Abstract
Surufatinib is a novel, China-developed small-molecule tyrosine kinase inhibitor that demonstrates high selectivity for VEGFR, FGFR1, and CSF1R. Surufatinib has been approved for the treatment of neuroendcrine tumors, including pancreatic neuroendocrine tumors (PNEN) and non-pancreatic neuroendocrine tumors (N-pNEN). The purpose of this retrospective study is to assess Surufatinib’s safety and effectiveness in patients with various advanced solid malignancies. The general clinical statistics and follow-up data of patients treated with Surufatinib for advanced solid tumors at Zhejiang Provincial People’s Hospital between January 2021 and April 2024 were gathered. Enhanced CT was used to assess the effectiveness during that time, and cases side effects were gathered. Survival rates of different diseases were analyzed using the Kaplan-Meier method. A total of 28 eligible patients were enrolled in this study. At the end of follow-up, treatment with Surufatinib resulted in the following outcomes: Complete response (CR) in 0 cases (0.0%), Partial response (PR) in 5 cases (17.9%), Stable disease (SD) in 7 cases (25.0%), and Progressive disease (PD) in 16 cases (57.1%). Objective response rate (ORR) and Disease control rate (DCR) were 17.9% and 42.9%, respectively. In the PNEN group, ORR was 33.3%, DCR was 66.7%, median progression-free survival (mPFS) was 11 months, while median overall survival (mOS) was 17 months. In the N-pNEN group, ORR was 14.3%, DCR was 42.3%, mPFS was 6 months and mOS was 7 months. ORR was 8.3%, DCR was 25%, mPFS was 2 months, and mOS was 2 months. The most common adverse reactions included hypoproteinemia, proteinuria, bone marrow suppression and gastrointestinal toxicity, and which of them were grade 1 to grade 2. In advanced solid tumors beyond PNEN, Surufatinib demonstrates clinically meaningful survival benefits for patients refractory to standard therapies, with a generally manageable safety profile.
Similar content being viewed by others
Introduction
Cancer remains a global public health priority and represents a leading cause of morbidity and mortality worldwide. China bears the highest cancer burden globally, with malignant tumor mortality constituting the primary cause of death among urban residents and individuals aged < 65 years in China1. Advances in clinical medicine have led to the emergence of numerous targeted therapies, which now represent a promising treatment option for patients with advanced malignancies. Notably, anti-angiogenic agents have achieved substantial breakthroughs in oncology, with clinical trials demonstrating their significant efficacy in prolonging overall survival2,3. Angiogenesis is a tightly regulated biological process orchestrated by the dynamic balance between pro-angiogenic and anti-angiogenic factors, which is essential for physiological tissue development and homeostasis4. Dysregulated cellular proliferation constitutes a defining characteristic of malignant transformation, wherein vascular endothelial growth factor (VEGF) functions as a principal modulator of tumor angiogenesis, playing an indispensable role in oncogenic progression5,6.
The primary medications that block the VEGF pathway are fuquitinib, bevacizumab, and anlotinib hydrochloride, among others7,8,9,10. Each medication differs from the others in terms of its indications, effectiveness, frequency of use, adverse effects, etc11. Among these, Surufatinib is a brand-new oral small-molecule tyrosine kinase inhibitor (TKI) of the receptors for vascular endothelial cell growth factor receptor (VEGFR), fibroblast growth factor receptor 1 (FGFR1) and colony stimulating factor-1 receptor (CSF-1R). By preventing tumor angiogenesis, it can stop the growth of tumor cells12. Overall survival was considerably increased by using surufatinib as a third-line or postline treatment for pancreatic (pNEN) and non-pancreatic neuroendocrine tumors (N-pNEN), according to the findings of the randomized, double-blind, placebo-controlled Phase 3 SANET-p and SANET-ep trials in Chinese patients. When compared to the placebo group, the median progression-free survival of individuals receiving surufatinib improved noticeably13,14. Based on these studies, Surufatinib was approved by the US FDA in April 2020 as the first anti-angiogenic agent for the treatment of neuroendocrine tumors, and in China in December 2020 for the treatment of N-pNEN, changing the landscape of neuroendocrine tumor treatment strategies.
To further examine the safety and effectiveness of Surufatinib in treating several advanced solid tumors, we carried out a real-world study, creating new therapy options for solid tumors.
Materials and methods
Cases selection criteria
Study enrollment is restricted to patients meeting all inclusion criteria specified below: (1) With histologically or cytologically confirmed advanced solid tumors; (2) Clinical stage IV with measurable target lesions; (3) The patient was treated with Surufatinib for more than one 28-day cycle during systemic therapy; (4) Eligible participants aged 18–75 years, regardless of sex, with body weight ≥ 40 kg; (5) Expected survival ≥ 3 months; (6) Availability of complete clinical data with documented informed consent obtained from all participants.
Cases exclusion criteria
Patients meeting any of the following exclusion criteria were ineligible for study participation: (1) Multiple primary tumors or pathology was unclear; (2) Incomplete, missing or difficult to obtain clinical data; (3) Cases were excluded for either inadequate Surufatinib exposure (< 1 treatment cycle) or unwillingness to participate in survival follow-up.
Treatment method
For a treatment cycle, the standard dosage of surufatinib (made by Hutchison Whampoa Pharma Shanghai Co, LTD) is 300 mg once daily, every four weeks. The dosage is changed based on the individual circumstances of enrolled patients and is combined with other anti-tumor therapies (chemotherapy, vaccination, etc.) until the disease progresses, the patient dies, has intolerable side effects, or refuses to continue treatment. Enhanced CT scans were performed every 8 weeks to monitor changes in target lesions, with treatment response evaluated according to Response Evaluation Criteria in Solid Tumors RECIST 1.1.
Evaluation of therapeutic effect and observation index
Objective efficacy evaluations
Target lesion responses were categorized according to Response Evaluation Criteria in Solid Tumors RECIST 1.1 based on contrast-enhanced CT findings, with the following classifications: (1) Complete response (CR): complete response was achieved, with disappearance of all target lesions (confirmed on subsequent imaging), no emergence of new lesions, and normalization of tumor markers sustained for ≥ 4 weeks; (2) Partial response (PR): The target lesions’ overall longest diameters decreased by at least 30% and remained that way for at least a week as compared to the baseline sum of the longest diameters; (3) Stable disease (SD): tumor measurements that neither shrank sufficiently to qualify for complete response nor grew sufficiently to constitute progressive disease (maintained for ≥ 6 weeks), with the reference value being the smallest sum of the longest diameters recorded since treatment initiation; (4) Progressing disease (PD): an increase of at least 20% in the total length and diameter of baseline lesions or the appearance of new lesions.
Objective response rate (ORR) and disease control rate (DCR), which are determined using the formulas Objective response rate = (CR + PR)/total cases ×100% and Disease control rate = (CR + PR + SD)/total cases ×100%, are included in the short-term efficacy evaluation.
Survival assessments
They comprised median overall survival (mOS) and median progression-free survival (mPFS). OS referred to the time interval between the first dose and the date of death from any cause, with the last known date of survival used as the deletion date for subjects who did not report death as of the time of analysis. PFS was defined as the time interval between the first dose and objective PD or death (no progression, death from any cause).
Statistical analysis
Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cutoff value, which was typically selected by maximizing Youden’s index. Youden’s index was calculated as: sensitivity − (1 - specificity), with the maximum value corresponding to the optimal cutoff threshold15 (Supplementary Fig. 1).
Statistical analyses were performed using SPSS 25.0 software (IBM Corp.). Fisher’s exact test was employed to compare differences in CR, PR, SD, PD, ORR, and DCR among patients with different clinical characteristics. Survival analysis was conducted using the Kaplan-Meier method to estimate OS and PFS. Cox proportional hazards regression models were utilized for survival and prognostic analysis. In the univariate Cox regression analysis, variables with P < 0.1 were subsequently incorporated into the multivariate Cox regression model.
Results
Clinical factors of the patients
A total of 28 patients with advanced solid tumors meeting the eligibility criteria were enrolled from Zhejiang Provincial People’s Hospital and treated with surufatinib. The cohort comprised 9 PNEN patients, 7 N-pNEN patients, and 12 patients with other tumor types. Baseline characteristics of all patients (Table 1).
Objective efficacy evaluations
The number of patients with the objective efficacy evaluation of CR, PR, SD, and PD following Surufatinib treatment was 0 (0.0%), 5 (17.9%), 7 (25.0%), and 16 (57.1%), in that order. DCR was 42.9%, and ORR was 17.9%. The table of objective efficacy evaluations for various tumor species was displayed (Table 2).
Curative effect
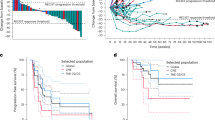
According to Kaplan-Meier analysis of tumor patients from different sources showed that mPFS for PNEN patients were 11 months(95%CI,6.3–24.0 months) and mOS for 17 months (95%CI, 9.7–37.2 months), which was basically similar to the results of SANET-p study. mPFS for N-pNEN patients was 6 months (95%CI, 3.2-1 months), lower than 9.2 months in previous studies. mOS was 7 months (95%CI, 3.7–17.4 months), mPFS was 2 months (95%CI, 0.3–3.7 months) and mOS was 2 months (95%CI, 0.3–3.7 months) for patients with other tumors. Subsequent investigation revealed that PNEN, N-pNEN, and other tumor patients’ mPFS and mOS did not differ statistically significantly (p = 0.091; p = 0.088) (Fig. 1).
Kaplan-Meier survival curves of PFS and OS in these three groups of tumor patients. (A) PFS in patients with pancreatic neuroendocrine tumors, non-pancreatic neuroendocrine tumors, and other tumors. (B) OS in patients with pancreatic neuroendocrine tumors, non-pancreatic neuroendocrine tumors, and other tumors. PFS, progression-free survival; OS, overall survival; mPFS, median progression-free survival; mOS, median overall survival.
Analysis of prognostic factors
Cox proportional hazards modeling and Kaplan-Meier survival analysis in neuroendocrine tumor patients treated with Surufatinib identified the following independent prognostic factors: for OS, fewer than 3 metastatic sites (Fig. 2A), Eastern Cooperative Oncology Group performance status (ECOG PS; Fig. 2B), and baseline free fatty acid (FFA) levels > 635.5 µmol/L (Fig. 2C); for PFS, fewer than 3 metastatic sites (Fig. 2D; Supplementary Table 1). Multivariate Cox regression analysis revealed no independent prognostic factors in other tumor types treated with Surufatinib (Supplementary Table 2).
Based on the important factors of survival and progression-free survival of patients with neuroendocrine tumors treated with solvatinib. (A) Patients with a number of metastatic sites < 3 had longer OS than patients with a number of metastatic sites > 3. (B) Patients with an ECOG score of 0–1 had a longer OS than patients with an ECOG score of > 0–1. Patients with FFA > 635.5µmol/L had longer PFS than those with less than 3 OS(D) metastatic sites. OS, overall survival; PFS, progression-free; ECOG PS, Eastern Cooperative Oncology Group Performance status. FFA, free fatty acid.
Adverse reactions
The most common AEs in this study included hypoalbuminemia (38.5%), proteinuria (25%), myelosuppression (14.3%), gastrointestinal reactions (14.3%), hypertriglyceridemia (14.3%), and hyperbilirubinemia (14.3%) (Table 3). Grade ≥ 3 AEs consisted of proteinuria (7.1%) and gastrointestinal reactions (7.1%), all of which resolved rapidly following symptomatic treatment or drug discontinuation. No grade 4 or higher AEs were observed in the study population.
Figure 2. Based on the important factors of survival and progression-free survival of patients with neuroendocrine tumors treated with solvatinib, (A) Patients with a number of metastatic sites < 3 had longer OS than patients with a number of metastatic sites > 3. (B) Patients with an ECOG PS of 0–1 had a longer OS than patients with an ECOG score of > 0–1. Patients with FFA > 635.5µmol/L had longer PFS than those with less than 3 OS(D) metastatic sites.OS, total lifetime; PFS, progression-free ; ECOG PS, Eastern Cooperative Oncology Group Performance status. FFA, free fatty acid.
Discussion
Targeted medicines are particularly noteworthy when the precision medicine age dawns. In order to suppress particular proteins linked to the development of malignant tumors, promote tumor cell apoptosis, and have a potent anti-tumor effect, targeted therapies examine or sequence the DNA or RNA of tumor cells and create medications based on the pertinent mutation sites. Additionally, it can lessen the harmful side effects on healthy cells. Small-molecule kinase inhibitors and macromolecular antibodies are the two primary forms of targeted therapy for malignancies. Small-molecule kinase inhibitors function by blocking the catalytic activity of kinases, such as gefitinib, which inhibits the epidermal growth factor (EGFR) of cancer cells and is authorized for the treatment of non-small cell lung cancer (NSCLC); macromolecular antibody drugs have an anti-tumor effect by blocking the binding of signaling molecules and antibodies3,16,17.
Both Surufatinib and fruquintinib are small-molecule, multi-target tyrosine kinase inhibitors (TKIs) independently developed by Hutchison Whampoa Pharma2. While fruquintinib primarily targets VEGFR-1, -2, and − 3, Surufatinib demonstrates broader kinase inhibition, additionally targeting FGFR1 and CSF-1R beyond the VEGFR family, resulting in a more extensive target profile compared to fruquintinib18. The elimination half-life (t1/2) of 300 mg of Surufatinib was comparable in tumor patients and healthy volunteers, with the mean t1/2 following a single oral dosage of the drug being 17.1 h. The geometric mean plasma peak concentration (Cmax) of surufatinib in tumor patients was 674 ng/mL. However, in advanced cancer patients, the mean Cmax of a single 5 mg oral dose of fruquintinib capsule is 195 ng/Ml. When administered at single doses ranging from 2 mg to 6 mg, the mean t1/2 of fruquintinib is 35.2 to 48.5 h. It can be concluded that the Cmax of Surufatinib is higher than that of fruquintinib, while the t1/2 of Surufatinib is the shortest. Due to its faster clearance in humans, Surufatinib thereby reduces the risk of drug toxicity accumulation in the body19,20,21. (Supplementary Fig. 2; Supplementary Table 3).
Surufatinib has been licensed for the treatment of advanced nonfunctional, well-differentiated (G1, G2), locally or metastatic, non-surgically resectable PNEN and N-pNEN. It also exhibits antitumor angiogenesis and prolongs OS and PFS in patients with neuroendocrine tumors.
13,14. Although previous studies by Mizuno Y et al. demonstrated that the anti-angiogenesis target kinase inhibitor TKI could treat PNEN, the efficacy in N-pNEN was unclear22. In this study, the survival data for PNEN (11 months) were generally consistent with previous findings from the SANET-p study (10.9 months). However, mPFS for N-pNEN patients was 6 months, which was lower than the 9.2 months observed in SANET-ep. Given the limited sample size of this real-world study, particularly in the N-pNEN subgroup (n = 7), these results should be considered exploratory and not directly comparable with data from large-scale clinical trials. Larger investigations are necessary to confirm the preliminary findings, which indicates that the prognosis of N-pNEN may be substantially worse than that of PNEN.
Patients with neuroendocrine tumors with fewer than three metastases, ECOG PS, and a baseline FFA level below 635.5 mmol/L were shown to have a longer OS based on the results of prognostic survival analysis of various tumor types based on baseline values of metabolic markers. Longer PFS is possible if there are fewer than three metastases. Prior research has demonstrated that a poor prognosis for neuroendocrine tumors is linked to increased levels of lactate dehydrogenase and platelets as well as the number of metastatic locations23,24. The findings of this study differ from previous reports, which may be attributed to the limited sample size. While these results require further validation, they provide promising preliminary evidence that warrants confirmation in larger-scale studies.
Despite Surufatinib’s current indication being restricted to neuroendocrine tumors, clinical trials have been carried out to treat thyroid and biliary tract cancers (BTC) due to its broad spectrum anti-VEGFR activity25,26. For BTC, clinical trial results of Surufatinib monotherapy in patients who failed first-line treatment showed: a 16-week progression-free survival rate of 46.33% (95% CI, 24.38–65.73), median PFS of 3.7 months, and median OS of 6.9 months. These findings indicate that Surufatinib demonstrates clinically meaningful activity in BTC treatment, with safety and tolerability profiles consistent with expectations. Furthermore, the comparative study of Surufatinib monotherapy versus capecitabine chemotherapy in BTC remains ongoing25. Given that only 4 BTC patients were included in the current study, survival analysis was not feasible. Therefore, we recommend further investigation with larger real-world datasets to validate these findings.
The optimal dosing strategy of Surufatinib for this tumor type requires further exploration. Real-world evidence indicates that merely 21.4% of patients maintained the standard 300 mg dose, with dose reductions primarily attributed to treatment-related adverse events or clinicians’ therapeutic choices when used in combination regimens. Since 100 mg of Surufatinib produced PR efficacy in one patient with pancreatic cancer, it is debatable if all patients require a dose of 300 mg. The number of cases in this study is small, and more cases are expected to be further explored. Our analysis revealed distinct efficacy patterns between dose cohorts: patients receiving the standard 300 mg dose of surufatinib achieved an ORR of 50.0% and DCR of 66.7%, whereas the reduced-dose group (< 300 mg) demonstrated significantly lower response rates (ORR: 9.1%; DCR: 36.4%). Notably, while the standard-dose group showed clinically meaningful improvements in both endpoints, the intergroup differences lacked statistical significance (Supplementary Table 4).
The adverse event profile of Surufatinib reflected characteristic anti-VEGFR class effects, with the most common treatment emergent adverse events being hypoalbuminemia, proteinuria, gastrointestinal toxicity, and elevated bilirubin a pattern consistent with the safety data reported in the pivotal SANET-ep study. The incidence of skin reactions such as hand-foot syndrome is lower than that of other similar drugs, which may be related to the difference in the past chemotherapy of selected cases. Compared to published literature, the incidence of grade 3/4 adverse events was decreased, and most adverse events were grade 1/2 in severity overall.
Surufatinib has been approved for the treatment of PNEN and N-pNEN patients and has demonstrated some success in advanced solid tumors that have not responded to conventional regimens. It has also been proven to enhance survival in these patients27,28,29,30. The following restrictions have an impact on the study’s findings: Because this is a single-center real-world retrospective study, the sample size is small, and there aren’t enough outcome events in some subgroups, which could skew the data’stability. To further confirm Surufatinib’s effectiveness in various tumor species, multicenter, prospective, large-sample cohort studies with a predetermined sample size and adequate statistical power are therefore desperately needed.
Conclusion
Surufatinib and fuquitinib work through separate mechanisms. While the latter can prevent tumor growth by selectively inhibiting the EGFR pathway, the former can achieve an anti-tumor impact by inhibiting VEGFR 1 and many targets of 2, 3, FGFR 1, and CSF-1R. With a generally manageable toxicity profile, Surufatinib has demonstrated comparable effectiveness trends in patients with PNEN and N-pNEN as well as some therapeutic promise in other advanced solid malignancies. As a result, patients with advanced solid tumors now have a new therapy option with Surufatinib.
Data availability
The data sets generated and analyzed during this period are not publicly available because the study data involves basic patient information. However, areavailable from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74(3), 229–263 (2024).
Dasari, A. et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): An international, multicentre, randomised, double-blind, phase 3 study. Lancet 402(10395), 41–53 (2023).
Hou, X. et al. Gefitinib plus chemotherapy vs gefitinib alone in untreated EGFR-Mutant non-small cell lung cancer in patients with brain metastases: The GAP BRAIN open-label, randomized, multicenter, phase 3 study. JAMA Netw. Open 6(2), e2255050 (2023).
Li, X. et al. New advances in the research of clinical treatment and novel anticancer agents in tumor angiogenesis. Biomed. Pharmacother. 163, 114806 (2023).
Giavazzi, R. et al. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am. J. Pathol. 162(6), 1913–1926 (2003).
Hicklin, D. J. & Ellis, L. M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 23(5), 1011–1027 (2005).
Garcia, J. et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 86, 102017 (2020).
Shen, G. et al. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol. 11(1), 120 (2018).
Yewale, C. et al. Epidermal growth factor receptor targeting in cancer: A review of trends and strategies. Biomaterials 34(34), 8690–8707 (2013).
Liang, L. et al. Autophagy Inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J. Exp. Clin. Cancer Res. 38(1), 71 (2019).
Deng, Y. Y. et al. Comparison of the efficacy and safety of fruquintinib and regorafenib in the treatment of metastatic colorectal cancer: A real-world study. Front. Oncol. 13, 1097911 (2023).
Lin, Q. et al. Structural basis and selectivity of Sulfatinib binding to FGFR and CSF-1R. Commun. Chem. 7(1), 3 (2024).
Xu, J. et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21(11), 1489–1499 (2020).
Xu, J. et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21(11), 1500–1512 (2020).
Mandrekar, J. N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 5(9), 1315–1316 (2010).
Hosomi, Y. et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J. Clin. Oncol. 38(2), 115–123 (2020).
Mok, T. S. et al. Updated overall survival in a randomized study comparing dacomitinib with gefitinib as first-line treatment in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. Drugs 81(2), 257–266 (2021).
Li, Y. et al. Combination of Anti-EGFR and Anti-VEGF drugs for the treatment of previously treated metastatic colorectal cancer: A case report and literature review. Front. Oncol. 11, 684309 (2021).
Lu, X. et al. Surufatinib for the treatment of advanced extrapancreatic neuroendocrine tumors. Expert Rev. Anticancer Ther. 21(9), 917–926 (2021).
Mei, Y. B. et al. Validated UPLC-MS/MS method for quantification of fruquintinib in rat plasma and its application to Pharmacokinetic study. Drug Des. Dev. Ther. 13, 2865–2871 (2019).
Cao, J. et al. A phase I study of safety and pharmacokinetics of fruquintinib, a novel selective inhibitor of vascular endothelial growth factor receptor-1, -2, and – 3 tyrosine kinases in Chinese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 78(2), 259–269 (2016).
Mizuno, Y. et al. Sunitinib shrinks NET-G3 pancreatic neuroendocrine neoplasms. J. Cancer Res. Clin. Oncol. 144(6), 1155–1163 (2018).
Gao, H. et al. Patterns and predictors of pancreatic neuroendocrine tumor prognosis: Are no two leaves alike? Crit. Rev. Oncol. Hematol. 167, 103493 (2021).
Sorbye, H. et al. European neuroendocrine tumor society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J. Neuroendocrinol. 35(3), e13249 (2023).
Xu, J. et al. A single-arm, multicenter, open-label phase 2 trial of Surufatinib in patients with unresectable or metastatic biliary tract cancer. Cancer 127(21), 3975–3984 (2021).
Chen, J. et al. Surufatinib in Chinese patients with locally advanced or metastatic differentiated thyroid Cancer and medullary thyroid cancer: A multicenter, Open-Label, phase II trial. Thyroid 30(9), 1245–1253 (2020).
Xu, J. M. et al. Sulfatinib, a novel kinase inhibitor, in patients with advanced solid tumors: Results from a phase I study. Oncotarget 8(26), 42076–42086 (2017).
Xu, J. et al. Surufatinib in advanced well-differentiated neuroendocrine tumors: A multicenter, single-arm, open-label, phase Ib/II trial. Clin. Cancer Res. 25(12), 3486–3494 (2019).
Das, M. Surufatinib in neuroendocrine tumours. Lancet Oncol. 20(4), e196 (2019).
Syed, Y. Y. Surufatinib: First approval. Drugs 81(6), 727–732 (2021).
Funding
This Research was supported by grants from the General Research Projects of Zhejiang Provincial Department of Education (Y202249311).
Author information
Authors and Affiliations
Contributions
Z.C. and K.C. made contribution to conception and design. H.Y. analyzed and interpreted the data. H.Y., H.Z., A.Q., X.C. performed the data collection. H.Y. and H.Z. prepared the final draft. F.Y. contributed extensively to the subsequent revisions of the manuscript, including critical data analysis and substantial improvements to the content. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Confirms that informed consent was obtained from all participants and their legal guardians.
Institutional review board
This study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital. Confirms that all experiments were performed in accordance with relevant named guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, HP., Zhao, HY., Qiu, AC. et al. Real world study on efficacy and safety of surufatinib in advanced solid tumors evaluation. Sci Rep 15, 16294 (2025). https://doi.org/10.1038/s41598-025-00974-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00974-8
Keywords
This article is cited by
-
Real-world application of surufatinib in the treatment of neuroendocrine tumors: a multi-center retrospective study in China
European Journal of Medical Research (2025)