Abstract
Breast cancer remains the most common malignancy among women and a leading cause of cancer-related mortality worldwide. Early detection through imaging is critical for improving patient prognosis. While conventional two-dimensional ultrasound (2D-US) is widely utilized in clinical practice, it has several limitations, including high operator dependency, limited reproducibility, and the lack of standardized examination protocols. To overcome these challenges, the Automated Breast Volume Scan (ABVS) has been developed as an advanced ultrasound technique, offering fully automated, high-resolution breast imaging with improved reproducibility and standardized acquisition. Furthermore, ultrasound elastography (UE) provides complementary functional information by assessing tissue stiffness, aiding in the differentiation between benign and malignant breast lesions. This study aimed to assess the diagnostic performance of ABVS combined with UE in distinguishing benign from malignant breast lesions. A prospective study was conducted involving 93 patients with a total of 103 breast lesions, all classified as Breast Imaging-Reporting and Data System (BI-RADS) category 4 or higher on conventional ultrasound. Between October 2023 and October 2024, all participants underwent both ABVS and UE, with pathological biopsy results serving as the diagnostic “gold standard”. Of the 103 lesions, 47 were confirmed malignant, while 56 were benign. The combined diagnostic approach yielded a sensitivity of 91%, specificity of 75%, and overall accuracy of 84%, demonstrating superior diagnostic performance compared to either ABVS or UE alone. Our findings indicate that the integration of ABVS and UE enhances diagnostic accuracy, improves lesion localization, reduces operator dependency, and strengthens the reliability of breast cancer detection. This combined imaging strategy holds significant potential for optimizing breast cancer screening and early diagnosis, ultimately contributing to better clinical outcomes.
Similar content being viewed by others
Introduction
Breast cancer is the most prevalent malignant tumor among women worldwide, ranking first in both incidence and mortality1. According to the Global Cancer Statistics 2020 report by the International Agency for Research on Cancer (IARC), breast cancer accounted for approximately 2.26 million new cases, surpassing lung cancer (2.2 million cases) as the most commonly diagnosed cancer globally2. Despite extensive global efforts to prevent and control breast cancer, its incidence continues to rise. A study analyzing global trends in breast cancer incidence and mortality from 1990 to 2019 reported a significant upward trend, with an average annual percentage change (AAPC) of 0.44%3. Projections suggest that the age-standardized incidence rate of breast cancer will continue to increase between 2020 and 2029. Early diagnosis and timely treatment are crucial for improving survival rates and quality of life in breast cancer patients. Studies indicate that early detection significantly improves survival outcomes. In the United States, for instance, the five-year relative survival rate for patients diagnosed is approximately 91%, whereas for those diagnosed when metastatic, the rate drops dramatically to 17%4. Therefore, enhancing early screening and diagnosis, alongside promoting healthy lifestyle interventions, is essential for reducing breast cancer incidence and improving patient survival rates.
Ultrasound imaging examinations play a crucial role in the early diagnosis of breast cancer. According to Siegel et al.5, breast cancer remains the most commonly diagnosed cancer among women worldwide, with early detection significantly improving prognosis and survival rates. Conventional two-dimensional (2D) ultrasound, ultrasound elastography, contrast-enhanced ultrasound (CEUS), mammography, and magnetic resonance imaging (MRI) are among the commonly used imaging modalities6. Conventional 2D ultrasound, widely utilized due to its non-invasive nature and real-time imaging capabilities, serves as a primary adjunctive diagnostic tool, particularly in women with dense breast tissue7. However, its application in breast cancer diagnosis is limited by operator dependence, poor reproducibility, and the lack of standardized protocols8. Studies have shown that the diagnostic accuracy of conventional ultrasound for breast cancer varies significantly, with reported sensitivity ranging from 65 to 97%, depending on the operator’s experience and lesion characteristics9. Moreover, the positive predictive value (PPV) of ultrasound alone is lower compared to mammography, particularly in detecting microcalcifications, a critical feature of early-stage breast cancer10. The development of AI-assisted ultrasound and multimodal imaging approaches is being explored to address these limitations and improve diagnostic performance11.
With advancements in technology and the continuous innovation of ultrasound equipment, ABVS has emerged as a novel imaging technique. ABVS allows for fully automated acquisition of whole-breast volumetric images, ensuring standardized and highly reproducible examinations, thus overcoming some of the limitations of conventional 2D ultrasound12. Additionally, ultrasound elastography provides valuable diagnostic insights by assessing the stiffness differences between lesions and surrounding tissues, aiding in the differentiation between benign and malignant nodules13.
This study aims to explore the combined application of ABVS and ultrasound elastography to comprehensively evaluate their advantages and diagnostic value in distinguishing benign from malignant breast lesions.
Methods
Study population
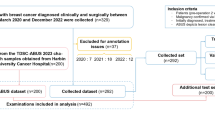
A total of 93 patients with 103 breast lesions who underwent ultrasound examinations at the Department of Ultrasound, Xiangyang No. 1 People’s Hospital from October 2023 and October 2024 were included in this study. All patients underwent ABVS and UE examinations. The patients’ ages ranged from 20 to 86 years, with an average age of (46.4 ± 14.3) years. Histopathological diagnoses were obtained for all cases. Inclusion criteria: (1) BI-RADS category 4 or higher on conventional ultrasound; (2) No prior clinical treatment for the lesions; (3) Availability of histopathological results. Exclusion criteria: (1) Presence of cystic nodules; (2) Incomplete clinical data; (3) Absence of pathological confirmation; (4) ABVS and UE not performed on the same lesion.
This study was approved by the Ethics Committee of Xiangyang No. 1 People’s Hospital (Grant No. 2021KYLX02), and informed consent was obtained from all patients. The study adhered to the Declaration of Helsinki, ensuring the protection of patient privacy and confidentiality of personal information. No hazardous experiments were conducted on patients.
ABVS examination
An intelligent automated breast volume ultrasound system (IBUS, BE3, Shantou Ultrasonic Instruments Institute Co., Ltd.) was used with a high-frequency linear volumetric probe (7–14 MHz). Patients were placed in a supine position with both arms raised above the head to ensure full exposure of both breasts. The probe was gently pressed against the breast for scanning in the medial, lateral, and central positions. For larger breasts, additional scanning planes were included. The acquired images were stored and uploaded to the ABVS imaging analysis workstation. Two radiologists, each with over eight years of experience in breast ultrasound, independently analyzed the images, evaluating features such as convergence sign, angulation, spiculation, and microcalcifications. Both radiologists were blinded to the patients’ clinical and pathological data and were not involved in the scanning procedures.
UE examination
A Samsung RS80A ultrasound system with a 7–14 MHz probe was used for the UE examination. Patients were positioned in a supine or semi-lateral position to ensure full exposure of the breast and axillary tissues. Initially, conventional two-dimensional ultrasound was performed to assess lesion morphology, margins, spiculation, internal echogenicity, and microcalcifications. The ultrasound mode was then switched to elastography, and patients were instructed to hold their breath while the probe was placed gently on the lesion for 2 s to stabilize the image. The results were evaluated by two radiologists with at least five years of experience in breast ultrasound, who were blinded to the clinical and pathological data and did not participate in the examination procedures.
Diagnostic criteria
The diagnostic criteria for breast lesions were based on the BI-RADS classification established by the American College of Radiology14. According to BI-RADS, category 0 indicates an incomplete assessment requiring further imaging evaluation. Category 1 is classified as negative with no abnormal findings, while category 2 represents benign lesions that require routine follow-up. Category 3 lesions are considered probably benign, but a shortened follow-up interval is recommended for monitoring.
Lesions categorized as BI-RADS 4 are considered suspicious for malignancy and require biopsy. This category is further divided into three subgroups: 4A, indicating a low suspicion of malignancy (≤ 10% malignancy rate); 4B, representing a moderate suspicion of malignancy (≤ 50% malignancy rate); and 4C, suggesting a high suspicion of malignancy (50–95% malignancy rate). Category 5 lesions are highly suggestive of malignancy and necessitate biopsy confirmation, while category 6 lesions have already been confirmed as malignant by pathology. In this study, BI-RADS categories 3–4A were classified as benign, while categories 4B–5 were classified as malignant.
For UE, lesion stiffness was evaluated using a modified BI-RADS-based 5-point elastography scoring system. Referring to Itoh et al.‘s15 elastic imaging hardness grading criteria, the lesions were divided into 5 levels: 1 point: the entire tumor deformed and the image was displayed in green. 2 points: Most of the tumors are deformed, but a small part is not deformed. The image shows that green and blue are mixed, mainly green. 3 points: The tumor boundary is deformed, the central part is not deformed, the image shows that the center of the lesion is blue and the periphery of the lesion is green. 4 points: The entire tumor was not deformed, and the image showed that the overall lesion was blue. 5 points: The entire tumor and the surrounding tissues did not deform, and the images showed that the lesions and surrounding tissues were blue. Among them, 1–3 points are considered benign, and 4–5 points are considered malignant.
Statistical analysis
Data analysis was performed using SPSS version 26.0. The normality of the data was assessed using the Shapiro-Wilk test. Continuous variables were presented as mean ± standard deviation (SD) for normally distributed data, and as median (interquartile range) for non-normally distributed data. For comparisons between groups (benign and malignant nodules), independent samples t-tests were used for normally distributed data, while the Mann-Whitney U test was applied for non-normally distributed data. The diagnostic performance of different examination methods (ABVS, UE, and combined ABVS + UE) was evaluated through sensitivity, specificity, and accuracy calculations. The Kappa coefficient was used to assess the agreement between the diagnostic methods and pathological findings, and the F1-score was calculated to evaluate the balance between precision and recall. The Net Reclassification Improvement (NRI) was calculated to compare the performance of the combined ABVS + UE method relative to ABVS and UE alone. A p-value < 0.05 was considered statistically significant.
Results
Pathological results
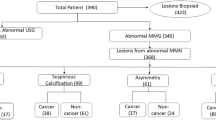
Pathological examination confirmed that among the 103 nodules from 93 patients, 47 were malignant, and 56 were benign (Table 1).
Comparison of ABVS coronal plane imaging features of benign and malignant breast nodules
The “convergence sign,” “angle formation,” and “spiculated” signs in the ABVS coronal plane (Fig. 1A, B, C). In this study, 85% (40/47) of malignant lesions exhibited the “convergence sign,” “angle formation,” and “spiculated” signs; among benign lesions, 25% (14/56) showed these signs. The difference was statistically significant (χ²=61.199, P = 0.000). The results suggest that the detection rate of malignant features such as the “convergence sign,” “angle formation,” and “spiculated” signs in the ABVS coronal plane is higher for malignant breast nodules than for benign ones (Table 2).
Comparison of UE scores between benign and malignant nodules
The UE score was used to assess the malignancy of the lesions (Fig. 2A, B). 87% (41/47) of malignant lesions had an elasticity score ≥ 4, while 70% (39/56) of benign lesions had an elasticity score < 4. The difference was statistically significant (χ²=31.329, P = 0.000). The results indicate that the UE score of malignant nodules is higher than that of benign nodules (Table 3).
(A) Pathological results confirmed as fibroma of the breast, with UE imaging predominantly rendered in green and an elastography score of ≤ 3. (B) Pathological results confirmed as invasive breast carcinoma of no special type, with UE imaging predominantly rendered in blue and an elastography score of ≥ 4.
Comparison of combined diagnostic results
In this study, based on pathological results, the combination of ABVS and UE was found to detect 91% (43/47) of malignant nodules and 75% (42/56) of benign nodules. The difference was statistically significant (χ²=45.544, P = 0.000) (Table 4).
Comparison of diagnostic performance of different examination methods
The sensitivity, specificity, and accuracy of the ABVS + UE combined examination were higher than those of the single ABVS or UE examination methods (P = 0.000) (Table 5).
The Kappa coefficient indicates the level of agreement between methods, with ABVS + UE showing the highest value (0.65), suggesting the best consistency in results. The F1-Score, a measure of the method’s accuracy, is also highest for ABVS + UE (82.69%), compared to 79.21% for ABVS and 78.10% for UE. The Net Reclassification Index for ABVS + UE shows an improvement of 6% compared to ABVS and 9% compared to UE, indicating a better reclassification of cases using the combined method (Table 6).
The ROC curve compares the performance of three models: “ABVS + UE,” “ABVS,” and “UE”. The “ABVS + UE” model, shown by the red curve, achieved the highest AUC of 0.83, indicating the best performance. The “ABVS” model (orange curve) had an AUC of 0.80, while the “UE” model (blue curve) scored 0.78 (Fig. 3).
Discussion
Breast cancer has a high incidence rate and a tendency for metastasis and recurrence. Studies have shown that the 5-year survival rate for breast cancer decreases as the clinical stage progresses. Early detection of breast cancer is therefore of significant importance. Previously, conventional two-dimensional ultrasound was commonly used for detection. According to relevant reports, this method faces challenges such as difficulty in spatial localization, longer detection time, and lower image recognition rates16. As an emerging ultrasound method, ABVS has the advantage of coronal plane imaging, which allows direct observation of tumor morphology in the coronal plane. It is well known that signs such as “convergence,” “angularity,” and “spiculations” are key indicators of malignant tumors. Breast ultrasound plays a critical role in detecting breast diseases, but a single ultrasound examination method cannot provide an accurate diagnosis. Therefore, a combination of multiple methods is necessary to improve the diagnostic rate of breast cancer17. The integration of Automated Breast Volume Scan (ABVS) and Ultrasound Elastography (UE) represents a significant advancement in the diagnostic evaluation of breast lesions, particularly in differentiating benign from malignant conditions.
The results of our study indicate that the combined use of ABVS and UE significantly enhances the differentiation of benign and malignant breast lesions, achieving a sensitivity of 91%, specificity of 75%, and overall accuracy of 84%. This performance surpasses that of either modality used independently, aligning with previous research that highlights the superior diagnostic capabilities of ABVS in breast imaging. For instance, Chen et al. demonstrated that ABVS provides a comprehensive analysis of 3D data, ensuring accurate and consistent results, which is crucial for effective diagnosis18. Furthermore, Ibraheem et al. noted that ABVS has shown promise in the diagnostic stage, enhancing breast cancer detection rates compared to traditional methods19. Our findings corroborate these assertions, suggesting that the automated nature of ABVS contributes to its reproducibility and reliability, thereby minimizing operator-related variability that often plagues conventional ultrasound techniques20.
Moreover, the incorporation of UE adds a critical layer of functional assessment by evaluating tissue stiffness, which is instrumental in distinguishing between benign and malignant lesions. Our results indicated that 87% of malignant lesions exhibited higher elasticity scores (≥ 4), while a significant proportion of benign lesions scored below this threshold. This is consistent with findings from Cheng et al., who reported that ultrasound elastography can achieve high sensitivity in breast cancer diagnosis, further supporting the utility of this modality in clinical practice21. The combination of ABVS and UE not only improves diagnostic accuracy but also enhances lesion localization, which is vital for guiding biopsies and subsequent treatment decisions.
The reduction of operator dependency is another significant advantage of the ABVS and UE integration. Traditional 2D ultrasound is known for its high operator dependence, which can lead to variability in diagnostic outcomes22. In contrast, ABVS offers a standardized acquisition process that minimizes the influence of individual operator skill levels. This is particularly relevant in settings where access to experienced radiologists may be limited. The findings of our study align with those of Liu et al., who emphasized that the automated nature of ABVS allows for more consistent imaging results, thereby improving the overall diagnostic process23. The ability to produce high-quality images with minimal operator intervention not only enhances diagnostic confidence but also facilitates broader implementation of advanced imaging techniques in diverse clinical settings.
Furthermore, the integration of ABVS and UE may contribute to improved clinical outcomes by enabling earlier detection of breast cancer. Early diagnosis is critical for enhancing survival rates, as evidenced by the stark contrast in five-year survival rates between early-stage and late-stage diagnoses24. Our study’s high sensitivity in detecting malignant lesions suggests that this combined approach could play a pivotal role in breast cancer screening programs, particularly for women with dense breast tissue where traditional imaging methods may fall short25. The ability to accurately identify lesions that may otherwise be missed is crucial for timely intervention and treatment, ultimately leading to better patient prognoses.
In addition to the clinical implications, the economic impact of adopting ABVS and UE in routine practice should also be considered. The potential for reduced false-negative rates and improved diagnostic accuracy may lead to fewer unnecessary biopsies and follow-up procedures, which can be both costly and emotionally taxing for patients26. By streamlining the diagnostic process and enhancing the accuracy of breast cancer detection, healthcare systems may realize significant cost savings while simultaneously improving patient care.
Despite the promising results, it is essential to acknowledge the limitations of our study. The sample size, while adequate for preliminary findings, may not fully represent the diverse spectrum of breast lesions encountered in clinical practice. Future studies with larger cohorts and multi-center designs are warranted to validate our findings and further explore the generalizability of the combined ABVS and UE approach27. Additionally, the potential for technological advancements in imaging modalities should be continuously evaluated to ensure that the most effective tools are utilized in breast cancer diagnosis.
In conclusion, the innovative integration of ABVS and ultrasound elastography presents a compelling advancement in the differentiation of benign and malignant breast lesions. Our findings demonstrate that this combined approach not only enhances diagnostic accuracy but also reduces operator dependency, thereby improving the reliability of breast cancer detection. As the landscape of breast imaging continues to evolve, the adoption of such advanced techniques holds significant promise for optimizing breast cancer screening and early diagnosis, ultimately contributing to improved clinical outcomes for patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Katsura, C., Ogunmwonyi, I., Kankam, H. K. & Saha, S. Breast cancer: presentation, investigation and management. Br. J. Hosp. Med. (Lond). 83 (2), 1–7 (2022).
Zhang, X. et al. Interpretation on the report of global cancer statistics 2022. Zhonghua Zhong Liu Za Zhi. 46 (7), 710–721 (2024).
Huang, J. et al. Global incidence and mortality of breast cancer: a trend analysis. Aging (Albany NY). 13 (4), 5748–5803 (2021).
Houghton, S. C. & Hankinson, S. E. Cancer progress and priorities: breast cancer. Cancer Epidemiol. Biomarkers Prev. 30 (5), 822–844 (2021).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48 (2023).
Madani, M., Behzadi, M. M. & Nabavi, S. The role of deep learning in advancing breast Cancer detection using different imaging modalities: a systematic review. Cancers (Basel) 14, 21 (2022).
Berg, W. A. & Vourtsis, A. Screening breast ultrasound using handheld or automated technique in women with dense breasts. J. Breast Imaging. 1 (4), 283–296 (2019).
Calas, M. J. G., Pereira, F. P. A., Gonçalves, L. P. & Lopes, F. Preliminary study of the technical limitations of automated breast ultrasound: from procedure to diagnosis. Radiol. Bras. 53 (5), 293–300 (2020).
Chen, H. L., Zhou, J. Q., Chen, Q. & Deng, Y. C. Comparison of the sensitivity of mammography, ultrasound, magnetic resonance imaging and combinations of these imaging modalities for the detection of small (≤ 2 cm) breast cancer. Med. (Baltim). 100 (26), e26531 (2021).
Brem, R. F., Lenihan, M. J., Lieberman, J. & Torrente, J. Screening breast ultrasound: past, present, and future. AJR Am. J. Roentgenol. 204 (2), 234–240 (2015).
Le, E. P. V., Wang, Y., Huang, Y., Hickman, S. & Gilbert, F. J. Artificial intelligence in breast imaging. Clin. Radiol. 74 (5), 357–366 (2019).
Wang, S. J. et al. Automated breast volume scanner (ABVS)-Based radiomic nomogram: a potential tool for reducing unnecessary biopsies of BI-RADS 4 lesions. Diagn. (Basel) 12, 1 (2022).
Sigrist, R. M. S., Liau, J., Kaffas, A. E., Chammas, M. C. & Willmann, J. K. Ultrasound elastography: review of techniques and clinical applications. Theranostics 7 (5), 1303–1329 (2017).
Liu, G. et al. BI-RADS 4 breast lesions: could multi-mode ultrasound be helpful for their diagnosis? Gland Surg. 8 (3), 258–270 (2019).
Itoh, A. et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 239 (2), 341–350 (2006).
Wang, L. Early diagnosis of breast cancer. Sens. (Basel) 17 (7), 1572 (2017).
Wang, X. L., Tao, L., Zhou, X. L., Wei, H. & Sun, J. W. Initial experience of automated breast volume scanning (ABVS) and ultrasound elastography in predicting breast cancer subtypes and staging. Breast 30, 130–135 (2016).
Chen, T. et al. A novel application of the automated breast volume scanner (ABVS) in the diagnosis of soft tissue tumors. Clin. Imaging 39 (3), 401–407 (2015).
Ibraheem, S. A. et al. Evaluation of diagnostic performance of automatic breast volume scanner compared to handheld ultrasound on different breast lesions: a systematic review. Diagn. (Basel) 12, 2 (2022).
Sherchan, A., Liang, J. T., Sherchan, B., Suwal, S. & Katwal, S. Comparative analysis of automated breast volume scanner (ABVS) combined with conventional hand-held ultrasound and mammography in female breast cancer detection. Ann. Med. Surg. (Lond.) 86 (1), 159–165 (2024).
Cheng, R., Li, J., Ji, L., Liu, H. & Zhu, L. Comparison of the diagnostic efficacy between ultrasound elastography and magnetic resonance imaging for breast masses. Exp. Ther. Med. 15 (3), 2519–2524 (2018).
Sharma, U. et al. Potential of Diffusion-Weighted imaging in the characterization of malignant, benign, and healthy breast tissues and molecular subtypes of breast Cancer. Front. Oncol. 6, 126 (2016).
Liu, J. et al. Metabolic reprogramming enables the auxiliary diagnosis of breast cancer by automated breast volume scanner. Front. Oncol. 12, 939606 (2022).
Johnson, A., Sarawagi, R., Malik, R., Sharma, J. & Bhagat, A. Utility of diffusion-weighted imaging in differentiating benign and malignant breast lesions. SA J. Radiol. 28 (1), 2952 (2024).
Zhang, X. et al. Diagnostic value of an automated breast volume scanner compared with a hand-held ultrasound: a meta-analysis. Gland Surg. 8 (6), 698–711 (2019).
Liu, J. et al. Diagnostic performance of combined use of automated breast volume scanning & hand-held ultrasound for breast lesions. Indian J. Med. Res. 154 (2), 347–354 (2021).
Xu, C. et al. Combined use of the automated breast volume scanner and the US elastography for the differentiation of benign from malignant lesions of the breast. BMC Cancer 14, 798 (2014).
Funding
The study was supported by The Hubei Provincial Technology Innovation Project (grant no. 2022BCE004).
Author information
Authors and Affiliations
Contributions
J.Q.Z. and L.G. conducted the study design; Y.P., L.M.Z., Y.H., and Z.X.W. data collection; Y.P. and L.G. performed the data analysis and interpretation; Y.P. wrote the draft of the manuscript; J.Q.Z. and L.M.Z. provided critical revisions of the manuscript and contributed to the overall conceptualization and supervision of the project. All authors approved the final version of the manuscript and agree to be accountable for the accuracy and integrity of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Ethics and Scientific Committee of Xiangyang No. 1 People’s Hospital (Grant No. 2021KYLX02) and complied with the principles of the Declaration of Helsinki.
Informed consent
All participants provided written informed consent for ultrasonography examinations. Written informed consent was obtained from the individual for the publication of any potentially identifiable images included in this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Peng, Y., He, Y. et al. Innovative integration of automated breast volume scan and ultrasound elastography for enhanced differentiation of benign and malignant breast lesions. Sci Rep 15, 18816 (2025). https://doi.org/10.1038/s41598-025-01061-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01061-8