Abstract
This work synthesized and evaluated a new series of quinolinvinyl-phenoxy-1,2,3-triazole-acetamide hybrids 9a-p against breast cancer cell lines, including MCF-7 and MDA-MB-231. Among 16 synthesized compounds, (E)-N-(4-chlorophenyl)-2-(4-((4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)acetamide (9f) was the most active compound on both cell lines, with IC50 values of 16.84 (MCF-7) and 21.78 µM (MDA-MB-231). 3D cell culture was also performed by providing an accurate in vitro model to test the most potent analog on the cancerous spheroids. Additionally, compound 9f demonstrated significant apoptosis induction in MDA-MB-231 cells, as evidenced by Annexin V-FITC/PI assays, with an increase in total apoptotic cells. Over-activation of caspase-3 was also observed in cell-based assays, indicating a potential apoptotic mechanism. DFT analysis revealed favorable electronic properties and electrostatic potential distributions that suggest these compounds’ high reactivity and binding affinity with biological targets. These results underscore the promising anticancer potential of the synthesized hybrids for further therapeutic applications.
Similar content being viewed by others
Introduction
Cancer is the main public health concern and cause of almost one in six deaths (16.8%) in the world, which has induced a huge economic burden on societies1. According to 2020 statistics from the International Agency for Research on Cancer (IARC), breast cancer has become the most common malignancy among women. It is the second leading cause of cancer-related death worldwide. It accounts for 11.7% of all tumor cases, surpassing lung, colorectal, prostate, stomach, liver, and cervical cancer2. In addition to surgery, hormone therapy, and radiotherapy, chemotherapy remains a powerful approach in the fight against breast cancer. However, challenges like metastasis, drug resistance, and recurrence have underscored the need for novel therapeutic agents. In this respect, both natural and synthetic small molecules are versatile candidates for breast cancer treatment3,4.
Apoptosis, known as Type I programmed cell death, is a vital and complicated process that enables an organism to eliminate unwanted cells. It has been considered a chief mechanism of cell death in chemotherapy5, particularly in managing excessive breast cancer cell proliferation6,7, which happens because of growth factors, DNA damage, reactive oxygen species (ROS), and ultraviolet (UV) radiation that promotes uncontrolled breast cancer cell proliferation through multiple signaling pathways, including the mitochondrial, FasL/Fas, PI3K/Akt, ROS, nuclear factor κB (NF-κB), and mitogen-activated protein kinase (MAPK) pathways. Thus, focusing on apoptotic pathways leads to an efficient therapeutic strategy for the treatment or management of breast cancer.
Caspases (cysteine-dependent aspartate-specific proteases) are a fundamental protease family that maintains cellular homeostasis by regulating cell death and inflammation8. Among 14 identified caspases, caspase-3 has been a versatile target as its cleavage and activation lead to apoptosis and following cancer cell death, playing an important role in the survival of breast cancer patients9.
Stilbene and its derivatives are distinguished scaffolds in medicinal chemistry due to their structural versatility and biological activity. They can tolerate E/Z isomerization, which directly affects the corresponding biological activity, such as antimicrobial, antioxidant, anti-inflammatory, anti-HIV, and anticancer properties10.
The anticancer activity of stilbene-containing compounds has gained an important place10,11. Stilbene and its natural derivatives such as resveratrol, piceatannol, and pterostilbene have shown potent anti-cancer activity. Also, there are two FDA-approved stilbene derivatives known as tamoxifen (A) and toremifene (B) (Fig. 1) for the prevention of breast cancer in women at high risk of developing the disease and treatment of advanced breast cancer in postmenopausal women, respectively12.
Building on the potent activity observed with stilbene derivatives, a series of quinolinvinyl-phenoxy-1,2,3-triazole-acetamides was designed, synthesized, and evaluated against breast cancer cell lines, including MCF-7 (breast cancer) and MDA-MB-231 (breast adenocarcinoma). A 3D cell culture model was also established to test the most potent analog on MDA-MB-231 cells, as a model for more aggressive and hormone-independent breast cancer. The potential of this analog to induce apoptosis or necrosis was assessed, along with its ability to activate caspase-3. Finally, DFT analysis further supported the high binding affinity of this analog to the proposed target.
Experimental
Materials and methods
All chemicals were purchased from Merck and Aldrich and used without purification. Also, the etoposide active ingredient was obtained from Actoverco (Iran) and resveratrol was received from GOLEXIR Company (Iran).
Thin layer chromatography (TLC) was performed using silica gel 60/Kieselguhr F254 precoated on Aluminum sheets (thickness 0.2 mm, Merck). The spots on the TLC plate were visualized using UV light. Melting points were determined on a Kofler hot stage apparatus and were uncorrected1H NMR (500 MHz) and13C NMR (125 MHz) spectra were measured with Varian-INOVA. The IR spectra were obtained on a Nicolet Magna FTIR 550 spectrophotometer (in KBr). The elemental analysis was performed on an Elementar Analysensystem GmbH VarioEL CHNS mode. MS spectra were measured on Waters Synapt G1 HDMS High Definition Mass Spectrometer and Agilent Technology (HP) mass spectrometer operating at an ionization potential of 70 eV. HPLC analysis was performed on a YL9100 HPLC system (Korea) equipped with a UV detector and RP column.
Synthesis of compound 3a-b
A mixture of 4-hydroxybenzaldehyde derivative 1a-b (1 mmol), propargyl bromide 2 (1.2 mmol), and potassium carbonate (1mmol) in DMF (8 mL) was stirred at 80 ˚C for 4 h. After completion of the reaction (checked by TLC), it was poured into cold water, the resulting precipitate was filtered and washed with water to give 4-(prop-2-yn-1-yloxy)benzaldehydes 3a-b.
Synthesis of compound 5a-b
Compound 3a-b (1 mmol) reacted with 2-methylquinoline 4 (1 mmol) in acetic acid (10 mL) in the presence of morpholine (1 mmol) under reflux conditions for 48–72 h. After completion of the reaction (checked by TLC), the mixture was poured into cold water, the precipitate was filtered and washed with petroleum ether to afford compound 5a-b.
Synthesis of compound 8a-p
Aniline or benzylamine derivatives 6a-p (1 mmol) reacted with chloroacetyl chloride 7 (1 mmol) in DMF (8 mL) at room temperature for 4–5 h. After completion of the reaction (checked by TLC), the mixture was poured into cold water, the precipitate was filtered and washed with water, yielding the desired methylene chloride derivatives 8a-p.
Synthesis of compound 9a-p
A solution of compound 8a-p (1.1 mmol), sodium azide (0.9 mmol), and triethylamine (1.3 mmol) in DMF (5 mL) stirred at room temperature for 1 h. Then, compound 5a-b (0.5 mmol), CuSO4.5H2O (7 mol%), and sodium ascorbate (7 mol%) were added to the reaction mixture and the reaction continued for 24 h. After completion of the reaction (checked by TLC), the mixture was poured into water, extracted with ethyl acetate, and dried over anhydrous Na2SO4. Evaporation of solvent and recrystallization from n-hexane and ethyl acetate gave the desired product.
(E)-N-Benzyl-2-(4-((4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9a ).
Yellow powder, yield 78%, mp = 187–189 ºC. IR (KBr): 3352, 1684, 1654, 2950, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 5.24 (s, 2 H, CH2), 5.22 (s, 2 H, CH2), 4.35 (d, J = 5.8 Hz, 2 H, CH2), 7.14 (d, J = 8.8 Hz, 2 H, H3’, H5’), 7.26–7.38 (m, 6 H, =CH, H2’’, H3’’, H4’’, H5’’, H6’’), 7.54 (t, J = 8.2 Hz, 1 H, H6), 7.71-0.76 (m, 3 H, H7, H2’, H6’), 7.81 (d, J = 16.3 Hz, 1 H, =CH), 7.85 (d, J = 8.6 Hz, 1 H, H3), 7.93 (d, J = 8.2 Hz, 1 H, H8), 7.99 (d, J = 8.2 Hz, 1 H, H5), 8.25 (s, 1 H, triazole), 8.33 (d, J = 8.6 Hz, 1 H, H4), 8.86 (t, J = 5.8 Hz, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 42.9, 52.1, 61.6, 115.6, 115.7, 120.3, 126.4, 126.7, 127.1, 127.5, 127.9, 128.2, 128.8, 129.2, 129.7, 130.2, 132.3, 134.2, 136.8, 139.2, 142.8, 148.2, 156.4, 159.1, 165.9. Anal. calcd. for C29H25N5O2: C, 73.25; H, 5.30; N, 14.73. Found: C, 73.61; H, 5.51; N, 14.50.
(E)-N-Phenyl-2-(4-((4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9b ).
Yellow powder, yield 75%, mp = 224–226 ºC. IR (KBr): 3350, 1682, 1666, 2958, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 5.36 (s, 2 H, CH2), 5.23 (s, 2 H, CH2), 7.07 (t, J = 7.5 Hz, 1 H, H4’’), 7.11 (d, J = 8.7 Hz, 2 H, H3’, H5’), 7.31–7.36 (m, 3 H, H3’’, H5’’, =CH), 7.52 (t, J = 8.0 Hz, 1 H, H6), 7.58 (d, J = 7.5 Hz, 2 H, H2’’, H6’’), 7.68–7.72 (m, 3 H, H7, H2’, H6’), 7.77–7.83 (m, 2 H, H3, =CH), 7.90 (d, J = 8.0 Hz, 1 H, H8), 7.96 (d, J = 8.0 Hz, 1 H, H5), 8.28–8.31 (m, 2 H, H4, triazole), 10.47 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 52.7, 61.6, 115.5, 119.7, 120.2, 124.2, 126.4, 126.8, 127.0, 127.3, 128.2, 129.0, 129.2, 129.4, 129.7, 130.2, 134.2, 136.8, 138.9, 142.9, 148.1, 156.4, 159.1, 164.6. Anal. calcd. for C28H23N5O2: C, 72.87; H, 5.02; N, 15.17. Found: C, 72.60; H, 5.25; N, 15.34.
(E)-2-(4-((4-(2-(Quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)-N-(p-tolyl)acetamide ( 9c ).
Yellow powder, yield 70%, mp = 240–242 ºC. IR (KBr): 3361, 1680, 1666, 2953, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 2.25 (s, 3 H, CH3), 5.25 (s, 2 H, CH2), 5.35 (s, 2 H, CH2), 7.13–7.15 (m, 4 H, H3’, H5’, H3’’, H5’’), 7.36 (d, J = 16.3 Hz, 1 H, =CH), 7.48 (d, J = 6.6 Hz, 2 H, H2’’, H6’’), 7.55 (t, J = 8.0 Hz, 1 H, H6), 7.71–7.76 (m, 3 H, H7, H2’, H6’), 7.81 (d, J = 16.3 Hz, 1 H, =CH), 7.84 (d, J = 8.5 Hz, 1 H, H3), 7.93 (d, J = 8.0 Hz, 1 H, H8), 7.98 (d, J = 8.0 Hz, 1 H, H5), 8.29 (s, 1 H, triazole), 8.33 (d, J = 8.5 Hz, 1 H, H4), 10.40 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 20.9, 52.7, 61.6, 115.6, 119.7, 120.3, 126.3, 126.8, 127.0, 127.4, 128.2, 129.0, 129.2, 129.7, 130.2, 132.3, 133.2, 134.2, 136.4, 136.8, 142.8, 148.1, 156.4, 159.1, 164.4. Anal. calcd. for C29H25N5O2: C, 73.25; H, 5.30; N, 14.73. Found: C, 73.41; H, 5.14; N, 14.55.
(E)-N-(2,4-Dimethylphenyl)-2-(4-((4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9d ).
Yellow powder, yield 71%, mp = 175–177 ºC. IR (KBr): 3358, 1680, 1665, 2950, 2828 cm− 1;1HNMR (500 MHz, DMSO-d6): 2.14–2.18 (m, 6 H, 2 × CH3), 5.25 (s, 2 H, CH2), 5.43 (s, 2 H, CH2), 7.08–7.09 (m, 2 H, H3’’, H5’’), 7.14 (d, J = 8.9 Hz, 2 H, H3’, H5’), 7.27 (d, J = 7.2 Hz, 2 H, H6’’), 7.37 (d, J = 16.3 Hz, 1 H, =CH), 7.54 (t, J = 8.0 Hz, 1 H, H6), 7.70–7.75 (m, 3 H, H7, H2’, H6’), 7.81 (d, J = 16.3 Hz, 1 H, =CH), 7.84 (d, J = 8.7 Hz, 1 H, H3), 7.93 (d, J = 8.0 Hz, 1 H, H8), 7.99 (d, J = 8.0 Hz, 1 H, H5), 8.30–8.33 (m, 2 H, H4, triazole), 9.80 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 18.5, 51.1, 61.6, 115.6, 115.7, 117.7, 120.3, 126.8, 127.1, 127.2, 127.4, 128.2, 129.0, 129.2, 129.7, 130.2, 132.3, 134.2, 134.7, 135.6, 136.8, 142.3, 142.8, 148.2, 156.4, 159.1, 164.5. Anal. calcd. for C30H27N5O2: C, 73.60; H, 5.56; N, 14.31. Found: C, 73.41; H, 5.75; N, 14.11.
(E)-N-(4-Methoxyphenyl)-2-(4-((4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9e ).
Yellow powder, yield 75%, mp = 236–238 ºC. IR (KBr): 3325, 1675, 1665, 2950, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 3.73 (s, 3 H, OCH3), 5.25 (s, 2 H, CH2), 5.34 (s, 2 H, CH2), 6.91 (d, J = 9.0 Hz, 2 H, H3’’, H5’’), 7.14 (d, J = 8.8 Hz, 2 H, H3’, H5’), 7.36 (d, J = 16.3 Hz, 1 H, =CH), 7.51 (d, J = 9.0 Hz, 2 H, H2’’, H6’’), 7.54 (t, J = 8.1 Hz, 1 H, H6), 7.71–7.74 (m, 3 H, H7, H2’, H6’), 7.81 (d, J = 16.3 Hz, 1 H, =CH), 7.84 (d, J = 8.5 Hz, 1 H, H3), 7.93 (d, J = 8.1 Hz, 1 H, H8), 7.98 (d, J = 8.1 Hz, 1 H, H5), 8.29 (s, 1 H, triazole), 8.33 (d, J = 8.5 Hz, 1 H, H4), 10.36 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 52.6, 55.6, 61.6, 114.5, 115.6, 120.3, 121.3, 126.8, 127.1, 127.4, 128.2, 129.0, 129.2, 129.7, 130.2, 131.3, 132.0, 134.2, 136.8, 142.8, 148.2, 156.0, 156.4, 159.1, 164.1. Anal. calcd. for C30H27N5O2: C, 70.86; H, 5.13; N, 14.25. Found: C, 70.61; H, 5.31; N, 14. 48.
(E)-N-(4-Chlorophenyl)-2-(4-((4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9f ).
Yellow powder, yield 72%, mp = 231–233 ºC. IR (KBr): 3350, 1675, 1665, 2950, 2820 cm− 1;1HNMR (500 MHz, DMSO-d6): 5.26 (s, 2 H, CH2), 5.39 (s, 2 H, CH2), 7.14 (d, J = 8.7 Hz, 2 H, H3’, H5’), 7.35–7.41 (m, 3 H, =CH, H3’’, H5’’), 7.54 (t, J = 8.1 Hz, 1 H, H6), 7.63 (d, J = 8.9 Hz, 2 H, H2’’, H6’’), 7.71–7.75 (m, 3 H, H7, H2’, H6’), 7.81 (d, J = 16.2 Hz, 1 H, =CH), 7.74 (d, J = 8.6 Hz, 1 H, H3), 7.93 (d, J = 8.1 Hz, 1 H, H8), 7.99 (d, J = 8.1 Hz, 1 H, H5), 8.31–8.33 (m, 2 H, H4, triazole), 10.62 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 52.7, 61.6, 115.6, 115.7, 120.3, 121.3, 126.4, 126.9, 127.1, 127.9, 128.2, 129.0, 129.2, 129.3, 129.7, 130.2, 132.3, 134.2, 136.8, 137.8, 148.2, 156.4, 159.1, 164.7. ESI-MS: [M + 1]+: 496.0520. Anal. calcd. for C28H22ClN5O2: C, 67.81; H, 4.47; N, 14.12. Found: C, 67.60; H, 4.27; N, 14. 28.
(E)-N-(2,4-Dichlorophenyl)-2-(4-((4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9g ).
Yellow powder, yield 78%, mp = 228–230 ºC. IR (KBr): 3330, 1675, 1665, 2952, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 5.25 (s, 2 H, CH2), 5.50 (s, 2 H, CH2), 7.13 (d, J = 8.8 Hz, 2 H, H3’, H5’), 7.36 (d, J = 16.4 Hz, 1 H, =CH), 7.44 (dd, J = 8.8, 2.4 Hz, 1 H, H5’’), 7.54 (t, J = 8.1 Hz, 1 H, H6), 7.71–7.76 (m, 4 H, H7, H2’, H6’, H6’’), 7.80 (s, 1 H, H3’’), 7.81–7.85 (m, 2 H, H3, =CH), 7.93 (d, J = 8.1 Hz, 1 H, H8), 7.98 (d, J = 8.1 Hz, 1 H, H5), 8.30 (s, 1 H, triazole), 8.32 (d, J = 8.6 Hz, 1 H, H4), 10.18 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 52.4, 61.6, 115.6, 118.5, 120.3, 125.1, 126.4, 126.9, 127.1, 127.3, 127.5, 128.1, 128.2, 129.0, 129.2, 129.7, 129.9, 130.2, 130.3, 133.9, 134.2, 136.8, 148.2, 156.4, 159.1, 165.5. MS (m/z, %): 91 (100), 144 (53), 246 (13), 283 (10), 529 (M+., 6)/531 (M + 2)+., 533 (M + 4)+. Anal. calcd. for C28H21Cl2N5O2: C, 63.41; H, 3.99; N, 13.20. Found: C, 63.22; H, 4.28; N, 13.47.
(E)-N-(4-Bromophenyl)-2-(4-((4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9 h ).
Yellow powder, yield 75%, mp = 239–240 ºC. IR (KBr): 3328, 1675, 1665, 2950, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 5.26 (s, 2 H, CH2), 5.39 (s, 2 H, CH2), 7.14 (d, J = 8.6 Hz, 2 H, H3’, H5’), 7.36 (d, J = 16.4 Hz, 1 H, =CH), 7.53–7.59 (m, 5 H, H6, H2’’, H3’’, H5’’, H6’’), 7.71–7.75 (m, 3 H, H7, H2’, H6’), 7.81 (d, J = 16.4 Hz, 1 H, =CH), 7.84 (d, J = 8.5 Hz, 1 H, H3), 7.93 (d, J = 8.2 Hz, 1 H, H8), 7.99 (d, J = 8.2 Hz, 1 H, H5), 8.31–8.33 (m, 2 H, H4, triazole), 10.64 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 52.7, 61.6, 115.6, 115.7, 120.3, 121.6, 126.4, 126.9, 127.1, 127.4, 128.2, 129.0, 129.2, 129.7, 130.2, 132.2, 132.3, 134.2, 136.8, 138.2, 148.2, 156.4, 159.1, 164.9. Anal. calcd. for C28H22BrN5O2: C, 62.23; H, 4.10; N, 12.96. Found: C, 62.51; H, 4.33; N, 13.28.
(E)-N-Benzyl-2-(4-((2-methoxy-4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9i ).
Yellow powder, yield 75%, mp = 129–131 ºC. IR (KBr): 3335, 1675, 1666, 2952, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 3.83 (s, 3 H, OCH3), 4.32 (d, J = 6.0 Hz, 2 H, CH2), 5.20 (s, 2 H, CH2), 5.18 (s, 2 H, CH2), 7.20–7.34 (m, 7 H, H2’, H5’, H6’, H2’’, H4’’, H6’’, =CH), 7.39 (t, J = 8.0 Hz, 2 H, H3’’, H5’’), 7.52 (t, J = 8.0 Hz, 1 H, H6), 7.72 (t, J = 8.0 Hz, 1 H, H7), 7.77 (d, J = 16.2 Hz, 1 H, =CH), 7.82 (d, J = 8.6 Hz, 1 H, H3), 7.90 (d, J = 8.0 Hz, 1 H, H8), 7.95 (d, J = 8.0 Hz, 1 H, H5), 8.21 (s, 1 H, triazole), 8.30 (d, J = 8.6 Hz, 1 H, H4), 8.84 (t, J = 6.0 Hz, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 42.8, 52.1, 55.9, 62.0, 110.3, 113.7, 120.2, 121.4, 126.4, 126.8, 127.2, 127.3, 127.5, 127.8, 128.2, 128.8, 129.0, 130.1, 130.2, 134.6, 136.8, 139.1, 142.8, 148.1, 148.8, 149.7, 156.4, 165.9. Anal. calcd. for C30H27N5O3: C, 71.27; H, 5.38; N, 13.85. Found: C, 71.52; H, 5.60; N, 13.59.
(E)-2-(4-((2-Methoxy-4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)-N-phenylacetamide ( 9j ).
Yellow powder, yield 73%, mp = 124–126 ºC. IR (KBr): 3322, 1680, 1668, 2950, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 3.84 (s, 3 H, OCH3), 5.20 (s, 2 H, CH2), 5.36 (s, 2 H, CH2), 7.07 (t, J = 8.5 Hz, 1 H, H4‘‘), 7.21 (d, J = 8.5 Hz, 1H, H5’), 7.25 (d, J = 8.5 Hz, 1H, H6‘), 7.32 (t, J = 8.5 Hz, 2H, H3’’, H5’’), 7.38–7.41 (m, 2H, H2’, =CH), 7.52 (t, J = 8.4 Hz, 1H, H6), 7.58 (d, J = 8.5 Hz, 2H, H2’’, H6’’), 7.72 (t, J = 8.4 Hz, 1H, H7), 7.77 (d, J = 16.3 Hz, 1H, =CH), 7.82 (d, J = 8.7 Hz, 1H, H3), 7.90 (d, J = 8.4 Hz, 1H, H8), 7.95 (d, J = 8.4 Hz, 1H, H5), 8.27 (s, 1H, triazole), 8.30 (d, J = 8.7 Hz, 1H, H4), 10.47 (s, 1H, NH)13CNMR (125 MHz, DMSO-d6): 52.7, 56.0, 62.0, 110.3, 113.7, 119.7, 120.2, 121.5, 124.2, 126.4, 126.9, 127.2, 127.3, 128.2, 129.0, 129.4, 130.1, 130.2, 134.6, 136.8, 138.9, 142.8, 148.1, 148.8, 149.7, 156.4, 164.6. ESI-MS: [M + 1]+: 492.0940. Anal. calcd. for C29H25N5O3: C, 70.86; H, 5.13; N, 14.25. Found: C, 70.59; H, 5.41; N, 14.58.
(E)-2-(4-((2-Methoxy-4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)-N-(p-tolyl)acetamide ( 9k ).
Yellow powder, yield 71%, mp = 203–205 ºC. IR (KBr): 3340, 1675, 1665, 2950, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 2.35 (s, 3 H, CH3), 3.84 (s, 3 H, OCH3), 5.19 (s, 2 H, CH2), 5.33 (s, 2 H, CH2), 7.11 (d, J = 8.2 Hz, 2 H, H3’’, H5’’), 7.21 (d, J = 8.3 Hz, 1 H, H5’), 7.25 (d, J = 8.3 Hz, 1 H, H6’), 7.38–7.41 (m, 2 H, H2’, =CH), 7.45 (d, J = 8.2 Hz, 2 H, H2’’, H6’’), 7.51 (t, J = 8.3 Hz, 1 H, H6), 7.72 (t, J = 8.3 Hz, 1 H, H7), 7.77 (d, J = 16.3 Hz, 1 H, =CH), 7.82 (d, J = 8.6 Hz, 1 H, H3), 7.91 (d, J = 8.3 Hz, 1 H, H8), 7.95 (d, J = 8.3 Hz, 1 H, H5), 8.26 (s, 1 H, triazole), 8.30 (d, J = 8.6 Hz, 1 H, H4), 10.38 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 20.9, 52.6, 55.9, 62.0, 110.3, 113.7, 119.7, 120.2, 121.4, 126.4, 126.9, 127.2, 127.3, 128.2, 129.0, 129.7, 130.1, 130.2, 133.2, 134.6, 136.3, 136.8, 142.8, 148.1, 148.8, 149.7, 156.4, 164.4. Anal. calcd. for C30H27N5O3: C, 71.27; H, 5.38; N, 13.85. Found: C, 71.58; H, 5.69; N, 14.15.
(E)-N-(2,4-Dimethylphenyl)-2-(4-((2-methoxy-4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9l ).
Yellow powder, yield 75%, mp = 240–242 ºC. IR (KBr): 3330, 1678, 1665, 2950, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 2.15–2.18 (m, 6 H, 2 × CH3), 3.86 (s, 3 H, OCH3), 5.22 (s, 2 H, CH2), 5.42 (s, 2 H, CH2), 7.08–7.10 (m, 3 H, H3’’, H5’’, H6’’), 7.23 (d, J = 8.5 Hz, 1 H, H5’), 7.27 (d, J = 8.5 Hz, 1 H, H6’), 7.40–7.43 (m, 2 H, H2’, =CH), 7.55 (t, J = 8.0 Hz, 1 H, H6), 7.75 (t, J = 8.0 Hz, 1 H, H7), 7.779 (d, J = 16.3 Hz, 1 H, =CH), 7.85 (d, J = 8.6 Hz, 1 H, H3), 7.94 (d, J = 8.0 Hz, 1 H, H8), 7.98 (d, J = 8.0 Hz, 1 H, H5), 8.29 (s, 1 H, triazole), 8.33 (d, J = 8.6 Hz, 1 H, H4), 9.80 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 18.5, 52.1, 56.0, 62.0, 110.3, 113.8, 114.6, 120.2, 121.5, 121.5, 126.4, 126.9, 127.2, 127.3, 127.4, 128.2, 129.0, 129.7, 130.1, 130.2, 134.6, 134.7, 135.6, 136.8, 142.8, 148.2, 148.8, 149.7, 156.4, 164.5. Anal. calcd. for C31H29N5O3: C, 71.66; H, 5.63; N, 13.48. Found: C, 71.32; H, 5.91; N, 13.67.
(E)-2-(4-((2-Methoxy-4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)-N-(4-methoxyphenyl)acetamide ( 9m ).
Yellow powder, yield 75%, mp = 177–179 ºC. IR (KBr): 3325, 1675, 1665, 2950, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 3.70 (s, 3 H, OCH3), 3.84 (s, 3 H, OCH3), 5.19 (s, 2 H, CH2), 5.32 (s, 2 H, CH2), 6.89 (d, J = 7.0 Hz, 2 H, H3‘‘, 5‘‘), 7.21 (d, J = 8.3 Hz, 1 H, H5‘), 7.24 (d, J = 8.3 Hz, 1 H, H6‘), 7.38–7.41 (m, 2 H, H2‘, =CH), 7.487.56 (m, H, H6, H2‘‘, 6‘‘), 7.72 (t, J = 8.0 Hz, 1H, H7), 7.77 (d, J = 16.2 Hz, 1H, =CH), 7.82 (d, J = 8.7 Hz, 1H, H3), 7.90 (d, J = 8.0 Hz, 1H, H8), 7.95 (d, J = 8.0 Hz, 1H, H5), 8.26 (s, 1H, triazole), 8.30 (d, J = 8.7 Hz, 1H, H4), 10.34 (s, 1H, NH)13CNMR (125 MHz, DMSO-d6): 52.6, 55.6, 55.9, 62.0, 110.3, 113.8, 114.5, 120.2, 121.2, 121.4, 126.4, 126.9, 127.2, 127.3, 128.2, 129.0, 130.1, 130.2, 131.9, 134.6, 136.8, 142.8, 148.2, 148.8, 149.7, 156.0, 156.4, 164.1. Anal. calcd. for C30H27N5O4: C, 69.08; H, 5.22; N, 13.43. Found: C, 68.81; H, 5.48; N, 13.19.
(E)-N-(4-Chlorophenyl)-2-(4-((2-methoxy-4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9n ).
Yellow powder, yield 72%, mp = 186–188 ºC. IR (KBr): 3331, 1675, 1648, 2950, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 3.84 (s, 3 H, OCH3), 5.20 (s, 2 H, CH2), 5.40 (s, 2 H, CH2), 7.21 (d, J = 8.5 Hz, 1 H, H5’), 7.25 (d, J = 8.5 Hz, 1 H, H6’), 7.37–7.41 (m, 4 H, H2’, H3’’, H5’’, =CH), 7.51 (t, J = 8.4 Hz, 1 H, H6), 7.60 (d, J = 8.9 Hz, 2 H, H2’’, H6’’), 7.71 (t, J = 8.4 Hz, 1 H, H7), 7.77 (d, J = 16.3 Hz, 1 H, =CH), 7.82 (d, J = 8.7 Hz, 1 H, H3), 7.90 (d, J = 8.4 Hz, 1 H, H8), 7.95 (d, J = 8.4 Hz, 1 H, H5), 8.27 (s, 1 H, triazole), 8.30 (d, J = 8.7 Hz, 1 H, H4), 10.62 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 52.7, 55.9, 62.0, 110.3, 113.8, 114.5, 120.2, 121.2, 121.4, 126.4, 126.9, 127.2, 127.3, 127.8, 128.2, 129.0, 129.3, 130.1, 130.2, 134.6, 136.8, 137.8, 142.9, 148.2, 148.8, 156.4, 164.9. Anal. calcd. for C29H24ClN5O3: C, 66.22; H, 4.60; N, 13.31. Found: C, 66.41; H, 4.28; N, 13.55.
(E)-N-(2,4-Dichlorophenyl)-2-(4-((2-methoxy-4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9o ).
Yellow powder, yield 70%, mp = 114–116 ºC. IR (KBr): 3329, 1677, 1668, 2950, 2825 cm− 1;1HNMR (500 MHz, DMSO-d6): 3.84 (s, 3 H, OCH3), 5.19 (s, 2 H, CH2), 5.47 (s, 2 H, CH2), 7.20 (d, J = 8.4 Hz, 1 H, H5’), 7.24 (dd, J = 8.4, 1.9 Hz, 1 H, H6’), 7.37–7.43 (m, 3 H, H2’, H5’’, =CH), 7.52 (t, J = 8.1 Hz, 1 H, H6), 7.68 (d, J = 2.3 Hz, 1 H, H3’’), 7.72 (t, J = 8.1 Hz, 1 H, H7), 7.75–7.83 (m, 2 H, H6’’, =CH), 7.82 (d, J = 8.6 Hz, 1 H, H3), 7.91 (d, J = 8.1 Hz, 1 H, H8), 7.95 (d, J = 8.1 Hz, 1 H, H5), 8.26 (s, 1 H, triazole), 8.31 (d, J = 8.1 Hz, 1 H, H4), 10.15 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 52.4, 56.0, 62.0, 110.3, 113.8, 114.5, 120.2, 121.3, 121.4, 126.4, 126.9, 127.2, 127.3, 128.2, 128.2, 129.0, 129.5, 130.1, 130.2, 133.8, 134.6, 136.8, 139.6, 146.2, 148.1, 148.7, 149.7, 156.4, 165.5. MS (m/z, %): 91 (100), 144 (47), 246 (25), 283 (13), 559 (M+., 1)/561 (M + 2)+., 563 (M + 4)+. Anal. calcd. for C29H23Cl2N5O3: C, 62.15; H, 4.14; N, 12.50. Found: C, 62.44; H, 4.33; N, 12.18.
(E)-N-(4-Bromophenyl)-2-(4-((2-methoxy-4-(2-(quinolin-2-yl)vinyl)phenoxy)methyl)-1 H-1,2,3-triazol-1-yl)acetamide ( 9p ).
Yellow powder, yield 75%, mp = 199–201 ºC. IR (KBr): 3334, 1675, 1665, 2951, 2822 cm− 1;1HNMR (500 MHz, DMSO-d6): 3.84 (s, 3 H, OCH3), 5.20 (s, 2 H, CH2), 5.36 (s, 2 H, CH2), 7.20 (d, J = 8.5 Hz, 1 H, H5‘), 7.24 (d, J = 8.5 Hz, 1 H, H6’), 7.38–7.41 (m, 2 H, H2’, =CH), 7.50–7.56 (m, 5 H, H6, H2’’, H3’’, H5’’, H6’’), 7.72 (t, J = 8.4 Hz, 1 H, H7), 7.77 (d, J = 16.3 Hz, 1 H, =CH), 7.82 (d, J = 8.5 Hz, 1 H, H3), 7.90 (d, J = 8.4 Hz, 1 H, H8), 7.95 (d, J = 8.4 Hz, 1 H, H5), 8.27 (s, 1 H, triazole), 8.30 (d, J = 8.5 Hz, 1 H, H4), 10.61 (s, 1 H, NH)13CNMR (125 MHz, DMSO-d6): 52.7, 56.0, 62.0, 110.3, 113.8, 115.9, 120.2, 121.4, 121.6, 126.4, 126.9, 127.2, 127.3, 128.2, 129.0, 130.1, 130.2, 132.2, 134.6, 136.8, 138.2, 142.9, 148.2, 148.8, 149.7, 156.4, 164.9. Anal. calcd. for C29H24BrN5O3: C, 61.06; H, 4.24; N, 12.28. Found: C, 60.84; H, 4.50; N, 12.52.
HPLC analysis
The HPLC analysis was performed using the following program: methanol (solvent A) and water, a gradient of 0–100% solvent A in 11 min, 1 min at 0%, to 50% within 3 min, to 100% at 6 min, to 0 within 5 min (total run time 11 min); flow rate, 1 mL/min; detection, 254 nm; injection volume, 20 µL.
Cytotoxic effect
The breast cancer cell lines MDA-MB-231 and MCF-7 were obtained from the National Cell Bank of Iran (Pasteur Institute, Iran). Cells were cultured in RPMI-1640 medium (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin). They were maintained at 37 °C in a humidified incubator with 5% CO₂. To achieve the MTT assay, cells were seeded in 96-well microplates at a density of 5 × 10⁴ cells per well in a volume of 100 µL/well. After overnight incubation at 37 °C, half of the growth medium was replaced with 50 µL of fresh medium containing various concentrations of the synthesized compounds, dissolved in DMSO. Each concentration was tested in triplicate, with the maximum DMSO concentration kept below 0.25% in all wells. After 48 h of incubation, the medium was removed, and 0.5 mg/mL MTT was added to each well. Plates were then incubated for an additional 4 h at 37 °C. Formazan crystals formed by viable cells were solubilized in 200 µL DMSO, and absorbance was measured at 570 nm to determine cell viability.
Acridine orange (AO)/ethidium bromide (EB) double staining analysis
The viability and morphological changes in the treated cells were evaluated using AO/EB double staining method. AO penetrates both viable and dead cells and emits green fluorescence while EB is taken up only by dead cells and emits red fluorescence after DNA intercalating. The cell suspension (3 × 105 cells/well) of MDA-MB-231 was cultured in 6-well plates and incubated overnight and the experiment was achieved according to the previous report in the literature13.
Analysis of annexin V/PI-stained cells by flow cytometry
To evaluate apoptosis and/or necrosis, MDA-MB-231 cells were treated with the potent analog and analyzed using the Annexin V-FITC apoptosis kit (BioVision). Initially, 3 × 10⁶ MDA-MB-231 cells were seeded in a 6-well cell culture plate with 3 mL of complete medium. After 24 h, cells were treated with the IC50 concentration of the compound for 4 h. The cells were then harvested, washed, and labeled with PI and FITC according to the manufacturer’s protocol. Finally, Annexin V-FITC/PI-stained cells were analyzed by flow cytometry to assess the extent of apoptosis and necrosis13.
Cytotoxicity analysis in monolayer cells and spheroids
To assess cell viability in 3D cell cultures, a cytotoxicity analysis was conducted following previously reported procedures for 24 h. MDA-MB-231 cells were seeded at a density of 7.5 × 10⁴ cells per well to form spheroids13,14.
Caspase-3 activity assay
Caspase-3 activity in cells was measured using the Caspase-3 Colorimetric Activity Assay Kit (BIOMOL International, USA) following previously reported protocols13. Briefly, MDA-MB-231 cells at a density of 3 × 10⁶ were treated with various concentrations of compounds 9f, 9j, and etoposide as the positive control. After 4 h of incubation, cells were washed with cold PBS and lysed with cell lysis buffer on ice. The lysates were then centrifuged, and supernatants were collected. Protein concentration in the supernatant was determined using the Bradford method. Equal amounts of protein (100 µg), along with 5 µL of colorimetric caspase-3 substrate (Ac-DEVD-pNA, 2 mmol/L) and assay buffer [NaCl, HEPES, DTT, CHAPS, EDTA, Glycerol], were added to each reaction mixture. The reactions were incubated for 3 h at 37 °C, and absorbance was measured at 405 nm using a microplate reader.
Results and discussion
Design
Focusing on the anti-cancer activity of the stilbene derivatives, aza-stilbenes have been found important in the drug discovery of anti-cancer agents via inhibition of protein-tyrosine kinase. Compound C (Fig. 2) potently inhibited p56lck (IC50 = 178 µM); however, the increase or reduction of methoxy groups afforded the reduced potency15. Also, some aza-stilbenes have depicted c-RAF/MEK/ERK inhibitory activity16. In this respect, compounds D and E were potent inhibitors with pIC50 = 8.4 and 8.2 (Fig. 2), respectively. The presence of a heterocyclic moiety (tetrazole) was found to be essential for inducing desired activity and the removal of this group led to the reduction of activity.
1,2,3-Triazole moiety as a bioisostere to amide, ester, and carboxylic acid is an important tool in the drug design of anti-cancer agents17. They have also been applied against breast cancer due to various properties such as the ability to form hydrogen bonding interactions, moderate dipole moment, and improved water solubility18,19. Additionally, different studies have confirmed the potency of phenoxy-1,2,3-triazole agents as effective cytotoxic agents. Compound F (Fig. 2) displayed good activity against MCF-7 breast cancer cells with IC50 = 8.48 µM, however, it showed low cytotoxicity against MCF-10 A (IC50 = 114.80 µM). However, the cyclization of the hydrazide moiety to thiazolidinone ring led to better activity on MCF-7 (IC50 = 4.38 µM), while lower activity toward MCF10A (IC50 = 170.60 µM)20. 1,2,3-Triazole-thymol hybrids were also found to be potent against breast cancer, and compound G (Fig. 2) demonstrated good activity against MCF-7 and MDA-MB-231 with IC50 values of 6.17 and 10.52 µM, respectively. Also, it remarkably inhibited thymidylate synthase enzyme and cell cycle arrest annexin V-induced apoptosis study of G revealed cell cycle arrest at the G2/M phase and induction of apoptosis in MCF-7 cells21.
Compound H (Fig. 2), having 3,4-dimethoxyphenyl chalcone moiety linked to a 1,2,3-triazole ring, the most potent derivative, inhibited the growth of MCF-7 with an IC50 value of 0.24 µM. However, flow cytometry analysis in RPMI-8226 cells revealed that H caused cell cycle arrest at the G2/M phase and induced apoptosis in a dose-dependent manner, which is associated with the induction of mitochondrial apoptotic pathway through inducing ROS accumulation, increasing Bax/Bcl-2 ratio, and activation of caspases 3, 7, and 9 22. Compound I (Fig. 2) was also potent against MCF-7 with an IC50 value of 18.03 µM and induced apoptosis through activation of caspase-3 and 7 23.
In this study, focusing on the stilbene-based structures, quinolinvinyl moiety was linked to 1,2,3-triazole-acetamides, and various substituents were introduced into the X and Y positions to explore their contributions to enhancing cytotoxic potency (Fig. 2).
Chemistry
The reaction of 4-hydroxybenzaldehyde or its derivative (3-methoxy-4-hydroxybenzaldehyde) 1a-b and propargyl bromide (2) in DMF at 80 ˚C afforded 4-(prop-2-yn-1-yloxy)benzaldehyde derivatives 3a-b, which reacted with 2-methylquinoline (4) in the presence of morpholine in refluxing acetic acid to give the condensed derivatives 5a-b. On the other hand, aniline or benzylamine derivatives 6a-p reacted with chloroacetyl chloride (7) in DMF at room temperature to obtain the desired methylene chlorides 8a-p. The target compounds 9a-p were prepared via the Click reaction of the compounds 5a-b and 8a-p in DMF, in the presence of sodium azide (NaN3), triethylamine (NEt3), catalytic amounts of CuSO4.5H2O, and sodium ascorbate (SA) at room temperature for 24 h (Fig. 3).
Biological evaluation
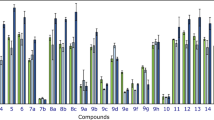
The cytotoxic activity of the synthesized compounds 9a–p was evaluated against MCF-7 (breast cancer) and MDA-MB-231 (triple-negative breast cancer) cell lines. The IC50 values are summarized in Table 1. The comparison revealed how substitutions at X and Y affect anticancer potency.
Group 1: Compounds 9a–h (X = H)
Unsubstituted compound (9a; Y = Benzyl) exhibited moderate to good activity with IC50 values of 41.68 µM against MCF-7 and 57.26 µM against MDA-MB-231. However, replacing benzyl with phenyl reduced the activity against MCF-7 (IC50 = 53.27 µM) and MDA-MB-231 (IC50 > 100 µM). The presence of electron-donating groups such as methylphenyl (9c and 9d) and methoxyphenyl (9e) at the Y position significantly diminished activity, indicating that such substitutions deteriorated the cytotoxicity of synthesized compounds.
Evaluation of halogenated derivatives depicted the highest anticancer potency. In detail, 9f (Y: 4-Cl) demonstrated the most promising inhibition, with IC50 values of 16.84 µM (MCF-7) and 21.78 µM (MDA-MB-231). 9g (Y: 2,4-diCl) and 9h (Y: 4-Br) also exhibited strong activity, although slightly less potent than 9f.
Group 2: Compounds 9i–p (X = MeO).
The unsubstituted compound 9i exhibited no cytotoxicity (IC50 > 100) against MCF-7 and MDA-MB-231 cell lines. In contrast to the first group, the substitution of benzyl with a phenyl group (compound 9j) significantly enhanced cytotoxicity, resulting in IC50 values of 27.68 µM against MDA-MB-231 and 18.57 µM against MCF-7. This shift highlighted the impact of structural modifications on the compound’s anticancer efficacy.
Like the first group, electron-donating groups (9k, 9l, and 9m) impaired activity, resulting in IC50 values > 100 µM.
Halogenated compounds retained good potency, and 9n (Y: 4-Cl) exhibited IC50 values of 27.52 µM (MCF-7) and 31.54 µM (MDA-MB-231). 9o (Y: 2,4-diCl) maintained potency with IC50 values of 23.00 µM (MCF-7) and 32.81 µM (MDA-MB-231).
Interestingly, compound 9p (Y: 4-Br) displayed a dropin activity (IC50 > 100 µM) to IC50 = 37.40 and 47.62 µM (compound 9h).
Overall, in most cases methoxy substitution at X position (X = OMe) generally reduced cytotoxicity compared to unsubstituted analogs (X = H), suggesting that the absence of this group favored higher activity. Electron-withdrawing halogen groups at the Y position (e.g. 4-Cl, 2,4-diCl) enhanced cytotoxic potency, with compounds 9f and 9g emerging as the most effective analogs. It seems that MCF-7 tends to be more sensitive to these compounds than MDA-MB-231 cells, potentially due to differences in cell types or drug uptake mechanisms.
Acridine orange (AO)/ethidium bromide (EB) double staining
The most active compound 9f was investigated for its potential to induce apoptosis in MDA-MB-231 cells morphologically using an acridine orange/ethidiumbromide (AO/EB) double staining test. MDA-MB-231 triple-negative breast cancer cells are highly aggressive and harder to treat in comparison to MCF-7 (ER+/PR+), so further study was conducted on MDA-MB-231.
Living cells were found to have a normal green nucleus, while apoptotic cells demonstrated orange-stained nuclei with chromatin condensation or fragmentation (Fig. 4). Based on the analysis of the AO/EB staining, compound 9f induced apoptosis due to clear chromatin condensation and nuclear fragmentation.
Annexin V-FITC and Propidium iodide (PI) double staining
The apoptosis-inducing potential of the most effective antiproliferative compound 9f was assessed in MDA-MB-231 cells using an Annexin V-FITC/PI assay (Fig. 5).
As shown in Fig. 5, a significant reduction in viable cells was demonstrated after treatment with 9f, indicating its strong apoptotic potency. Signs of apoptosis, such as nuclear condensation and fragmentation, were observed at a concentration of IC50 value of 21.78 µM. In untreated cells, the percentage of total apoptosis was 2.19% (1.99% early and 0.2% late apoptosis). In contrast, cells treated with 9f exhibited an increase to 18.79% total apoptosis (18.1% early and 0.69% late apoptosis). For comparison, cells treated with etoposide at IC50 concentration showed a total apoptosis rate of 36.85% (34.4% early and 2.45% late apoptosis). Notably, there was no significant difference in necrotic cell percentages between the treatment and control groups.
3D multicellular spheroid culture
The cytotoxicity of compound 9f was evaluated in a 3D multicellular spheroid model of MDA-MB-231 cells (Fig. 6). After 24 h of exposure to various concentrations of 9f, the compound exhibited an IC50 value of 25.67 ± 3.56 µM. The IC50 value of 9f in the 3D spheroid model was slightly times higher than that observed in traditional monolayer cell cultures due to the reduced sensitivity of solid tumor models.
Caspase-3 activation assay
Caspase-3 is a key enzyme in the apoptotic process, playing a critical role in cell death. To determine if apoptosis induced by compounds is dependent on caspase-3 activity, a caspase-3 colorimetric assay in MDA-MB-231 cells was conducted. In this assay, MDA-MB-231 cells were treated with the IC50 concentration of 9f and 9j, and a caspase-3-specific substrate (acetyl-Asp-Glu-Val-Asp) labeled with the chromophore p-nitroaniline (pNA) was added. The results showed a significant increase, approximately fourfold, in caspase-3 activity in cells treated with 9f and 9j compared to the control wells (Fig. 7). This increase strongly indicated the cytotoxic activity of these derivatives in MDA-MB-231 cells through caspase-3 activation, supporting the compound’s role in apoptosis induction.
DFT analysis
Density Functional Theory (DFT) is a powerful computational method used to determine the electronic structures of atoms and molecules in drug design and discovery. It plays a critical role in predicting the electronic properties of drug candidates, as well as their interactions with biological targets. By modeling these interactions at the quantum mechanical level, DFT helps to provide precise information on molecular behavior.
In this study, DFT calculations were performed to determine the Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) energies, as well as the HOMO-LUMO energy gap for compounds 9f and 9j using the B3LYP/6-31G(d, p) method (Fig. 8). These values offer significant insight into the compounds’ chemical reactivity, stability, and potential electronic transitions. In the molecular orbital diagrams, the red regions indicate the positive phase, while the green regions represent the negative phase. For compound 9f, the HOMO and LUMO orbitals are predominantly distributed across the quinoline and phenoxy linker, reflecting a balanced electron distribution. The calculated HOMO-LUMO energy gap for this compound is -4.06 eV, suggesting a higher reactivity which typically indicates increased electron mobility and a great tendency for interactions. In contrast, compound 9j shows the LUMO orbitals primarily localized on the quinoline ring, while the HOMO orbitals are mostly situated on the methoxyphenoxy linker. The calculated energy gap for 9j is -3.98 eV, implying reactivity, though slightly less than compound 9f.
Figure 9 illustrates the Electrostatic Potential (ESP) maps for compounds 9f and 9j, where blue, red, and green represent positive, negative, and neutral electrostatic potential regions, respectively. ESP maps help visualize the electron density distribution across a molecule, which is critical for understanding how it may interact with other molecules, particularly biological macromolecules like enzymes. Positive regions (blue) are likely to form favorable interactions with electron-rich species, such as nucleophiles or negatively charged residues on enzymes, while negative regions (red) are prone to interact with electrophiles or positively charged areas.
For compound 9f, the NH group of the quinoline exhibits blue coloration, suggesting a region of positive potential, while the 1-NH of the 1,2,3-triazole moiety is also located in the blue region. The chlorine substituent appears in green, indicating a neutral charge distribution.
In compound 9j, electronegative groups such as the methoxy and carbonyl groups are situated in the red areas, indicating regions of negative potential where the electron density is higher. The quinoline ring shows a green color, reflecting a neutral electrostatic potential, while the 1-NH of the 1,2,3-triazole moiety and the NH of the amide linker are represented in blue, indicating regions of positive potential where the electron density is lower.
The balanced distribution of electron density in compounds 9f and 9j suggests they may engage in both hydrogen bonding and electrostatic interactions, enhancing their potential to bind effectively with biological enzymes.
Conclusion
This study successfully synthesized a series of quinolinvinyl-phenoxy-1,2,3-triazole-acetamide compounds 9a-p as anticancer agents. SAR analysis against MCF-7 and MDA-MB-231 cell lines revealed that substitutions at the X and Y positions significantly influenced cytotoxicity. Notably, halogenated derivatives, particularly 9f (Y: 4-Cl), exhibited the highest potency, with IC50 values of 16.84 µM for MCF-7 and 21.78 µM for MDA-MB-231. In contrast, introducing methoxy groups into the X position mostly reduced anticancer efficacy. Biological evaluations indicated that compound 9f induced apoptosis in MDA-MB-231 cells, evidenced by significant increases in total apoptotic rates and caspase-3 activation. The compound’s efficacy was also confirmed in a 3D multicellular spheroid model, indicating its potential for solid tumor treatment. DFT analysis provided insights into the electronic properties of compounds 9f and 9j, revealing favorable electron distributions that could enhance binding interactions with biological targets. Overall, these findings highlight the potential of synthesized derivatives as anticancer agents by evaluating their mechanisms of action in cancer treatment.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 74, 229–263 (2024).
Arnold, M. et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66, 15–23 (2022).
Zhang, J. et al. Natural products and derivatives for breast cancer treatment: from drug discovery to molecular mechanism. Phytomedicine, 155600 (2024).
James, N., Owusu, E., Rivera, G. & Bandyopadhyay, D. Small molecule therapeutics in the pipeline targeting for triple-negative breast cancer: origin, challenges, opportunities, and mechanisms of action. Int. J. Mol. Sci. 25, 6285 (2024).
Debatin, K. M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 53, 153–159 (2004).
Yuan, L. et al. Promoting apoptosis, a promising way to treat breast cancer with natural products: A comprehensive review. Front. Pharmacol. 12, 801662 (2022).
Liu, X., Miao, M., Sun, J., Wu, J. & Qin, X. PANoptosis: a potential new target for programmed cell death in breast cancer treatment and prognosis. Apoptosis 29, 277–288 (2024).
Shi, Y. Mechanisms of caspase activation and Inhibition during apoptosis. Mol. Cell. 9, 459–470 (2002).
Srivastava, N. & Saxena, A. K. Caspase-3 activators as anticancer agents. Curr. Protein Pept. Sci. 24, 783–804 (2023).
Sepehri, S., Khedmati, M., Yousef-Nejad, F. & Mahdavi, M. Medicinal chemistry perspective on the structure–activity relationship of Stilbene derivatives. RSC Adv. 14, 19823–19879 (2024).
De Filippis, B. et al. Anticancer activity of stilbene-based derivatives. ChemMedChem 12, 558–570 (2017).
Yadav, M. et al. Oestrogen receptor positive breast cancer and its embedded mechanism: breast cancer resistance to conventional drugs and related therapies, a review. Open. Biology. 14, 230272 (2024).
Jamali, T., Kavoosi, G., Safavi, M. & Ardestani, S. K. In-vitro evaluation of apoptotic effect of OEO and thymol in 2D and 3D cell cultures and the study of their interaction mode with DNA. Sci. Rep. 8, 15787 (2018).
Abolhasani, M. H., Safavi, M., Goodarzi, M. T., Kassaee, S. M. & Azin, M. Identification and anti-cancer activity in 2D and 3D cell culture evaluation of an Iranian isolated marine microalgae Picochlorum Sp. RCC486. DARU J. Pharm. Sci. 26, 105–116 (2018).
Cushman, M., Nagarathnam, D., Gopal, D. & Geahlen, R. L. Synthesis and evaluation of new protein-tyrosine kinase inhibitors. Part 1. pyridine-containing Stilbenes and amides. Bioorg. Med. Chem. Lett. 1, 211–214 (1991).
McDonald, O. et al. Aza-stilbenes as potent and selective c-RAF inhibitors. Bioorg. Med. Chem. Lett. 16, 5378–5383 (2006).
Slavova, K. I., Todorov, L. T., Belskaya, N. P., Palafox, M. A. & Kostova I. P. Developments in the application of 1, 2, 3-triazoles in cancer treatment. Recent Pat. Anti-cancer Drug Discov. 15, 92–112 (2020).
Wu, X., Wang, J., Xia, S., Cheng, S. & Shi, Y. 2, 3-triazole derivatives with anti-breast cancer potential. Curr. Top. Med. Chem. 22 (1), 1406–1425 (2022).
Song, J., Zhang, S., Zhang, B. & Ma, J. The anti-breast cancer therapeutic potential of 1, 2, 3‐triazole‐containing hybrids. Arch. Pharm. 357, 2300641 (2024).
Şenol, H., Ağgül, A. G., Atasoy, S. & Güzeldemirci, N. U. Synthesis, characterization, molecular Docking and in vitro anti-cancer activity studies of new and highly selective 1, 2, 3-triazole substituted 4-hydroxybenzohyrdazide derivatives. J. Mol. Struct. 1283, 135247 (2023).
Alam, M. M. et al. Design, synthesis and molecular Docking studies of thymol based 1, 2, 3-triazole hybrids as thymidylate synthase inhibitors and apoptosis inducers against breast cancer cells. Bioorg. Med. Chem. 38, 116136 (2021).
Ashour, H. F., Abou-Zeid, L. A., Magda, A. A. & Selim, K. B. 1, 2, 3-Triazole-Chalcone hybrids: synthesis, in vitro cytotoxic activity and mechanistic investigation of apoptosis induction in multiple myeloma RPMI-8226. Eur. J. Med. Chem. 189, 112062 (2020).
Bimoussa, A. et al. Novel Bis-1, 2, 3-triazole-thiazolidinone hybrid as anticancer agents that induce apoptosis and molecular modeling study. Future Med. Chem., 1–18 (2024).
Acknowledgements
The authors acknowledged the support from the National Institute for Medical Research Development with project No. 4000287.
Author information
Authors and Affiliations
Contributions
MS conducted biological assays. AI performed DFT analysis and wrote the manuscript. SFM synthesized compounds. MM contributed to the synthesis of compounds. SFD contributed to the conduction of biological assays. TA contributed to the identification of compounds. MS designed the project, supervised all steps, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Safavi, M., Iraji, A., Farid, S.M. et al. Synthesis and evaluation of quinolinvinyl-phenoxy-1,2,3-triazole-acetamide hybrids against breast cancer. Sci Rep 15, 17058 (2025). https://doi.org/10.1038/s41598-025-01051-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01051-w