Abstract
Peatlands are believed to be important as significant long-term global carbon sinks of atmospheric CO2. The ignition of flammable dry peat substrate, either naturally or anthropogenically, may lead to periods of extensive peat fires, the dynamics of which we aim to clarify. However, existing records are largely historically limited or do not accurately identify fire severity and nutrient status. In this study, we test the isotope compositions of carbon and nitrogen to determine fire severity archived in peat deposits. Eight fire periods were determined using microcharcoal analysis. High fire severity results in the alteration of organic matter to pyrogenic organic matter resulting in the depletion of δ13Corg (~1‰), however the δ13Corg remains enigmatic due to variation of organic sources and its combustion efficiencies. Following heavy loss of nitrogen by high fire severity, regrowing vegetation gains nitrogen via atmospheric fixation, subsequently resulting in a lower δ15Ntot (-1–1‰). During the undisturbed accumulation of peat, residual ash from past fires and the depth of the water table can be factors for enrichment of nitrogen. This condition led to N2-fixation by microbial activity and nitrification processes, resulting in higher δ15Ntot values as well more luxuriant plant growth, along with variable values of δ13Corg. This improves the current understanding of the carbon and nitrogen isotopes fractionation pathways during fires and are potentially useful proxies to identify fire severity.
Similar content being viewed by others
Introduction
Peatlands are believed to be important as significant long-term global carbon sinks to sources of atmospheric CO2 and are vulnerable to global climate change1,2. As the climate continues to change globally, it is expected that all aspects of the fire regime, including severity, will display immediate responses, increasing future fire impacts on soil, water and vegetation3. Once ignited, either naturally or anthropogenically, dry peat substrate fires cause peatlands to become a major source of carbon emissions4,5, and it could also influence atmospheric nutrient deposition6. Incomplete combustion of organic material during fires produces pyrogenic organic matter7,8, while fires could release organically bound nutrients through combustion; thus, the residual ash and charcoal are typically rich in nutrients, e.g., nitrogen (N), phosphorus (P), potassium (K), magnesium (Mg), sulfur (S), and calcium (Ca)9-12.
Fires recorded in the Kapuas coastal wetlands is known as prevalent factor in peat ecological changes, e.g., degree of preservation, nutrient status and paleovegetation shift13. At least eight fire events were reported, with decreasing intensity towards the top of the core. The fire peaks coincide with the change of vegetation from mixed-riparian forest to an open vegetation community13. Longer and higher fire frequencies, indicating more severe fires resulting in heavy losses of N and increasing C/N as the mire thickened and became nutrient depleted. In contrast, fire could also result in nutrient enrichment in mire deposition as indicated by low C/N ratios and increasing arboreal or tree communities13. However, existing records are largely historically limited or do not accurately identify fire severity and nutrient status; therefore, there is a need to develop new proxies that can extend our fire records significantly.
Stable isotopes are increasingly being used to detect and understand causes of environmental change. The natural abundance of 13C and 15N in soil and peat can be used to evaluate the origin of soil organic matter and the relative importance of N sources to plant nutrition14-17. Additionally, the proportion of two H stable isotopes (protium 1H and deuterium 2H) present in biomass and the relationship of these isotopes to water dynamics has served to enlighten past and modern climate changes through soil-plant-atmosphere interactions18,19. With regards to δ13C, depletion of carbon isotopes relative to source vegetation following charring has been widely reported due to the thermal degradation of cellulose20-23. However, high temperatures of charring could affect thermolabile molecules like polysaccharides, which leads to 13C-enrichment18,24,25. In addition, combustion of organic matter (OM), e.g., plants and detritus, releases 15N-depleted gases, and the mineralization, (de)nitrification, and associated N losses can be accelerated following fires26,27. Furthermore, 15N is not only enriched by peat decomposition, but can also be enriched by rainfall and leaf litter decomposition in standing water, and residual OM becomes enriched in 15N, most commonly several years to decades after disturbance26-30. Given the wide use of isotope proxies to paleoecology and paleofire sciences, it is known that a single proxy should be interpreted with caution to ensure consistent and robust interpretation of peat conditions.
This study aims to investigate the stable isotope composition of δ13C and δ15N as well as other ecological proxies of bulk peat deposits to identify the characteristics and severity of past fire periods. Macroscopically, alternating charcoal-rich layers indicated by solid-black layers and well-preserved plant fragments were identified in this core, indicating the presence of fire and non-fire activity in this core. We expect that sediment layers deposited during and/or after fire periods will exhibit a distinct isotope composition, since the combustion process releases a significant amount of C and N in volatile form. Contributions from plant matter are also expected to change with severity, thus reflecting exposure to elevated temperatures. Thus, the high sensitivities of peat deposits to environmental changes are well-suited to palaeoecological investigations. These efforts were tested on peat deposits from the Kapuas coastal wetlands, West Kalimantan, that were affected by both long-term natural and anthropogenic fire periods (Fig. 1 A and B).
Methods
Core sampling
Core samples were obtained in continuous 50 cm increments using a hand-operated MacCaulay peat sampler (refer to as a Russian D-corer). Sample coring was stopped once non-peat sediment was obtained in the base (Fig. 1C). This core penetrates peat layers to 480 cm in depth with 20 cm of organic-rich mud layers at the base. Core samples were described according to a field classification proposed and modified after32, see Fig. 1C. Color indexes for macroscopic description were described according to Munsell Soil Color Chart 10YR and a squeezing test proposed by31.
Map of wetlands distributions in Kalimantan Island, Indonesia, red square indicating the location of research area (Kapuas Coastal Wetlands). (B) Location of the KP-17 core. (C) Stratigraphy of peat obtained from KP-17 core, peat type was described based on Wust et al.31 and their visual appearance comparison with Munsell-Soil Color Chart. Note: black line indicating charcoal-rich layer and brown line indicating well-preserved plant fragments.
Microcharcoal analysis
Subsamples of 1 cm3 were taken from the cores every 2 cm (n = 250) for microcharcoal analysis. Charcoal fragments were dispersed in 1 mol/L HCl at 800C for 4 h and washed with pure water for 24 h to disaggregate the sediments. The samples were gently washed through sieves with mesh sizes of 125 and 250 µm to collect particles 125–250 µm in size (micro-charcoal) and dried in an oven at 600C32,33. Charcoal fragments, that is black, opaque, angular fragments showing cellular features, were identified and counted under a stereomicroscope and polarized reflected light microscope for further identification of the charcoal internal structure. All charcoal grains in each sample were counted and the number expressed as charcoal abundance based on sample volume (particles cm−3). Charcoal abundance was normalized using a Z-score value prior to further interpretation to determine its significance33.
Elemental and isotope characterization
The replicate sub-samples were then acid The replicate sub-samples were then acid treated using 1 N HCl (with block heater at 700C for 1 hour), diluted using Milli-Q water to remove the carbonates and dried at 600C for 48 h. Then, ~1.5 mg (±0.15) of finely grounded samples were weighed and prepared with tin capsules using a microbalance. The TCorg and TN content (w.t.%) was determined by high temperature combustion using Flash 2000 Organic Elemental Analyzer. The N2 and CO2 gas product of the combustion was then directed to a Thermo Finnigan Delta V Advantage isotope ratio mass spectrometer (IRMS) using continuous-flow (ConFlo-IV) for δ13Corg and δ15Ntot measurements. All these analyses were conducted at the Atmosphere and Ocean Research Institute (AORI), the University of Tokyo, Japan. The δ13Corg and δ15Ntot are reported as per mil (‰) deviation from the carbon isotope composition of the Vienna Pee Dee Belemnite (VPDB) and atmospheric air (N2) nitrogen isotope composition, respectively. The internal standard was IA-R001 - 13C/15N Wheat Flour with following analytical precision (SD) for δ13CVPDB (−26.43 ± 0.08; 40.2%; n = 15) and δ15NAir (+2.55 ± 0.22; 1.88%; n = 20).
Statistical analysis
Overall depth trends for microcharcoal, TCorg, TN, δ13Corgand δ15Ntot were visualized using RStudio version 4.4.0, the black line shows the moving average line using the SMA (Simple Moving Average) function setting from ggplot2 R Package. PCA is a variable reduction method that produces a smaller number of artificial variables, called Principal Components (PCs), which are visualized with scaled 2D biplots when evaluating PCA findings. More than 60% of the total variance is explained by PC1 and PC2 in this paper. For the principal component analysis (PCA), all data were preprocessed by scaling data using Z-score normalization and computed in RStudio version 4.4.0 with the prcomp function from the factoextra R Package. Since there is no standardization for fire severity, here we apply a definition of fire severity categorized based on the abundance of microcharcoal, normalized using Z-score value34. Three categories were used in this study to identify the fire significance; no fire (<−0.2), low (−0.2–0.2) and high (>0.2) are used to describe fire intensity in further discussions. In addition, a significant test of microcharcoal and peat chemical properties was assessed with two-tailed t tests and calculated Bias Factor (loge(BF01)) were conducted using ggstatsplot package from Rstudio ver 4.4.0.
Results
Peat physical and chemical properties
Charcoal abundance decreases upwards, with eight highly oxidized periods identified in the core. The bottom section shows more frequent highly oxidized periods with period #1 at depth 480 − 444 cmbs (126.5 particle/cm3; 1.32), period #2 at 426 − 388 cmbs (121.8 particle/cm3; 1.22), and period #3 at 366 − 348 cmbs (131.7 cm/cm3; 1.42). The middle section (hemic peat layers) exhibits four periods; period #4 at 332 − 302 cmbs (137.4 particle/cm3; 1.52), period #5 at 262 − 256 cmbs (139.5 particle/cm3; 1.56), period #6 at 244 − 232 cmbs (109.3 particle/cm3; 0.98), and period #7 at 134 − 122 cmbs (77.5 particle/cm3; 0.37). Lastly, period #8 at 14 − 0 cmbs (73.4 particle/cm3; 0.29) lies within the uppermost layer (Fig. 2).
The TCorg of KP-17 core shows wide variation from 7.75 to 31.73 w.t.% (20.02 w.t.% on average; n = 6) for the organic-rich mud layer and 39.82–55.70 w.t.% (50.27 w.t.% on average; n = 244) for the peat layer. The TCorg highlights a general slight decreasing trend with depth. However, an increase in TCorg content is related to the high occurrence of microcharcoal layers. This is supported by the δ13Corg analysis, which shows an increasing trend with depth, the values ranging from − 27.20 to −31.04‰ with an average of −29.79 (n= 250). The more negative value of δ13Corg occurs adjacent with the microcharcoal layer. The total nitrogen content (TN) for organic-rich mud layer varies from 0.33 to 0.99 w.t.% (0.67 w.t.% on average) and 0.64–1.77 w.t.% (1.13 w.t.% on average) for the peat layer. The N content displays a general decreasing trend with depth and more constant values associated with charcoal-rich layers at 225–425 cmbs. The δ15Ntot values show distinctly more negative values related to the fire-affected layers, with values ranging from − 0.73‰ to 2.12‰ with an average of (0.82‰; n= 250). However, from 0 to 225 cmbs, the wide variety of fluctuations in nitrogen and δ15Ntot values makes it difficult to pinpoint the effects of fire periods during this time. The C/N ratio shows a fluctuating pattern and a general increasing trend with depth, ranging in value from 23.43 to 81.85 with an average of 45.41 (n = 250), Fig. 2.
Statistical analysis
The principal component analysis (PCA) revealed that the first two axes together explained 66.2% and 62.5% of the total variation in peat chemical properties (Fig. 3 A and B). The PCA of microcharcoal abundances and peat-chemical properties shows similar fire severity variations, with high fire severity clustering with microcharcoal abundance along PC1 42.4% and 43.1% for peat samples only and included sediments, respectively. Trend shows that non-fire and low fire severity peat layers clustering within the TN and δ15Ntot. These patterns suggest two mechanisms to explain the trend within fire severity and peat chemical properties: (1) higher intensity of fire resulting in loss of nitrogen and depleted δ15Ntot composition, and (2) there is a nutrient and/or microbial activity enhancement during the non-fire periods, whereas the non-fire peat layers showing greater TN and enriched δ15Ntot composition. In addition, a combination of both mechanisms may have a role creating this fire severity trend in peat chemistry.
PC2 only represents 23.8% and 19.4% of the variation in peat chemical properties, showing fire characterized by the greater TCorg content. Moreover, it shows a clear cluster of sediment layer in which mainly composed by clay with TCorg content < 45% w.t. A significant correlation between fire severity and TCorg, however, does not show a clear correlation between its isotope composition. This suggests that the δ15Corg compositions may not contribute fundamentally to the fire severity and its variations is more responsible to vegetation source variations. Based on paired two-tailed t tests of the peat layers, TCorg (t(238) = −4.65, p = < 0.001), TN (t(238) = −3.55, p= < 0.001) and δ15Ntot (t(238) = −4.68, p = < 0.001) within Bias Factor (loge(BF01)) lower than −3.40, indicating a significant correlation with the presence of microcharcoal content. While δ13Corg (t(238) = 1.79, p = 0.07) with Bias Factor (loge(BF01) = 0.72), shows a negligible correlation with the presence of microcharcoal.
Principal Component Analysis (PCA) based on peat chemical properties and color-coded by the fire severity. Vectors and score plots indicate the direction of the increasing gradient of each variable, while arrow lengths are proportional to the strength of the correlation with the PCA. (A) Only peat samples performed in analysis; (B) sediments samples are included in analysis.
Discussion
Assessing the combined ability of the proxies to identify fire severity
The advantages of analyzing C/N ratios, δ13Corg and δ15Ntot isotopes in parallel is the combined understanding of the geochemical and biological changes occurring during fire periods. The extent of these changes is generally described as fire severity, a metric for quantifying fire effects on ecosystems. High fire severity broadly defined by the high microcharcoal distribution and accumulation rate33-35, depletion of carbon8,20–24, and nitrogen isotopes26,27,42,43. Compared to the microcharcoal data, isotope composition of organic matter in peat shows a synchronous pattern. The higher abundances of microcharcoal show an isotope composition of depleted carbon and nitrogen. At the bottommost part, fire event #1 is characterized by more positive δ13Corg and the tipping-point of the δ15Ntot. The peak in fire event #1 of enriched δ13Corg and δ15Ntot is attributed to water-table fluctuation during early peat deposition, which could promote nutrient availability to the ecosystems through its fertilizing effect. The C/N ratio shows a gradual increase from <20 to 50, indicating vegetational changes from aquatic to C3 plants. The enriched δ13Corg of up to −27.20 and more depleted δ15Ntot up to 0.42 indicates that the organic matter could be derived from C4 plants or freshwater macroalgae, while the interaction between fire severity and sufficient water-table depth also can be a driving factor for fungi to grow in humid-wet peat conditions. This is evidenced by the presence of mixed-riparian forest pollen (e.g., Pandanus, Celtis and Antidesma) gradually replaced by open vegetation communities such as Piperaceae, Poaceae, and Cyperaceae13. This stage is recognized as the initiation stage of peat-forming, where flooding is dominant and accelerates organic matter alteration by microbial/fungal activity as indicated by high funginite content13.
High fire severity was observed at fire periods #2, #3 and #4, those periods identified by the distinct depletion of δ13Corg and δ15Ntot. High fire severity leads to alteration of organic matter to pyrogenic organic matter, an incomplete combustion product of terrestrial biomass produced by fire8. The majority of pyrolysis experiments converting organic matter to pyrogenic organic matter suggests that the depleted carbon isotope fractionation is on the order of 1–2‰8,35. The peat deposit in the Kapuas wetland, showing a pattern of depletion of δ13Corg with the presence of fire and higher concentration of C, indicates alteration of organic matter to pyrogenic organic matter. Moreover, the formation of pyrogenic organic matter may generally reflect paleovegetation change in correspondence to an increase in the relative abundance of C4 plants or shrubs-dominated vegetation36,37,38,39. During fire periods, N held in vegetation, litter, and soil can be volatilized, reducing total ecosystem N content resulting in N-poor environments40,41. Heavy loss of N by high fire severity and the following increase in N by atmospheric fixation through plant regrowth subsequently results in lower δ15Ntot26,27. Such a trend was observed in Kapuas wetlands; the depletion of δ15Ntot coincides with microcharcoal abundances, indicating that high fire severity plays an important role in the heavy loss of N. The ecosystem shifted into nitrogen- and nutrient-poor conditions, and regrowing vegetation was dominated by N-fixing species, reflected by the relative increase of C4 plants or more open vegetation communities following fire periods. These periods were demarcated by layers with the highest percentages of grass, fern and shrub pollen, which typically indicate forest openings or canopy gaps13. Moreover, the proportion of oxidized organic matter was greater than that of degraded organic matter during these periods, as indicated by the high amounts of inertinite macerals13.
Though periods #5, #6, #7 and #8 were identified by the presence of microcharcoal, the severity is lower than previous periods. The low-moderate fire severity is characterized by the insignificant changes of pyrogenic organic matter depicted with insignificant changes of δ13Corg. However, the δ15Ntot consistently shows a lower peak at these periods, although it shows more positive values compared to high severity periods. This suggests that the variation of isotope compositions in these periods is mainly controlled by the decomposition and alteration of organic matter rather than fire severity. With respect to isotope composition, it is reported that degraded organic matter was dominant during these periods and mangrove-associated taxa development was driven by tidal fluctuation in coastal intertidal zones13. Furthermore, fungal remains and/or multicellular sclerotia is relatively higher compared to high severity fire periods13, indicating the decomposition of peat in aerobic conditions which promotes the utilisation of 14N isotopes by micro-organisms while leaving behind 15N.
Differences of carbon and nitrogen fractionation pathways
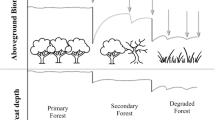
Based on the above discussion, fire severity, vegetation changes and water table conditions are the dominant factors for the carbon and nitrogen isotopes composition in peat deposits in the Kapuas Wetlands. These conditions commonly have different geochemical and isotope compositions, which later affect nutrient content and organic matter composition in peat, reflecting different growing conditions. As a result, we postulate there are two models of disturbed conditions by high fire severity and undisturbed condition during low or no fire periods that can be used to explain the processes responsible for the variability of δ13Corg and δ15Ntot in peat deposits from the Kapuas Wetlands (Fig. 4). Through the utilization of various physical and chemical proxies, distinct outcomes regarding carbon and nitrogen fractionation pathways in response to fire severity may arise to accommodate the findings of higher δ15Ntot composition in Air Hitam and Sungai Buluh peatlands, Jambi42and lower δ15Ntot composition in Kalampangan peatlands, Kalimantan43.
During fire periods, higher severity results in heavy loss of nitrogen through volatilization and reduces total ecosystem nitrogen (Fig. 4 A). Thus, exacerbated by low water table conditions, the ecosystem becomes nitrogen-poor following the burning and death of remaining vegetation. The nitrogen-poor conditions and associated nutrient depletion allows regrowing vegetation to be accommodated by N-fixing species drawing N from the atmosphere. The regrowing vegetation is dominated by a relatively higher population of C4 plants e.g., shrubs, macroalgae and open vegetation communities. These processes result in a low δ15Ntot signal in peat deposits. In addition, fire severity leads to the greater alteration of organic matter to pyrogenic organic matter, which subsequently leads to a lower δ13Corg signal. High severity fire may cause surface and crown/canopy fires which are recognized as having temperatures ranging from >500°C to >1000°C44–47. The thermal degradation of cellulose at high temperature is extensive, and thermal reactions involving lignin components would have given rise to the 6-aromatic carbon rings48, resulting in δ13Corg depletion and formation of inertinite and microcharcoal abundances20,21,23,49. Furthermore, high fire severity which involves the forest canopy, are often ignited by surface fires and can lead to significant tree mortality and vegetation changes50,51, thus converting the vegetation coverage into open vegetation taxa such as grasses, herbs, ferns, and pioneer vegetation13,42,52,53.
Outside of fire periods, residual ash from past fires transported to the study site, as well as in-situ deposition, both play an important role in the enrichment of nitrogen in peat deposits through mineralization and nitrification processes54,55(Fig. 4B). Low to moderate severity fires often promote soil nutrient availability and allows for the growth of larger and more luxuriant plants56. A high-water table could possibly enhance nutrient enrichment through leaching of ash and/or allowing N2 fixation by microbial and bacterial growth. This could also lead to a high decomposition rate of organic matter during peat accumulation and significant enrichment of TN and δ15Ntot in organic matter57,58. On the other hand, during low severity or undisturbed conditions, fire may have a more selective effect on plants by fracturing the three-dimensional structure of the aliphatic domain of suberin18. This thermal cracking may produce black charcoal yet still retain a noticeable amount of lignin-like compounds, resulting in higher δ13Corg values59,60. With respect to low severity and temperature of fire, less significant changes on the vegetation community would likely have led to the stabilization of a proportion of microbial processed organic matter13. During these more stable conditions, the organic matter is altered/humified by microbial-processes and/or methanogenic bacteria18,57. These combined processes during low-severity or undisturbed conditions might lead to variation of δ13Corg values.
Proposed conceptual models to describe the dynamics of carbon and nitrogen fractionation pathways in response to fire severity. (A) The carbon and nitrogen fractionation pathways during high fire severity of peat deposits results in lower δ13Corg and δ15Ntot isotope values. (B) The undisturbed condition of peatlands results in higher δ15Ntot value and variable δ13Corg value.
This study shows that using single proxy of isotope could lead multiple interpretation, specifically in defining fire occurrence and severity. In response to fire, carbon isotope (δ13Corg) composition could be varied due to difference of thermostable compounds and vegetation type of organic matter. Fire may be producing microcharcoal with insignificant change of isotope composition or even higher δ13Corg. Moreover, this study clearly shows that distinct depletion of δ15Ntot indicate the heavy loss of nitrogen through combustion process. While, during undisturbed conditions, depletion of δ13Corg and enriched δ15Ntot consistently showing the degradation processes by microbial activity plays an important role on the isotope signal in peat deposits. Using δ13Corg and δ15Ntot as a single proxy solely should be taking with a careful consideration; therefore, coupling of these isotopes provides strong evidence of the fire severity, and by combining biological proxy could give a better understanding and robust interpretations on peat ecological changes.
Notwithstanding the lack of age-constrained peat deposits documented in this study, a set of radiocarbon dates and microcharcoal abundances showed that in lowland areas of West Kalimantan, fire occurred throughout the last 3200 years, peaking at 1300–1600 CE61. This period of high intensity fire coincides with regional droughts induced by ENSO intensification and regional demographic changes. Evidence of fire in lowland areas suggests that the main driving factors are anthropogenic, for example, the practice of swidden agriculture in which forests are converted to fire-prone fern, shrub, and grass61. Fire records from the previous study in nearby areas mentioned above shows a consistent result with our results, indicating that fires become major ecological disturbances leading to ecological changes converting forests to open vegetation communities47,61. However, the origin of fire in this studied area remains unclear.
Limitations of this study
Though this study demonstrates the significance of isotope signals for identifying fire severity on tropical peat deposits; since there is no standardization on categorizing the level or magnitude of fire events, there may be potential discrepancies when similarly evaluated at other sites. Latitudinal constraint drives different of climatic condition, vegetation type and coverage, mean atmospheric temperature, peat recalcitrant which could lead various isotope signals. At high latitude, where the region is relatively low temperature and seasonally frozen, depleted δ15N is related to the low N content peat conditions62,63, rather than the presence of fires. High latitude peatlands mostly deposited under anaerobic conditions62, which lead vegetations absorb N nutrients from the atmosphere instead of peat substrate due to the preferential absorption by plants for lighter 14N, which results in low δ15N (< 3.0‰) in plant foliage and leaf litter64. Moreover, different vegetation coverage and fire type could lead different combustion efficiency in releasing reactive nitrogen to atmosphere65. Needle leaf forests extensively covered the boreal region and the smoldering fire occurs are highly efficient in releasing NH3 rather than NOx65, therefore it should be taking account of a possible different signals of δ13C and δ15N in boreal fires, compared to the tropical area.
In addition, fire may have selective effect on thermal cracking of lipid moieties and may retaining a thermostable organic matter such as lignin or known as combustion effect/efficiency, resulting in higher δ13C59,60. Isotope signals revealed by this study is reflected by the bulk samples, therefore specific plant particles such as cork, leaves, stem which composed of different lignin compositions might responsible for the difference of carbon isotope compositions and proportions. However, the combustion efficiency and fuel type in this study is not defined as the factor of the fire severity.
In future research, it is imperative to enhance the evaluation of fire origin and its response to ecological changes. For instance, this study should be tested in different geological or geographical settings to assess the relationship between combustion efficiency and ecological communities to the isotope signals. Emphasis should be placed on validating the origin and duration of fires by age-constraints to distinguish between local or regional drivers.
Conclusion
Our study investigates the potential of carbon (δ13Corg) and nitrogen (δ15Ntot) isotopes as recorders of fire severity and ecological changes in peat deposits. We have examined the δ13Corg and δ15Ntotin bulk peat deposit in Kapuas Wetlands. High severity fires recorded in peat deposit show depleted δ15Ntot values and varies values of δ13Corg. The heavy loss of N through combustion results in lower δ15Ntot, and the alteration of organic matter to pyrogenic organic matter due to fire leads to δ13Corg depletion. In addition, high fire severity plays an important role in vegetational changes, allowing the regrowth of N-fixing species, which draw N from the atmosphere, to become relatively more populated by open vegetation communities. On the other hand, in prevailing fire-free conditions, residual ash from past fires and the depth of the water table can be factors for enrichment of nitrogen. The nutrient-rich conditions allow N2-fixation by microbial and bacterial growth, resulting in higher δ15Ntot values. In addition, during the nutrient-rich or undisturbed conditions, organic matter led to altered/humified by microbial-derived soil organic matter and/or methanogenic bacteria and allows more luxuriant plant growth and more variable δ13Corg during peat accumulation. From these findings, the difference in carbon and nitrogen pathways in response to fire severity and their effects on ecological changes were identified. We postulate that these pathways are applicable to most peat deposits in equatorial latitudes around the world.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Frokling, S. & Roulet, N. T. Holocene radiative forcing impact of Northern peatland carbon accumulation and methane emissions. Glob Change Biol. 13 – 5. 1079–1088. https://doi.org/10.1111/j.1365-2486.2007.01339.x (2007).
Kleinen, T., Brovkin, V., von Bloh, W., Archer, D. & Munhoven, G. Holocene carbon cycle dynamics. Geophys. Res. Lett. 37, L02705. https://doi.org/10.1029/2009GL041391 (2010).
Bento-Gonçalves, A., Vieira, A., Úbeda, X. & Martin, D. Fire and soils: key concepts and recent advances. Geoderma 191, 3–13. https://doi.org/10.1016/j.geoderma.2012.01.004 (2012).
Page, S. E. et al. The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420, 61–65. https://doi.org/10.1038/nature01141.1 (2002).
van der Werf, G. R. et al. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009). Atmos. Chem. Phys. 10, 11707–11735. https://doi.org/10.5194/acp-10-11707-2010 (2010).
Mahowald, N. M. et al. Atmospheric global dust cycle and iron inputs to the ocean. Global Biogeochem. Cycles. 19, GB4025. https://doi.org/10.1029/2004GB002402 (2005).
Braadbaart, F. & Poole, I. Morphological, chemical and physical changes during charcoalification of wood and its relevance to archaeological contexts. J. Archaeol. Sci. 35, 2434–2445. https://doi.org/10.1016/j.jas.2008.03.016 (2008).
Bird, M. I. & Ascough, P. L. Isotopes in pyrogenic carbon: A review. Org. Geochem. 42 (12), 1529–1539. https://doi.org/10.1016/j.orggeochem.2010.09.005 (2012).
Etiegni, L. & Campbell, A. G. Physical and chemical characteristics of wood Ash. Bioresour Technol. 37 (2), 173–178. https://doi.org/10.1016/0960-8524(91)90207-Z (1991).
Ponette-González, A. G. et al. Biomass burning drives atmospheric nutrient redistribution within forested peatlands in Borneo. Environ. Res. Lett. 11, 085003. https://doi.org/10.1088/1748–9326/11/8/085003 (2016).
Tata, H. L. & Narendra, B. H. Mawazin. Forest and land fires in Pelalawan district, Riau, Indonesia: drivers, pressures, impacts and responses. Biodiversitas 19 (2), 544–551. https://doi.org/10.13057/biodiv/d190224 (2018).
Wasis, B., Saharjo, B. H. & Putra, E. I. Impacts of peat fire on soil flora and fauna, soil properties and environmental damage in Riau Province, Indonesia. Biodiversitas 20 (6), 1770–1775. https://doi.org/10.13057/biodiv/d200639 (2019).
Patria, A. A., Obrochta, S. P. & Anggara, F. Tracing highly oxidized events and its response to peat dynamic from the Northwest Kapuas coastal wetlands, Indonesia. Int. J. Coal Geol. 303, 104751. https://doi.org/10.1016/j.coal.2025.104751 (2025).
Reis, F. B. R. et al. Nodulation and nitrogen fixation by Mimosa spp. In the Cerrado and Caatinga biomes of Brazil. New. Phytol. 186, 934–946. https://doi.org/10.1111/j.1469-8137.2010.03267.x (2010).
Lamb, A. L., Wilson, G. P. & Leng, M. J. A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Sci. Rev. 75, 29–57. https://doi.org/10.1016/j.earscirev.2005.10.003 (2006).
Buso Junior, A. A. From an estuary to a freshwater lake: a paleo-estuary evolution in the context of holocene sea-level fluctuations, southeastern Brazil. Radiocarbon 55 (2–3), 1735–1746. https://doi.org/10.1017/S0033822200048657 (2013).
Silva Neto, E. C. et al. Palaeoenvironmental records of histosol pedogenesis in upland area, Espírito Santo state (SE, Brazil). J. South. Am. Earth Sci. 95, 102301. https://doi.org/10.1016/j.jsames.2019.102301 (2019).
Jiménez-Morillo, N. T. et al. Fire effects on C and H isotopic composition in plant biomass and soil: bulk and particle size fractions. Sci. Total Environ. 749, 141417. https://doi.org/10.1016/j.scitotenv.2020.141417 (2020).
Sachse, D. et al. Molecular paleohydrology: interpreting the hydrogen-isotopic composition of lipid biomarkers from photosynthesizing organisms. Annu. Rev. Earth Planet. Sci. 40, 221–249. https://doi.org/10.1146/annurev-earth-042711-105535 (2012).
Turekian, V. C. et al. Causes of bulk carbon and nitrogen isotopic fractionations in the products of vegetation Burns: laboratory studies. Chem. Geol. 152, 181–192. https://doi.org/10.1016/S0009-2541(98)00105-3 (1998).
Czimczik, C. I. et al. Effects of charring on mass, organic carbon, and stable carbon isotope composition of wood. Org. Geochem. 33, 1207–1223. https://doi.org/10.1016/S0146-6380(02)00137-7 (2002).
Hall, G., Woodborne, S. & Scholes, M. Stable carbon isotope ratios from archaeological charcoal as palaeoenvironmental indicators. Chem. Geol. 247, 384–400. https://doi.org/10.1016/j.chemgeo.2007.11.001 (2008).
Bird, M. I. & Ascough, P. L. Isotopes in pyrogenic carbon: a review. Org. Geochem. 42, 1529–1539. https://doi.org/10.1016/j.orggeochem.2010.09.005 (2012).
González-Pérez, J. A. et al. Compound-specific stable carbon isotopic signature of carbohydrate pyrolysis products from C3 and C4 plants. J. Sci. Food Agric. 96 (3), 948–953. https://doi.org/10.1002/jsfa.7169 (2015).
Schleser, G. H. Investigations of the δ13C pattern in leaves of Fagus sylvatica L. J. Exp. Bot. 41, 565–572. https://doi.org/10.1093/jxb/41.5.565 (1990).
Davidson, E. A. et al. Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature 447, 995–998. https://doi.org/10.1038/nature05900 (2007).
Dunnette, P. V. et al. Biogeochemical impacts of wildfires over four millennia in a Rocky mountain subalpine watershed. New. Phytol. 203 (3), 900–912. https://doi.org/10.1111/nph.12828 (2014).
Fiorentino, G. et al. Stable isotopes in archaeobotanical research. Veg. Hist. Archaeobot. 24, 215–227. https://doi.org/10.1007/s00334-014-0492-9 (2015).
Muller, J. et al. The use of principle component analyses in characterising trace and major elemental distribution in a 55kyr peat deposit in tropical Australia: implications to paleoclimate. Geochim. Cosmochim. Acta. 72, 449–463. https://doi.org/10.1016/j.gca.2007.09.028 (2008).
Turner, M. G. et al. Inorganic nitrogen availability after severe stand-replacing fire in the Greater Yellowstone ecosystem. Proc. Natl. Acad. Sci. USA. 104, 4782–4789. (2007). https://doi.org/10.1073/pnas.0700180104
Wüst, R. A. J., Bustin, R. M. & Lavkulich, L. M. New classification systems for tropical organic-rich deposits based on studies of the Tasek Bera basin, Malaysia. Catena 53, 133–163. https://doi.org/10.1016/S0341-8162(03)00022-5 (2003).
Esterle, J. S. Trends in Petrographic and Chemical Characteristics of Tropical Domed Peats in Indonesia and Malaysia as Analogues for Coal Formation (Doctorate dissertation). University of Kentucky, Lexington, Kentucky (1990).
Stevenson, J. & Haberle, S. Macro Charcoal Analysis: A Modified Technique Used by the Department of Archaeology and Natural History (Australian National University, 2005).
Yamamoto, M. et al. Tropical Western Pacific hydrology during the last 6,000 years based on wildfire charcoal records from Borneo. Geophys. Res. Lett. 48 https://doi.org/10.1029/2021GL093832 (2021). e2021GL093832.
Wang, X., Cui, L., Xiao, J. & Ding, Z. Stable carbon isotope of black carbon in lake sediments as an indicator of terrestrial environmental changes: an evaluation on paleorecord from Daihai lake, inner Mongolia, China. Chem. Geol. 347, 123–134. https://doi.org/10.1016/j.chemgeo.2013.03.009 (2013).
Bird, M. I. & Gröcke, D. R. Determination of the abundance and carbon isotope composition of elemental carbon in sediments. Geochim. Cosmochim. Acta. 61, 3413–3423. https://doi.org/10.1016/S0016-7037(97)00157-9 (1997).
Clark, J. S., Grimm, E. C., Lynch, J. & Mueller, P. G. Effects of holocene climate change on the C4 grassland/woodland boundary in the Northern plains, USA. Ecology 82, 620–636. https://doi.org/10.1890/0012-9658(2001)082 (2001). [0620:EOHCCO]2.0.CO;2.
Jia, G., Peng, P. A., Zhao, Q. & Jian, Z. Changes in terrestrial ecosystem since 30 ma in East Asia: stable isotope evidence from black carbon in the South China sea. Geology 31, 1093–1096. https://doi.org/10.1130/G19992.1 (2003).
Zhang, E. et al. Linkages between climate, fire and vegetation in Southwest China during the last 18.5 ka based on a sedimentary record of black carbon and its isotopic composition. Palaeogeogr Palaeoclimatol Palaeoecol. 435, 86–94. https://doi.org/10.1016/j.palaeo.2015.06.004 (2015).
Johnson, D. & Turner, J. Nitrogen budgets of forest ecosystems: a review. Ecol. Manag. 318, 370–379. https://doi.org/10.1016/j.foreco.2013.08.028 (2014).
Lindaas, J. et al. Emissions of reactive nitrogen from Western U.S. Wildfires during summer 2018. J. Geophys. Res: Atmos. 125 https://doi.org/10.1029/2020JD032657 (2021). e2020JD032657.
Hapsari, K. A. et al. Late holocene ENSO-related fire impact on vegetation, nutrient status and carbon accumulation of peatlands in Jambi, Sumatra, Indonesia. Rev. Palaeobot Palynol. 293, 104482. https://doi.org/10.1016/j.revpalbo.2021.104482 (2021).
Yulianto, E., Hirakawa, K. & Tsuji, H. Charcoal and organic geochemical properties as evidence of holocene fires in tropical peatland, central Kalimantan, Indonesia. Tropics 14, 55–63. https://doi.org/10.3759/tropics.14.55 (2004).
Hayasaka, H. & Putra, E. I. Reassessment of peatland fires in central Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 8, 959. https://doi.org/10.1088/1755-1315/959/1/012053 (2021).
Marcelli, T. et al. Fire spread across pine needle fuel beds: characterization of temperature and velocity distribution within the fire plume. Int. J. Wildland Fire. 13 (1), 37–48. https://doi.org/10.1071/WF02065 (2004).
Guo, H. et al. Transition from surface fire to crown fire and effects of crown height, moisture content and tree flower. Fire Technol. 60, 1403–1419. https://doi.org/10.1007/s10694-022-01262-x (2022).
Maezumi, S. Y. et al. A modern analogue matching approach to characterize fire temperatures and plant species from charcoal. Palaeogeogr Palaeoclimatol Palaeoecol. 578, 110580. https://doi.org/10.1016/j.palaeo.2021.110580 (2021).
Spiker, E. G. & Hatcher, P. G. The effects of early diagenesis on the chemical and stable carbon isotopic composition of wood. Geochim. Cosmochim. Acta. 51, 1385–1391. https://doi.org/10.1016/0016-7037(87)90323-1 (1987).
Guo, Y. & Bustin, R. M. FTIR spectroscopy and reflectance of modern charcoals and fungal decayed Woods: implications for studies of inertinite in coals. Int. J. Coal Geol. 37, 29–53. https://doi.org/10.1016/S0166-5162(98)00019-6 (1998).
Thompson, J. R. & Spies, T. A. Vegetation and weather explain variation in crown damage within a large mixed-severity wildfire. Ecol. Manag. 258, 1684–1694. https://doi.org/10.1016/j.foreco.2009.07.031 (2009).
Pérez-Izquierdo, L. et al. Crown-fire severity is more important than ground-fire severity in determining soil fungal community development in the boreal forest. J. Ecol. 109, 504–518. https://doi.org/10.1111/1365-2745.13529 (2021).
Biagioni, S. Unravelling the past 1,000 years of history of human–climate– landscape interactions at the Lindu plain, Sulawesi, Indonesia. Veget Hist. Archaeobot. 25, 1–17. https://doi.org/10.1007/s00334-015-0523-1 (2016).
Hapsari, K. A. et al. Environmental dynamics and carbon accumulation rate of a tropical peatland in central Sumatra, Indonesia. Quat Sci. Rev. 169, 173–187. https://doi.org/10.1016/j.quascirev.2017.05.026 (2017).
Ponette-González, A. G., Curran, L. M. & Pittman, A. M. Biomass burning drives atmospheric nutrient redistribution within forested peatlands in Borneo. Environ. Res. Lett. 11, 085003. https://doi.org/10.1088/1748-9326/11/8/085003 (2016).
Tata, H. L. et al. Domestication of Dyera polyphylla (miq.) steenis in peatland agroforestry systems in Jambi, Indonesia. Agroforest Syst. 90, 617–630. https://doi.org/10.1007/s10457-015-9837-3 (2016).
Certini, G. Effects of fire on properties of forest soils: a review. Oecologia 143, 1–10. https://doi.org/10.1007/s00442-004-1788-8 (2005).
Anderson, R. A. et al. Elemental and isotopic carbon and nitrogen records of organic matter accumulation in a holocene permafrost peat sequence in the East European Russian Arctic. J. Quat Sci. 27, 545–552. https://doi.org/10.1002/jqs.2541 (2012).
Krüger, J. P. et al. Biogeochemical indicators of peatland degradation – a case study of a temperate bog in Northern Germany. Biogeosciences 12, 2861–2871. https://doi.org/10.5194/bg-12-2861-2015 (2015).
De la Rosa, J. M. et al. Direct detection of black carbon in soils by Py-GC/MS, Carbon-13 NMR spectroscopy and thermogravimetric techniques. Soil. Sci. Soc. Am. J. 72, 258–267. https://doi.org/10.2136/sssaj2007.0031 (2008).
Jiménez-Morillo, N. T. et al. Fire effects in the molecular structure of soil organic matter fractions under Quercus suber cover. Catena 145, 266–273. https://doi.org/10.1016/j.catena.2016.06.022 (2016).
Hendricks, L. B., Anshari, G. Z. & Gavin, D. G. Fire in the rainforest: a 3200-year history of fire in a West Kalimantan, tropical rainforest. Ecosphere 15, e4918. https://doi.org/10.1002/ecs2.4918 (2024).
Hodgkins, S. B. et al. Tropical peatland carbon storage linked to global latitudinal trends in peat recalcitrance. Nat. Commun. 9, 3640. https://doi.org/10.1038/s41467-018-06050-2 (2018).
Damman, A. W. H. Regulation of nitrogen removal and retention in Sphagnum bogs and other peatlands. Oikos 51, 291–305. https://doi.org/10.2307/3565310 (1988).
Osaki, M. et al. . Tropical peat and peatland definition in Indonesia. In Tropical Peatland Ecosystem (eds Osaki, M. et al.) 137–147 (Springer, 2016). https://doi.org/10.1007/978-4-431-55681-7.
Chen, Q. et al. Unexpected high ammonia emissions from boreal fires in 2021 and 2023. Gephys Res. Lett. 52 e2024GL112396 (2025).
Acknowledgements
The authors acknowledge the financial support received from the Ministry of Education, Sports, Science and Technology of Japan (MEXT) scholarship. The authors also express their gratitude to A. Fahrialam, M. N. A. A. Danny, A. A. Annifari, for their generous support during field investigation and sample collection. The authors extend their thankful to Y. Yokoyama and Y. Miyairi (Atmosphere and Ocean Research Institute, The University of Tokyo) for their help during sample preparation and analysis.
Author information
Authors and Affiliations
Contributions
(A) A. P: conceptualization, formal analysis, methodology, visualization, writing – original draft. S. P. O: resources, supervision, writing – review & editing. T. M: formal analysis, methodology, writing – review. (B) C. B: writing – review & editing. F. A: resources, writing – review & editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Patria, A.A., Obrochta, S.P., Miyajima, T. et al. Identification of fire severity in peat deposits using carbon and nitrogen isotopes. Sci Rep 15, 17291 (2025). https://doi.org/10.1038/s41598-025-01123-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01123-x