Abstract
Sarcopenia, characterized by the progressive loss of skeletal muscle mass and strength, significantly impacts the people, leading to increased frailty and mortality. The atherogenic index of plasma (AIP), a biomarker for lipid imbalance, may be linked to sarcopenia due to shared pathways of inflammation and metabolic dysregulation. Data from the National Health and Nutrition Examination Survey (NHANES) 2011–2018 cycles were analyzed. The AIP was calculated as the logarithm of the ratio of triglycerides to High density lipoprotein cholesterol. Sarcopenia was defined using the appendicular skeletal muscle mass index (ASMBMI) adjusted for body mass index (BMI). Multivariable linear regression and logistic regression models were employed to assess the association between AIP and ASMBMI, as well as sarcopenia. Restrictive cubic spline curves were utilized to analyze potential nonlinear associations between AIP and outcome indicators. Additionally, subgroup analyses and intergroup interaction tests were performed. Elevated AIP levels were associated with decreased ASMBMI and an increased risk of sarcopenia. After adjusting for confounding factors, the association between AIP and ASMBMI remained significant (Beta [95% CI] = -0.02 [-0.03, -0.01], P < 0.001). AIP was significantly associated with sarcopenia (OR [95% CI] = 2.6 [1.78, 3.81], P = < 0.001). AIP is significantly associated with reduced muscle mass and potentially with sarcopenia, suggesting that lipid metabolism plays a critical role in muscle health. Identifying AIP as a modifiable risk factor could have important public health implications for managing sarcopenia.
Similar content being viewed by others
Introduction
Sarcopenia is a progressive loss of skeletal muscle mass and strength that significantly impacts the US population. This condition leads to increased frailty, disability, and mortality, posing a major public health challenge1,2. According to the SPRINT study of the US population, there were 48.6 cases per 1000 people per year, with a Hazard ratio of 1.703. Sarcopenia not only contributes to physical decline but also increases the risk of falls, fractures, and hospitalization4. While numerous factors such as aging, physical inactivity, and poor nutrition are well-established contributors to sarcopenia, emerging evidence suggests that metabolic and cardiovascular factors also play crucial roles5,6. Systemic inflammation, oxidative stress, and hormonal changes are known to exacerbate muscle degradation and functional decline7. Recent epidemiological studies indicate that metabolic syndrome, characterized by a cluster of conditions including dyslipidemia, affects about 30% population of the United States and is linked to increased risk of sarcopenia8,9.
The atherogenic index of plasma (AIP) is a novel biomarker calculated as the logarithm of the ratio of triglycerides to high-density lipoprotein cholesterol10,11. In large-scale epidemiological studies, AIP has been shown to correlate with increased risk of cardiovascular events and is used to assess lipid abnormalities12,13. Elevated AIP levels, indicating a higher atherogenic potential, are prevalent in populations with metabolic disorders, affecting approximately 30% of adults. Elevated AIP levels indicate dyslipidemia, a disorder characterized by abnormal lipid metabolism14. Dyslipidemia is associated with systemic inflammation, oxidative stress, and insulin resistance15. This suggests that AIP may play a role in the onset and progression of sarcopenia by disrupting normal muscle metabolism and promoting catabolic processes.
Considering the shared pathways of inflammation, oxidative stress, and metabolic dysregulation, it is plausible that higher AIP levels could be linked to an increased risk of sarcopenia16. Epidemiological studies have shown that conditions linked to high AIP, such as metabolic syndrome, are associated with increased prevalence of sarcopenia8,17,18. It has been reported that individuals with metabolic syndrome are at higher risk of developing sarcopenia compared to those without metabolic syndrome19. Furthermore, studies have demonstrated that metabolic syndrome, characterized by a cluster of conditions including dyslipidemia, hypertension, insulin resistance, and abdominal obesity, is closely linked to inflammation and oxidative stress, both of which are critical factors in the pathogenesis of sarcopenia20,21. These pathways are known to contribute to both cardiovascular diseases and muscle degeneration, suggesting a potential intersection where AIP could influence muscle health22. However, most previous studies have focused on broader metabolic markers or individual lipid components, without considering the complex markers provided by AIP.
The primary objective of this study is to investigate the association between AIP and sarcopenia in a cross-sectional study, using data from NHANES. Using population-based samples to enhance the generality of the findings. This will help elucidate whether AIP, as a marker of lipid imbalance, is a significant predictor of sarcopenia. Understanding this relationship could provide insights into the role of lipid metabolism in muscle health. Given the increasing aging population, identifying modifiable risk factors such as AIP could have significant implications for public health, potentially alleviating the burden of sarcopenia on healthcare systems worldwide.
Method
Data sources
The data for this study were acquired from the website of the US National Health and Nutrition Examination Survey (NHANES) (https://www.cdc.gov/nchs/nhanes/index.htm). NHANES is a comprehensive, nationally representative study of the US population, employing a multistage, stratified approach to provide detailed information on study design, interviews, demographics, and more. The study protocol was approved by the Ethical Review Board of the National Center for Health Statistics, and participants provided informed consent. All methods in this study were performed in accordance with Continuation of Protocol #2011–17 and did not involve limited access data.
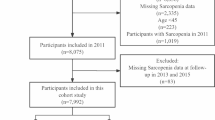
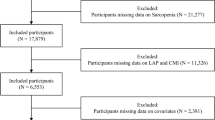
In this study, we analyzed NHANES data collected between 2011 and 2018, excluding participants who did not have triglycerides, high-density lipoprotein (HDL), dual-energy X-ray absorptiometry (DXA) data, Body Mass Index (BMI) and other covariates. A final total of 3,397 patients were included (Fig. 1).
Assessment of atherogenic index of plasma
AIP, as a composite index, can be directly calculated from clinical data. AIP was defined as log10 (triglycerides/HDL cholesterol) with triglycerides and HDL cholesterol expressed in mmol/L10. The AIP levels were categorized into quartiles (Q1, Q2, Q3, Q4) based on interquartile range.
Assessment of sarcopenia
Sarcopenia was defined based on the guidelines established by the Foundation for the National Institutes of Health (FNIH) and was characterized by skeletal muscle mass adjusted by body mass index (ASMBMI, ASMBMI = ALM/BMI) < 0.512 for females and < 0.789 for males23,24. NHANES used dual-energy X-ray absorptiometry (DXA) to measure the sum of four limbs’ muscle mass (appendicular lean mass, ALM). Participants with a height > 192.5 cm, weight > 136.4 kg, and pregnant individuals were excluded since these participants could not perform the DXA test.
Covariates
Drawing from existing literature25,26, we integrated clinically relevant covariates into our study. In addition to references, we mainly included variables related to body metabolism and lipids, which was more in line with our study. Covariates in this study included demographic information: age, sex, race, the ratio of family income to poverty, laboratory data: body mass index (BMI), total cholesterol (TC), systolic blood pressure (SBP), diastolic blood pressure (DBP), diet and lifestyle: alcohol, smoke, comorbidities: hypertension, hypercholesteremia, diabetes, arthritis, congestive heart failure, coronary heart disease, angina pectoris, stroke, emphysema, cancer. BMI was calculated by dividing the body weight (kg) by the square of height (m2). Comorbidities (hypertension, hypercholesteremia, diabetes, arthritis, congestive heart failure, coronary heart disease, angina pectoris, stroke, emphysema, cancer) were identified based on participants’ self-reports. Smoking history and alcohol consumption were measured by the question ‘Have you smoked at least 100 cigarettes in your life?’ And ‘Have you consumed at least 12 alcoholic beverages of any type in any given year?/In your entire life have you had at least 1 drink of any kind of alcohol, not counting small tastes or sips?’ (yes/no) to classify as active and non-active.
Statistical analysis
The categorical variables were described using frequencies and proportions, and group differences were assessed using either the χ2 test or Fisher’s exact test. Continuous variables were presented as either the mean (SD, standard deviation) or median (IQR, interquartile range), depending on their distribution. Group differences were compared using either the student t-test or nonparametric tests. Weighted linear regression and weighted logistic regression models were constructed with continuous AIPs and AIP segmented according to quartiles (using the first quartile’s AIP as the reference group) as exposure variables, while ASMBMI and sarcopenia served as outcome variables respectively. These confounders were gradually incorporated into the model for adjustment: Model 1 was unadjusted, Model 2 was adjusted for age, gender, race, and family income-to-poverty ratio, and Model 3 was adjusted for age, gender, race, family income-to-poverty ratio, total cholesterol, systolic blood pressure, diastolic blood pressure, body mass index, hypertension, hypercholesterolemia, diabetes, arthritis, congestive heart failure, coronary heart disease, angina pectoris, stroke, emphysema, cancer, smoke and alcohol adjustments. Trend tests were performed. Multicollinearity in the multivariate model was evaluated by computing variance inflation factor (VIF) and removing variables with a VIF over 5. Restricted cubic spline modeling was utilized to identify potential nonlinear associations between AIP and sarcopenia risk. Subgroup analyses were conducted to assess result consistency among different subgroups. The findings are displayed in forest plots. The weight calculation method is derived from the US National Health and Nutrition Examination Survey website. This study’s specific weight was calculated by dividing the current year’s weight by 4 (2011–2018). Using R programming language, we use specific weights, Masked variance pseudo-PSU and Masked variance pseudo-stratum to carry out our complex sampling weighting. The survey software package considered sampling design complexity in all analyses. The studies used R programming language (version 4.3), with statistical significance set at P-value < 0.05.
Results
Population characteristics
A total of 3,397 participants eventually participated in this study. Of all participants, 1,622(47.7%) were female and 1,775(52.3%) were male. We describe baseline data for participants grouped according to their AIP level and whether they had sarcopenia (Tables 1, 2). In this cross-sectional study, a notable association was observed between elevated AIP levels and increased prevalence of sarcopenia, with the highest AIP quartile (Q4) indicating the highest risk. As AIP increase, there was a significant uptrend in diastolic blood pressure, obesity indices, diabetes mellitus prevalence, angina pectoris incidence, total cholesterol levels, systolic blood pressure, and body mass index (Table 1). Participants with diabetes, arthritis, congestive heart failure, coronary heart disease, stroke, and angina were more likely to have sarcopenia (Table 2).
Association of AIP with sarcopenia
Table 3 presents the results of the association analysis between the AIP and sarcopenia. This table utilizes three distinct statistical models to assess the impact of AIP on the risk of sarcopenia (Model 1 was unadjusted, Model 2 was adjusted for age, gender, race, and family income-to-poverty ratio. Model 3 was adjusted for age, gender, race, family income-to-poverty ratio, total cholesterol, systolic blood pressure, diastolic blood pressure, body mass index, hypertension, hypercholesterolemia, diabetes, arthritis, congestive heart failure, coronary heart disease, angina pectoris, stroke, emphysema, cancer, smoke, alcohol adjustments).
We performed weighted linear regression a nalyses with ASMBMI as the outcome. When AIP is included in the model as a continuous variable, results showed a significant increase in AIP in the unadjusted (Beta [95% CI] = 0.03 [0.01, 0.05], P-value = 0.006), partial adjusted (Beta [95% CI] = −0.07 [−0.08, −0.06], P-value < 0.001), and fully adjusted (Beta [95% CI] = −0.02 [−0.03, −0.01], P-value < 0.001) models, ASMBMI were significantly lower with higher AIP. When AIP is included in the model as a categorical variable, patients with higher AIP had significantly lower ASMBMI than patients with lower AIP (Q1) in unadjusted (Q4: Beta [95% CI] = 0.02 [0, 0.04], P-value = 0.039), partially adjusted (Q4: Beta [95% CI] = −0.07 [−0.08, −0.06], P-value < 0.001) and fully adjusted model (Q4: Beta [95% CI] = −0.02 [−0.03, −0.01], P-value < 0.001) (Table 3).
Subsequently, we performed weighted logistic regression analyses with sarcopenia as the outcome. When AIP is a continuous variable, the results showed that the risk of sarcopenia was significantly higher with higher AIP in the unadjusted (OR [95% CI] = 4.08 [2.91, 5.74], P-value < 0.001), partial adjusted (OR [95% CI] = 2.6 [1.78, 3.81], P-value < 0.001), and fully adjusted (OR [95% CI] = 1.05 [0.66, 1.66], P-value = 0.844) models, sarcopenia risk was significantly higher with elevated AIP. When AIP is a categorical variable, patients with higher AIP had significantly higher risk of sarcopenia than those with lower AIP (Q1) in unadjusted (Q4: OR [95% CI] = 3.39 [2.29, 5.03], P-value < 0.001), partial adjusted (Q4: OR [95% CI] = 2.04 [1.34, 3.11], P-value = 0.001) (Table 3).
Potential nonlinear relationship between AIP and sarcopenia
Panels A, B, and C show the results from the weighted linear regression analysis with ASMBMI as the outcome variable. In Model 1 (Panel A), there is a significant nonlinear relationship between AIP and ASMBMI (p-nonlinearity = 0.001). When AIP is in the range of −0.3225 to 0.1479(25%, 75%), the higher AIP level is, the lower ASMBMI is. Model 2 (Panel B), adjusted for additional confounding factors, confirmed a significant nonlinear association (p nonlinearity < 0.001) and showed a similar pattern, with a more pronounced decline in ASMBMI at moderate AIP levels. Model 3 (Panel C), including further adjustments, maintains a significant nonlinear relationship (p-nonlinearity < 0.001), indicating a robust association between AIP and ASMBMI. Panels D, E, and F show the results of logistic regression analysis with sarcopenia as a binary outcome variable. In Model 1 (Panel D), there was a significant nonlinear relationship between AIP and sarcopenia OR (p-nonlinearity = 0.01), and the higher AIP level was when AIP was in the range of −0.3225 to 0.1479(25%, 75%), the higher sarcopenia OR was. Model 2 (Panel E), adjusted for additional confounding factors, shows a non-significant nonlinear association (p-nonlinearity = 0.111), although the trend towards an increase in OR remains clear at higher AIP levels. Model 3 (Panel F), which includes further adjustments, also shows a non-significant nonlinear relationship (p-nonlinearity = 0.172), indicating that although the trend persists, the strength of the association is weakened after full adjustment (Fig. 2).
Results of restrictive cubic spline analysis. Model 1 was unadjusted, Model 2 was adjusted for age, gender, race, and family income-to-poverty ratio. Model 3 was adjusted for age, gender, race, family income-to-poverty ratio, total cholesterol, systolic blood pressure, diastolic blood pressure, body mass index, hypertension, hypercholesterolemia, diabetes, arthritis, congestive heart failure, coronary heart disease, angina pectoris, stroke, emphysema, cancer, smoke, alcohol adjustments.
Subgroup analysis
The results of the subgroup analysis in Fig. 3 illustrate the associations between the AIP and two distinct outcome variables: ASMBMI (left side) and sarcopenia (right side). The analysis encompasses subgroups categorized by age, gender, race, hypertension, hypercholesterolemia, diabetes, arthritis, CHF, cancer, alcohol use, and smoking status. Significant associations with AIP were observed across most subgroups for ASMBMI. Notably, a significantly positive relationship between AIP and ASMBMI was observed in younger individuals (< 40 years) and those without diabetes, arthritis, cancer. Interaction effects were noted for diabetes (P for interaction = 0.032) and alcohol use (P for interaction = 0.005). For sarcopenia, higher AIP levels were generally associated with increased odds across most subgroups. Significant associations were found across age, gender race hypertension hypercholesterolemia diabetes arthritis CHF cancer alcohol use and smoking status. Specifically older adults (≥ 40 years) females and individuals without diabetes cancer or arthritis showed higher odds of sarcopenia with increasing AIP levels. Race demonstrated significant interaction effects suggesting variability in the AIP-sarcopenia relationship among different racial groups (Fig. 3).
Discussion
Our study has uncovered a significant correlation between the AIP and sarcopenia. Elevated AIP levels were found to be associated with an increased risk of sarcopenia across various demographic and health-related subgroups, while also demonstrating an inverse relationship with participants’ ASMBMI. These findings mutually reinforce each other, highlighting the potential role of dyslipidemia, as indicated by AIP, in the pathogenesis of sarcopenia. After full adjustment, every 1 unit increase in AIP was associated with a 0.02 decrease in ASMBMI (P < 0.001) and a 3.39-fold (95%CI :2.29–5.03) increase in the risk of sarcopenia for the highest quartile of AIP. Although Beta was small in absolute terms, the cumulative effect at the population level was significant: a rise in AIP from Q1 to Q4 could result in a loss of 0.5–1.0 kg of muscle mass, equivalent to 5–10% of total muscle mass in older adults, sufficient to impair functional independence. The magnitude of this effect is comparable to the effect of diabetes (OR≈2.0) OR hyperlipidemia (OR≈3.0), but AIP’s intervention ability makes it of greater public health value. The strong association observed, particularly among older adults and females, is consistent with previous research suggesting that metabolic dysregulation plays a crucial role in muscle degeneration and functional decline within these populations27,28,29.
The study utilized three distinct statistical models to evaluate the impact of AIP on sarcopenia. Initial unadjusted models indicated a significant association between elevated AIP, decreased ASMBMI, and an increased risk of sarcopenia. Upon adjusting for confounding factors such as age, sex, race, household income to poverty ratio, total cholesterol, blood pressure, BMI, and chronic disease in the model, the relationship between AIP and ASMBMI as well as sarcopenia remained statistically significant but exhibited reduced strength. This attenuation may be attributed to confounding factors obscuring the link between AIP and sarcopenia; for instance, variables like age and gender could influence lipid metabolism and muscle mass directly30,31,32. Moreover, chronic diseases (e.g., hypertension or diabetes) might further complicate this association by inducing systemic inflammation and metabolic disorders33,34,35. In the fully adjusted model, while the negative correlation between AIP and ASMBMI remained significant (Beta [95% CI] = −0.02 [−0.03, −0.01], P < 0.001), the connection with sarcopenia was no longer statistically significant (OR [95% CI] = 1.05 [0.66, 1.66], P = 0 0.844), indicating that confounding factors had some influence on the relationship between AIP and sarcopenia22. This provides us with a more nuanced comprehension of this relationship. Given these findings, we further explored the non-linear relationships and conducted subgroup analyses to better understand the underlying dynamics. The non-linear relationship analyses revealed that AIP and sarcopenia risk displayed different patterns across various ranges of AIP levels. Subgroup analyses across diverse demographic and health-related subgroups indicated consistent associations, yet the strength and significance varied, underscoring the complexity of the AIP-sarcopenia relationship, and suggesting that specific population characteristics might modulate this association. These insights underscore the need for a deeper understanding of the interplay between lipid metabolism and muscle health, particularly in vulnerable populations such as the elderly36,37,38.
The robust correlation between AIP and sarcopenia, as revealed by our study, necessitates a deeper exploration into the underlying biological mechanisms that connect these two conditions. This transition will help bridge the statistical findings with the biological implications, providing a comprehensive understanding of their interplay. The increase in AIP may impact the onset and progression of sarcopenia through diverse biological mechanisms39. Firstly, elevated AIP indicates an imbalance in the ratio of triglycerides to HDL cholesterol in plasma, which can result in atherosclerosis and microvascular dysfunction, thereby affecting the blood supply and nutrient delivery to skeletal muscle40,41,42,43. Secondly, high AIP levels may be linked to heightened systemic inflammatory responses, where chronic inflammation affects muscle protein synthesis and promotes muscle degradation, as well as oxidative stress that damages muscle cells and diminishes their regenerative capacity, both contributing to muscle catabolism44,45,46,47. Additionally, elevated AIP may be associated with insulin resistance, which plays a pivotal role in regulating the equilibrium between muscle protein synthesis and breakdown48. Insulin resistance could contribute to sarcopenia by increasing muscle protein breakdown while decreasing glucose uptake by muscle cells and subsequent muscular atrophy49,50,51. Understanding these mechanisms can aid in identifying potential therapeutic targets for mitigating the effects of dyslipidemia on muscular health.
Despite the strengths of our study, several limitations must be acknowledged. First, the cross-sectional design precludes the establishment of causal relationships between AIP and sarcopenia. Longitudinal studies are needed to confirm these findings and explore the temporal dynamics of this association. Second, according to the EWGSOP2 definition of sarcopenia (a reduction in muscle mass, quality, and physical performance), the diagnosis of sarcopenia in this study was inadequate. This would have biased the outcome. Third, the key modifiable factors influencing muscle health-such as dietary protein intake, dietary patterns, sleep patterns, micronutrient intake, physical activity and exercise-were not systematically assessed in the NHANES dataset. Additionally, genetic predisposition to sarcopenia and chronic low-grade inflammation biomarkers (e.g., CRP, IL-6) were not included in the adjustment, potentially introducing residual confounding. These factors may confound the observed relationship through independent pathways of muscle metabolism regulation. Lastly, the generalizability of our findings may be limited to the study population, and further research is needed to confirm these results in different demographic and clinical settings.
Conclusions
Our study demonstrates a significant association between the AIP and sarcopenia, with elevated AIP levels linked to reduced skeletal muscle mass and increased sarcopenia risk. These findings underscore the importance of lipid metabolism in muscle health.
Data availability
Data availability The dataset(s) supporting the conclusions of this article is(are) available in the NHANES website (https://www.cdc.gov/nchs/nhanes/).
References
Shen, Y. et al. Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J. Cachexia Sarcopenia Muscle 14(3), 1199–1211 (2023).
Zuo, X. et al. Sarcopenia and cardiovascular diseases: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 14(3), 1183–1198 (2023).
Mirzai, S. et al. Relationship between sarcopenia and intensive blood pressure control efficacy and safety: A secondary analysis of SPRINT. Hypertension 81(8), e77–e87 (2024).
Beaudart, C. et al. Sarcopenia and health-related quality of life: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 14(3), 1228–1243 (2023).
Costa, M. S. D., Pontes, K., Guedes, M. R., Barreto Silva, M. I. & Klein, M. Association of habitual coffee consumption with obesity, sarcopenia, bone mineral density and cardiovascular risk factors: A two-year follow-up study in kidney transplant recipients. Clin. Nutr. 42(10), 1889–1900 (2023).
Barbalho, S. M. et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications Int. J. Mol. Sci. 21(10), 3607 (2020).
Sasaki, K. I. & Fukumoto, Y. Sarcopenia as a comorbidity of cardiovascular disease. J. Cardiol. 79(5), 596–604 (2022).
Nishikawa, H., Asai, A., Fukunishi, S., Nishiguchi, S. & Higuchi, K. Metabolic syndrome and sarcopenia. Nutrients 13(10), 3519 (2021).
Li, W., Qiu, X., Ma, H. & Geng, Q. Incidence and long-term specific mortality trends of metabolic syndrome in the United States. Front. Endocrinol. (Lausanne) 13, 1029736 (2022).
Beauchet, O. et al. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J. Hypertens. 31(8), 1502–1516 (2013).
Wu, J., Zhou, Q., Wei, Z., Wei, J. & Cui, M. Atherogenic index of plasma and coronary artery disease in the adult population: A meta-analysis. Front. Cardiovasc. Med. 8, 817441 (2021).
Fernández-Macías, J. C., Ochoa-Martínez, A. C., Varela-Silva, J. A. & Pérez-Maldonado, I. N. Atherogenic index of plasma: Novel predictive biomarker for cardiovascular illnesses. Arch. Med. Res. 50(5), 285–294 (2019).
Xiao, S. et al. Association of systemic immune inflammation index with estimated pulse wave velocity, atherogenic index of plasma, triglyceride-glucose index, and cardiovascular disease: A large cross-sectional study. Med. Inflamm. 2023, 1966680 (2023).
Alifu, J. et al. Association between the atherogenic index of plasma and adverse long-term prognosis in patients diagnosed with chronic coronary syndrome. Cardiovasc. Diabetol. 22(1), 255 (2023).
Ménégaut, L. et al. Inflammation and oxidative stress markers in type 2 diabetes patients with advanced carotid atherosclerosis. Cardiovasc. Diabetol. 22(1), 248 (2023).
Sárközy, M. et al. Mechanisms and modulation of oxidative/nitrative stress in type 4 cardio-renal syndrome and renal sarcopenia. Front. Physiol. 9, 1648 (2018).
Tong, Q. et al. Metabolic syndrome and its association with components of sarcopenia in older community-dwelling Chinese. J. Biomed. Res. 36(2), 120–126 (2022).
Kim, S. H. et al. Association between sarcopenia level and metabolic syndrome. PLoS ONE 16(3), e0248856 (2021).
Zhang, H. et al. Association between sarcopenia and metabolic syndrome in middle-aged and older non-obese adults: A systematic review and meta-analysis. Nutrient 10(3), 364 (2018).
Iantomasi, T. et al. Oxidative stress and inflammation in osteoporosis: Molecular mechanisms involved and the relationship with microRNAs. Int. J. Mol. Sci. 24(4), 3772 (2023).
Pinheiro, L. et al. Prevalence of associations among sarcopenia, obesity, and metabolic syndrome in Brazilian older adults. Front. Med. (Lausanne) 10, 1206545 (2023).
Damluji, A. A. et al. Sarcopenia and cardiovascular diseases. Circulation 147(20), 1534–1553 (2023).
Li, W. et al. Associations of blood metals with liver function: Analysis of NHANES from 2011 to 2018. Chemosphere 317, 137854 (2023).
Batsis, J. A., Mackenzie, T. A., Jones, J. D., Lopez-Jimenez, F. & Bartels, S. J. Sarcopenia, sarcopenic obesity and inflammation: Results from the 1999–2004 national health and nutrition examination survey. Clin. Nutr. 35(6), 1472–1483 (2016).
Leem, A. Y. et al. Sarcopenia is associated with cardiovascular risk in men with COPD, independent of adiposity. Respir. Res. 23(1), 185 (2022).
Lopes, L. C. C. et al. Sex and population-specific cutoff values of muscle quality index: Results from NHANES 2011–2014. Clin. Nutr. 41(6), 1328–1334 (2022).
Hermans, M. P., Ahn, S. A. & Rousseau, M. F. The atherogenic dyslipidemia ratio [log(TG)/HDL-C] is associated with residual vascular risk, beta-cell function loss and microangiopathy in type 2 diabetes females. Lipid. Health Dis. 11, 132 (2012).
Moura, F. A., Freitas, W. M. & Sposito, A. C. Emergent cardiovascular risk factors in the very elderly. Expert Rev. Cardiovasc. Ther. 10(10), 1221–1225 (2012).
Rossi, F. E. et al. Influence of skeletal muscle mass and fat mass on the metabolic and inflammatory profile in sarcopenic and non-sarcopenic overfat elderly. Ag. Clin. Exp. Res. 31(5), 629–635 (2019).
Penner, A. L. et al. Oxylipin profiles and levels vary by skeletal muscle type, dietary fat and sex in young rats. Appl. Physiol. Nutr. Metab. 46(11), 1378–1388 (2021).
Sato, T. et al. Intracellular lipid content of liver and skeletal muscle in patients with adult growth hormone deficiency without diabetes mellitus. Obes. Res. Clin. Pract. 6(4), e263-346 (2012).
Ko, B. J. et al. Relationship between low relative muscle mass and coronary artery calcification in healthy adults. Arterioscler. Thromb. Vasc. Biol. 36(5), 1016–1021 (2016).
Cohen, S., Nathan, J. A. & Goldberg, A. L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 14(1), 58–74 (2015).
Rohm, T. V., Meier, D. T., Olefsky, J. M. & Donath, M. Y. Inflammation in obesity, diabetes, and related disorders. Immunity 55(1), 31–55 (2022).
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25(12), 1822–1832 (2019).
Wu, X. et al. Sarcopenia prevalence and associated factors among older Chinese population: Findings from the China health and retirement longitudinal study. PLoS ONE 16(3), e0247617 (2021).
Petermann-Rocha, F. et al. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 13(1), 86–99 (2022).
Cruz-Jentoft, A. J. et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ag. 43(6), 748–759 (2014).
Constantin-Teodosiu, D. & Constantin, D. Molecular mechanisms of muscle fatigue. Int. J. Mol. Sci. 22(21), 11587 (2021).
Sun, T., Ma, Z., Gao, L., Wang, Y. & Xie, H. Correlation between sarcopenia and arteriosclerosis in elderly community dwellers: A multicenter study. J. Nutr. Health Ag. 25(5), 692–697 (2021).
Pizzimenti, M. et al. Sarcopenia and peripheral arterial disease: A systematic review. J. Cachexia Sarcopenia Muscle 11(4), 866–886 (2020).
Liu, H. M. et al. Sarcopenia-related traits and coronary artery disease: A bi-directional mendelian randomization study. Ag. (Albany NY) 12(4), 3340–3353 (2020).
Hida, T. et al. Sarcopenia and physical function are associated with inflammation and arteriosclerosis in community-dwelling people: The Yakumo study. Mod. Rheumatol. 28(2), 345–350 (2018).
Strasser, B., Wolters, M., Weyh, C., Krüger, K. & Ticinesi, A. The effects of lifestyle and diet on gut microbiota composition, inflammation and muscle performance in our aging society. Nutrient 13(6), 2045 (2021).
Pérez-Baos, S. et al. Mediators and patterns of muscle loss in chronic systemic inflammation. Front. Physiol. 9, 409 (2018).
Antuña, E. et al. Inflammaging: Implications in Sarcopenia. Int. J. Mol. Sci. 23(23), 15039 (2022).
Livshits, G. & Kalinkovich, A. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ag. Res. Rev. 56, 100980 (2019).
Krentz, A. J., Viljoen, A. & Sinclair, A. Insulin resistance: A risk marker for disease and disability in the older person. Diabet. Med. 30(5), 535–548 (2013).
Xu, H. et al. Association between macro- and microvascular damage and sarcopenia index in individuals with type 2 diabetes mellitus. Appl. Physiol. Nutr. Metab. 49(6), 762–772 (2024).
Tanaka, K. I., Kanazawa, I. & Sugimoto, T. Reduced muscle mass and accumulation of visceral fat are independently associated with increased arterial stiffness in postmenopausal women with type 2 diabetes mellitus. Diabet. Res. Clin. Pract. 122, 141–147 (2016).
Hashimoto, Y. et al. Relationship between serum creatinine to cystatin C ratio and subclinical atherosclerosis in patients with type 2 diabetes. BMJ Open Diabet. Res. Care. 10(3), e002910 (2022).
Acknowledgements
Data availability The dataset(s) supporting the conclusions of this article is(are) available in the NHANES website (https://www.cdc.gov/nchs/nhanes/). The data used in this study were from the NHANES. We thank all the staff of and participants in the NHANES for their contribution. This research is financially supported by the project from Zunyi Science and Technology Plan Project Contract (Zun Shi Ke He HZ No. (2023):106).
Author information
Authors and Affiliations
Contributions
Zeli Tang: Conceptualization, Data curation, Formal analysis, Writing—original draft, Project administration. Jing Li: Conceptualization, Data curation, Formal analysis, Writing—original draft, Project administration. Xia Zhang: Writing—review & editing. Yanling Zheng: Writing—review & editing. Jie Yv: Writing—review & editing. Correspondence to Zeli Tang.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the NCHS Research Ethics Review Board (ERB) (Continuation of Protocol #2011–17).
Consent to statement
All participants provided informed consent to participate.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Tang, Z., Zhang, X. et al. Relationship between atherosclerotic burden and sarcopenia in U.S. adults: A cross-sectional study based on the NHANES database. Sci Rep 15, 16793 (2025). https://doi.org/10.1038/s41598-025-01133-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01133-9