutectic solvents. J. Mol. Liq. 276, 794 (2019).
Seiler, J. et al. Refrigeration below zero C: adsorption chillers using water with ethylene glycol as antifreeze. Int. J. Refrig. 77, 39 (2017).
Welsch, F. The mechanism of ethylene glycol ether reproductive and developmental toxicity and evidence for adverse effects in humans. Toxicol. Lett. 156, 13 (2005).
Patočka, J. & Hon, Z. Ethylene glycol, hazardous substance in the household. Acta Med. 53, 19 (2010).
Rajput., S. K. et al. Overview, toxicological profile, challenges, and future perspectives. Hazard. Chem., 205 (2025).
Saadat Niavol, S. et al. Ethylene glycol sensing properties of hydrothermally grown featherlike ZnO nanopowder with abundant oxygen vacancies. J. Mater. Res. 38, 1211 (2023).
Kuang, C. et al. A novel approach for fabricating NiO Hollow spheres for gas sensors. Phys. E. 97, 314 (2018).
Zhou, X. Highly sensitive acetone gas sensor based on porous ZnFe2O4 nanospheres. Sens. Actuators B: Chem. 206, 577 (2015).
Wang, L. et al. Ordered mesoporous carbon-supported CoFe2O4 composite with enhanced lithium storage properties. J. Mater. Sci. 52, 6265 (2017).
Lorenzi, G. et al. Spectroscopic study of a Ni-bearing gahnite pigment. J. Eur. Ceramic Soc. 26, 317 (2006).
Su, C. et al. Hierarchical heterojunction microrods of MgO/Co3O4/ZnO for enhanced conductometric detection of ethylene glycol. ACS Appl. Nano Mater. 7, 21983 (2024).
Liu., M. M. et al. ZnO/ZnCo2O4 composite prepared by one-step hydrothermal method for high-performance ethylene glycol sensor. Ceram. Int. 48, 22305 (2022).
Liao, Z. et al. Highly sensitive toluene sensor based on silver Ear-Like F-doped Co3O4 templated from Co(OH)F guided by SiO2 nanospheres. Chem. Eng. J. 498, 155688 (2024).
Li, L. et al. Porous ZnFe2O4 nanorods with net-worked nanostructure for highly sensor response and fast response acetone gas sensor. Sens. Actuators B: Chem. 248, 85 (2017).
Gao, X. et al. Highly sensitive and selective H2S sensor based on porous ZnFe2O4 nanosheets. Sens. Actuators B: Chem. 246, 662 (2017).
Dong, C. et al. Monodisperse ZnFe2O4 nanospheres synthesized by a nonaqueous route for a highly selective low-ppm-level toluene gas sensor. Sens. Actuators B: Chem. 239, 1231 (2017).
Raut, S. S. et al. First report on synthesis of ZnFe2O4 thin film using successive ionic layer adsorption and reaction: approach towards Solid-State symmetric supercapacitor device. Electrochim. Acta. 198, 203 (2016).
Chen, Y. et al. CO sensing properties and mechanism of Pd doped SnO2 thick-films. Appl. Surf. Sci. 428, 207 (2018).
He, L. et al. Preparation and characterization of high mobility Nb-doped SnO2 transparent conducting films. Mater. Sci. Forum. 993, 869 (2020).
Li, L. et al. Improved H2 sensing properties of Co-doped SnO2 nanofibers. Sens. Actuators B: Chem. 150, 806 (2010).
Liao, Z. et al. Porous rod-like Co(OH)F/Co3O4 heterojunction-based acetic anhydride gas sensor with high response and low operating temperature. Sens. Actuators B. 399, 134815 (2024).
Azmoodeh, Z. et al. Hydrogen gas sensing feature of polypyrrole nanofibers assisted by spinel ZnMn2O4 microspheres in dynamic conditions. Int. J. Hydrog. Energy. 47, 29971 (2022).
Bagheri Khatibani, A. & Abbasi., M. Effect of Fe and Co doping on ethanol sensing property of powder based ZnO nanostructures prepared by sol–gel method. J. Solgel Sci. Technol. 86, 255 (2018).
Azmoodeh, Z. et al. Improving H2 gas sensing with ZnMn2O4/Polypyrrole nanocomposite. Int. J. Hydrog. Energy. 85, 854 (2024).
Azmoodeh, Z. et al. A stunning sensitive isopropanol gas sensor based on ZnMn2O4 microspheres. Phys. B: Condens. Matter. 698, 416752 (2025).
Li, L. et al. Porous ZnFe2O4 nanorods with net-worked nanostructure for highly sensor response and fast response acetone gas sensor. Sens. Actuators B. 248, 85 (2017).
Kapse, S. D. et al. Characteristics of high sensitivity ethanol gas sensors based on nanostructured spinel Zn1 – xCoxAl2O4. Curr. Appl. Phys. 12, 307 (2012).
Zhang, P. et al. Synthesis and optical property of one-dimensional spinel ZnMn2O4 nanorods. Nanoscale Res. Lett. 6, 2 (2011).
Saadat Niavol, S. et al. Enhancing both methylene blue photocatalytic degradation and ethanol sensing performances of ZnO/rGO nanocomposite through the variation of GO amount. Appl. Phys. A. 128, 733 (2022).
Hashemi Karouei, S. F. et al. Characterization and gas sensing properties of graphene/polyaniline nanocomposite with long-term stability under high humidity. J. Mater. Sci. 56, 4239 (2021).
Chung, P. H. et al. A sensitive visible light photodetector using Cobalt-Doped zinc ferrite oxide thin films. ACS Appl. Mater. Interfaces. 13, 6411 (2021).
Zhou, X. et al. Nanosheet-assembled ZnFe2O4 hollow microspheres for high-sensitive acetone sensor. ACS Appl. Mater. Interfaces. 7(28), 15414–15421 (2015).
Qu, C. et al. Selectivity tailoring of polyaniline-based gas sensors towards amine homologs by adsorption regulation. Sens. Actuators B. 427, 137177 (2025).
Akyasan, A. et al. Protic Ionic Liquid Based Potentiometric Sensors: High Selectivity Detection of Silver(I) Ions, Chem. Select, 10 (2025).
Yang, H. et al. A simple gas sensor based on zinc ferrite Hollow spheres: highly sensitivity, excellent selectivity and long-term stability. Sens. Actuators B. 280, 34 (2019).
Hashemi Karouei, S. F. Prominent ethylene glycol sensing of sol-gel derived ZnO and ZnO: Cu nanostructures. J. Sol- Gel Sci. Technol. 111, 739 (2024).
Han, T. et al. Rough SmFeO3 nanofibers as an optimization ethylene glycol gas sensor prepared by electrospinning. Mater. Lett. 268, 127575 (2020).
Su et al. Boosting ethylene glycol sensing performance with dendritic hierarchical CuO/Co3O4 heterojunction nanowire. ACS Appl. Nano Mater. 20, 19249 (2023).
Song, G., Xin, F. & Yin, X. Photocatalytic reduction of carbon dioxide over ZnFe2O4/ TiO2 nanobelts heterostructure in cyclohexanol. J. Colloid Interface Sci. 442, 60 (2015).
Wang, Y. et al. A gas sensor based on Ag-modified ZnO flower-like microspheres: Temperature-modulated dual selectivity to CO and CH. Surf. Interfaces. 4, 101110 (2021).
Krishna., K. G. et al. Enhanced isopropanol gas sensing with ZnFe2O4 and ZnCuFe2O4 spinel nanoferrites at room temperature. Inorg. Chem. Commun. 174, 114051 (2025).
Sun, B. et al. Enhanced formaldehyde gas sensing properties of p-LaFeO3/n-Fe2O3 composite nanofibers synthesized by electrospinning method. Sens. Actuators B. 426, 137010 (2025).
Zhang, J. et al. Effect of Co doping on chemosorbed oxygen accumulation and gas response of SnO2 under dynamic program cooling. Sens. Actuators: B Chem. 340, 129810 (2021).
Acknowledgements
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R340), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
The authors would like to thank Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R340), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
A.S.: Writing, Methodology, Validation, Investigation, Supervision. R.M.A.: Conceptualization, Writing, Formal analysis, Visualization, Resources.J.A.A.: Writing, Methodology, Investigation, Software, Funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sumayli, A., Almotawa, R.M. & Alamoudi, J.A. Development of a high performance ethylene glycol gas sensor using cobalt doped porous ZnFe2O4 nanostructures. Sci Rep 15, 16876 (2025). https://doi.org/10.1038/s41598-025-00941-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00941-3
Keywords
This article is cited by
-
A superior ethylene glycol gas sensor based on cobalt doped highly porous ZnFe2O4 microspheres
Scientific Reports (2025)
-
Enhanced photocatalytic degradation of tetracycline using cobalt-substituted ZnFe2O4 porous microspheres under visible-LED irradiation
Scientific Reports (2025)
-
Breathtaking competition in ethylene glycol sensing of ZnCo2O4 and ZnCo2O4/graphene nanoparticles
Journal of Sol-Gel Science and Technology (2025)
-
Efficient photocatalytic degradation of acetaminophen using cobalt-doped ZnFe2O4 spinel as a promising solution for pharmaceutical wastewater treatment
Journal of Saudi Chemical Society (2025)
-
Fabulous sensing of N,N-Dimethylformamide (DMF) Vapor Using Hydrothermally Prepared Zinc Oxide/Graphene Nanoplatelets Nanocomposite
Journal of Electronic Materials (2025)