Abstract
This study aimed to investigate the predictive ability of Hounsfield unit (HU) measurements for microorganism growth observed microbiologically in stone cultures after stone surgery and to create a model by adding other predictive factors for predicting stone culture positivity. Patients who underwent percutaneous nephrolithotomy, retrograde intrarenal surgery, or ureteroscopy and had stone cultures performed were included in the study. Demographic and clinical data, including age, gender, body mass index, stone size, stone volume, hydronephrosis grade, stone location, energy used, presence of preoperative stent or nephrostomy, operation duration, midstream urine culture, renal pelvic urine culture, stone culture results, and postoperative fever and systemic inflammatory response syndrome criteria, were recorded. Non-contrast computed tomography images were used to measure the HU at the stone’s core (HUcore), the proximal surface of the stone periphery in the collecting system (HUproximal), the distal surface of the stone periphery in the collecting system (HUdistal), and the average HU of the stone periphery (HUperiphery mean). Absolute and relative differences between these values were calculated. A total of 383 patients were included, with microorganism growth observed in the stone cultures of 75 patients (19.6%). Radiological cut-off values distinguishing stones with positive cultures included RelativeHUdifference distal & proximal < 81.8, HUproximal < 807.0, and AbsoluteHUdifference core & periphery mean > 179.5. Factors associated with a statistically significant increase in the likelihood of positive stone cultures included longer operation duration (odds ratio [OR] = 1.102, 95% confidence interval [CI]: 1.053–1.154, p < 0.001), higher preoperative hydronephrosis grade (OR = 1.898, 95% CI: 1.289–2.795, p < 0.001), and the presence of preoperative stents or nephrostomy (OR = 4.232, 95% CI: 1.551–11.543, p = 0.005) in addition to the identified radiological HU cut-off values. HU values, as a radiological parameter, can predict microorganism growth in stone cultures, enabling identification of patients at risk for postoperative infectious complications.
Similar content being viewed by others
Introduction
Urinary stone disease (USD) is a condition that negatively affects renal function and quality of life and is a common urological disorder with a globally increasing incidence1. When stones obstruct the urinary system, surgical intervention is often required based on the stone’s size and location2. Surgical complications after urinary system stone disease vary between 5% and 25%, and the majority (1–6.6%) are postoperative infections3. Bacterial colonies on the stone surface can worsen the disease course and lead to sepsis-related comorbidities. Therefore, infectious complications are among the most critical issues following stone surgery4,5,6. To identify microorganisms responsible for infections associated with stones, it is essential to cultivate the organism microbiologically in a culture medium. To this end, microbiological diagnostic strategies such as midstream urine culture (MSUC), prior to surgery, renal pelvic urine culture (RPUC) obtained from the upper urinary tract during the operation, and stone culture (SC) are employed7,8,9. The collection of stone samples for SC after lithotripsy is strongly recommended by European Association of Urology guide postoperative antibiotic selection10,11,12.
The Hounsfield Unit (HU) is a parameter used in computed tomography (CT), defined based on predetermined values of 0 for water and − 1,000 for air, and measures the standardized linear attenuation coefficient of a substance. In a study comparing HU measurements of the renal papillae between stone-forming and non-stone-forming patients, the average density in stone formers was shown to be over 50 HU, and it was emphasized that CT could be used as a screening tool for future nephrolithiasis in the coming years13. Clinically, it provides insights into stone composition and hardness, assisting in the selection of treatments targeting the stone and in predicting post-treatment outcomes. In another study evaluating stone measurements and stone analysis results on CT images, maximum, minimum, and average HU values were compared, and high sensitivity and specificity results were reported in identifying monomineral stones14. Recently, HU has also been reported as a potential indicator for distinguishing pyonephrosis from hydronephrosis and predicting infectious complications15,16,17. It was shown that the HU of all patients with pyonephrosis and positive urine cultures was significantly higher than that of patients with hydronephrosis15.
SC results obtained during the operation take at least 24–48 h to be reported, and this is a long period in cases where infective complications develop in terms of patient mortality and morbidity18. New studies targeting stone components and structure to predict infective complications early are becoming popular19. Stones contain bacteria and their metabolites, which have been proven to be strongly associated with postoperative infection20,21. It has also recently been demonstrated that bacteria affect stone formation not only in infection stones but also in other stone compositions22. Bacterial colonization on the stone surface and the kidney’s own microbiota may trigger stone formation23. Bacteria ascending from the lower urinary tract to the upper urinary tract may first colonize the distal surface of the stone, then the proximal surface, or bacteria arriving via the hematogenous route, may colonize the proximal surface and then the central and distal surfaces24. This study was designed to focus on different regions of the stone in CT images using the HU difference between two different points and to contribute to predicting and diagnosing stones that will show growth in SC. Based on this hypothesis, the current study aimed to predict SC results obtained during surgery in patients undergoing operations for USD by utilizing HU parameters measured using preoperative non-contrast computed tomography (NCCT).
Methods
Following approval by the Clinical Research Ethics Committee of Adiyaman University (decision number: 2023/1–5), a retrospective review of data was conducted for patients who underwent surgery for USD at the Urology Department of Adiyaman University between January 2015 and June 2024. All methods were conducted in accordance with the relevant guidelines and regulations. Informed consent was obtained from all patients and/or their legal guardians. Patients aged over 18 years who had undergone percutaneous nephrolithotomy (PCNL), retrograde intrarenal surgery (RIRS), or ureteroscopy (URS) and whose SCs were submitted were included in the study. Patients who did not undergo SC or RPUC analysis, those with contaminated culture results, those for whom standardized NCCT imaging was unavailable, and those with incomplete or inaccessible clinical data were excluded.
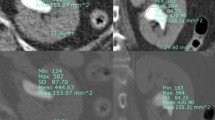
Demographic and clinical data, including age, gender, body mass index, stone size, stone volume, hydronephrosis grade (categorized per the classification recommended by the Society for Fetal Urology), stone localization and position, energy used for stone fragmentation, presence of preoperative stents or nephrostomy, MSUC, SC, RPUC, postoperative fever, and systemic inflammatory response syndrome (SIRS), were recorded. Radiological stone measurements were obtained using the abdominal window of NCCT images with high magnification of the stone and its surroundings. All cases were scanned using a 128-detector CT scanner (GE Healthcare). Axial images were obtained with a slice thickness of 0.625 mm and subsequently reformatted in coronal and sagittal planes. The preoperative NCCT scans of all patients were evaluated independently by one radiologist and one urologist. Stone size was defined as the largest dimension of the stone measured in millimeters on axial NCCT images. In cases of multiple stones, the parameters of the largest stone were measured. Stone volume, defined as the surface area of the stone in the maximum section, was calculated using a validated formula (volume = length × width × depth × π × 0.167) and expressed in mm3. For each stone, absolute HU values were measured. A region of interest of 5 mm² was selected in the NCCT coronal image at the largest stone cross-section. HU measurements were taken separately for the stone core (HUcore), the proximal surface of the stone periphery within the collecting system (HUproximal), and the distal surface of the stone periphery within the collecting system (HUdistal) (Fig. 1).
The absolute difference between HUproximal and HUdistal was recorded as AbsoluteHUdifference distal & proximal; that between HUcore and HUdistal as AbsoluteHUdifference core & distal; that between HUcore and HUproximal as AbsoluteHUdifference core & proximal; and that between HUcore and the average HU of the two peripheral surfaces (HUperiphery mean) as AbsoluteHUdifference core & periphery mean. In addition, the relative difference between HUproximal and HUdistal was computed as RelativeHUdifference distal & proximal; that between HUcore and HUdistal as RelativeHUdifference core & distal; that between HUcore and HUproximal as RelativeHUdifference core & proximal, and that between HUcore and HUperiphery mean as RelativeHUdifference core & periphery mean. Absolute HU differences were calculated as simple subtractions, while relative differences were expressed as percentages of the respective values.
All patients had negative MSUC and received prophylaxis with a third-generation cephalosporin (ceftriaxone sodium 1 g, intravenously) in accordance with the AUA Guidelines on Urological Infections. If MSUC was positive, surgery was performed under antibiotic treatment for one to seven days. Surgeries were performed under general or regional anesthesia. Fever was defined as a body temperature exceeding 38 °C. SIRS was defined based on the presence of at least two of the following criteria: fever < 36 °C or > 38 °C, heart rate > 90 beats/minute, respiratory rate > 20 breaths/minute, and white blood cell count < 4,000/mm³ or > 12,000/mm³.
Stones were fragmented using a pneumatic lithotripsy device (Swiss LithoClast® Master) and a 30 W holmium laser (Dornier Medilas® H Solvo) and collected in a sterile manner with foreign body forceps. Stone fragments were divided into smaller pieces in sterile liquid thioglycollate media and sent to the microbiology laboratory along with urine samples for SC analysis. Bacteria were identified using both conventional microbiological methods and the BD Phoenix™ automated microbiology system (Becton-Dickinson & Co., NJ, USA). The antibiotic susceptibility of the isolates was tested using the BD Phoenix™ automated microbiology system (Becton-Dickinson & Co., NJ, USA), based on the current Breakpoint Table of the European Committee on Antimicrobial Susceptibility Testing, version 10.0, 2020. Stone analysis was performed using the X-ray diffraction (XRD) method, which identified powdered stone samples using an XRD analyzer equipped with a copper tube, operating in the 2–30° range (MTA Ankara, Turkey). All data were analyzed by dividing patients into subgroups: Group A included patients with positive SC, and Group B included those with negative SC. Comparisons between the two groups were made accordingly.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics version 25 software (IBM Corporation, Armonk, NY, US). The Kolmogorov-Smirnov and Levene’s tests were respectively used to investigate whether the assumptions of normal distribution and homogeneity of variances were met. Categorical data were expressed as numbers and percentages, while quantitative data were given as median (25 th −75 th percentile) values. The Mann-Whitney U test was applied for comparisons of the continuous variables for which parametric assumptions were not met. Unless otherwise stated, Pearson’s χ2 test was used in the analysis of categorical data. In all 2 × 2 contingency tables to compare categorical variables, the Yates-corrected χ2 test was used when one or more of the cells had an expected frequency of 5–25, and Fisher’s exact test was used when one or more of the cells had an expected frequency of 5 or less. In RxC cross-tabulations (if at least one of the categorical variables in the row or column had more than two results), if the expected frequency was below 5 in at least ¼ of the cells, the Fisher-Freeman-Halton test was used. Receiver operating characteristic (ROC) curve analyses were performed to determine the cut-off levels for each radiographic measurement as a predictor of Group A. Youden’s (J) index method was used to identify the best cut-off values when the area under the curve (AUC) value indicated statistically significant results. For the measurements with significant AUCs, sensitivity and specificity values corresponding to each data point were calculated. The optimal cut-off point for each parameter was determined as the value at which the sum of sensitivity and specificity was maximized. At these cut-off points, sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy rates were calculated. Intraclass correlation coefficients (ICCs) and 95% confidence intervals (CIs) were calculated to determine the degrees of reliability between the measurements that were obtained from the urologist and the radiologist. The McNemar test was performed to examine whether the difference in proliferation frequencies among SC, MSUC, and RPUC was statistically significant. To determine the best predictors of Group A, multiple logistic regression analysis was undertaken via the forward likelihood ratio procedure. Any variable whose univariable test had a p-value less than 0.25 was accepted as a candidate for the multivariable model. Odds ratios (ORs), 95% CIs, and Wald statistics for each independent variable were also calculated. A p-value of less than 0.05 was considered statistically significant. Figure 2 presents the data analysis flowchart.
Results
Data from 1,071 patients were retrospectively reviewed, and 383 patients who met the inclusion criteria were enrolled in the study. SC positivity was identified in 75 patients (19.6%). SC positivity was identified in 75 patients (19.6%). Candida was isolated in three patients, Enterococcus faecalis in eight, Escherichia coli in 13, Klebsiella pneumoniae in 10, Proteus in nine, Pseudomonas in seven, Staphylococcus in 22, and Streptococcus in three. Comparisons of the demographic and clinical characteristics of the groups are presented in Table 1, while comparisons of radiological measurements are shown in Table 2. When the groups were compared based on HU parameters, the values of HUproximal, HUdistal, AbsoluteHUdifference distal & proximal, and RelativeHUdifference distal & proximal were statistically significantly lower in Group A, while AbsoluteHUdifference core & distal, AbsoluteHUdifference core & proximal, AbsoluteHUdifference core & periphery, RelativeHUdifference core & distal, RelativeHUdifference core & proximal, and RelativeHUdifference core & periphery measurements were statistically significantly higher in this group (p < 0.01).
According to ROC analysis, except for HUcore, all other radiological measurements had statistically significant AUC values for distinguishing between the groups (p < 0.01) (Table 3). Independent of other factors, the likelihood of SC positivity was significantly increased in stones with a Relative HUdifference distal & proximal of < 81.8 (OR = 2.930, 95% CI: 1.376–6.237, p = 0.005), a HUproximal periphery of < 807.0 (OR = 2.367, 95% CI: 1.224–4.579, p = 0.010), and an AbsoluteHUdifference core & periphery of > 179.5 (OR = 2.060, 95% CI: 1.026–4.135, p = 0.042).
ROC curves for HUcore, HUproximal periphery, and HUdistal periphery are presented in Fig. 3; those for AbsoluteHUdifference distal & proximal, AbsoluteHUdifference core & distal; AbsoluteHUdifference core & proximal, and AbsoluteHUdifference core & periphery in Fig. 4; and those for RelativeHUdifference distal & proximal, RelativeHUdifference core & distal, RelativeHUdifference core & proximal, and RelativeHUdifference core & periphery in Fig. 5.
The most discriminative factors for distinguishing the groups were further analyzed using multivariate forward logistic regression. A stepwise forward modeling approach was employed to derive the final model. Two alternative models were derived. In Model 1, the type of surgery was included as a candidate variable in the regression model, whereas in Model 2, it was excluded. According to Model 1, independent of other factors, compared to cases who underwent PCNL, the likelihood of detecting growth in stone cultures was statistically significantly higher in cases who underwent URS (OR = 52.763, 95% CI: 8.857–314.322, p < 0.001) and RIRS (OR = 8.223, 95% CI: 1.825–37.055, p = 0.006). The likelihood of SC positivity increased significantly as the duration of the operation increased (OR = 1.102, 95% CI: 1.053–1.154, p < 0.001). Higher preoperative hydronephrosis grade (OR = 1.898, 95% CI: 1.289–2.795, p < 0.001) and the presence of preoperative stents or nephrostomy (OR = 4.232, 95% CI: 1.551–11.543, p = 0.005) were also associated with a significantly increased likelihood of SC positivity. In addition, independent of other factors, having RelativeHUdifference distal&proximal < 81.8 (OR = 2.930, 95% CI: 1.376–6.237, p = 0.005), HUproximal periphery < 807.0 (OR = 2.367, 95% CI: 1.224–4.579, p = 0.010), and AbsoluteHUdifference core & periphery > 179.5 (OR = 2.060, 95% CI: 1.026–4.135, p = 0.042) significantly increased the likelihood of detecting growth in stone culture. In Model 2, unlike Model 1, the likelihood of culture growth in renal stones was statistically significantly higher compared to ureteral stones (OR = 12.397, 95% CI: 3.663–41.958, p < 0.001) (Table 4).
Using the cut-off values obtained from the ROC analysis for radiological measurements, true-negative and true-positive rates were compared across surgery types. Regarding HUproximal periphery, HUdistal periphery, AbsoluteHUdifference distal & proximal, RelativeHUdifference core & distal, and RelativeHUdifference core & periphery, RIRS group had statistically significantly higher true-negative rates compared to PCNL group (p < 0.05). For HUproximal periphery, both PCNL and RIRS groups had statistically significantly higher true-negative rates compared to URS group (p < 0.05). On the other hand, regarding AbsoluteHUdifference distal & proximal, AbsoluteHUdifference core & proximal, and AbsoluteHUdifference core & periphery, URS group had statistically significantly higher true-negative rates compared to PCNL group (p < 0.05). Lastly, when examining HUproximal periphery, HUdistal periphery, AbsoluteHUdifference distal & proximal, AbsoluteHUdifference core & distal, AbsoluteHUdifference core & proximal, AbsoluteHUdifference core & periphery, RelativeHUdifference distal & proximal, RelativeHUdifference core & distal, RelativeHUdifference core & proximal, and RelativeHUdifference core & periphery, URS group had statistically significantly higher true-positive rates compared to PCNL group (p < 0.05) (Table 5).
The agreement between measurements by the urologist and the radiologist was evaluated using ICCs and 95% CIs. The radiological measurements by both evaluators, including stone volume, stone size, HUcore, HUproximal, and HUdistal showed strong agreement, as shown in Table 6.
There were 34 patients with observed infectious complications. Among these, 26 patients (76.5%) had SC positivity, while MSUC and RPUC were positive in 21 (61.8%) and 12 (35.3%) cases, respectively, as shown in Table 7.
SC positivity was less frequently observed in all surgical procedures compared to MSUC (p < 0.05), as shown in Table 8.
Discussion
Based on the hypothesis that microorganisms colonizing the surface of stones create regional differences in stone density, this study investigated the HU values of the stone core, distal, and proximal regions in stones with SC positivity and investigated the absolute and relative differences in these values. Multivariate logistic regression analysis identified the most predictive factors for determining SC positivity. Independent of other factors, RelativeHUdifference distal & proximal < 81.8, HUproximal < 807.0, and AbsoluteHUdifference core & periphery mean > 179.5, measured on NCCT, were found to be statistically significant cut-off values for predicting SC positivity. These findings suggest that preoperative HU parameters derived from NCCT could serve as a guide in predicting SC positivity in patients undergoing surgery for USD.
NCCT is the gold standard imaging modality for evaluating USD, providing predictive information for treatment planning by determining stone size (length and volume), location, and HU25. Several approaches, including the difference between central and peripheral HU, the stone heterogeneity index, the attenuation/size ratio, and combinations of mean and maximum HU values, have been employed to characterize stone compositions26,27,28,29. It has been shown that mean and maximum HU values are significantly higher in calcium oxalate stones compared to uric acid stones in distinguishing uric acid stones from calcium oxalate stones26. In a study comparing core HU, periphery HU, absolute and relative HU differences, and HU density of calcium oxalate, uric acid, and cystine stones, calcium oxalate stones could be predicted with high accuracy, whereas cystine and uric acid stones could not be differentiated due to overlap27. Additionally, some studies have reported that mean and maximum HU can be used to make more accurate predictions in identifying uric acid stones28. The initial use of HU for identifying infectious conditions was evaluated in a study by Yuruk et al., which used HU values to differentiate between pyonephrosis and hydronephrosis, reporting higher HU cut-off values for pyonephrosis15. Similarly, other studies demonstrated that the HU values of fluid in the dilated renal collecting system were lower in individuals with positive MSUCs compared to those with negative cultures16,17. HU values are defined across a spectrum, ranging from − 1,000 for air, 0 for water, and − 100 to 100 for most soft tissues, to approximately 2,000 for dense bone (e.g., cochlea) and over 3,000 for metals.
In this study, all HU parameters except for HUcore showed statistically significant AUC values for identifying SC positivity. The findings suggest that stones with SC positivity have lower mean HU values, possibly attributable to the biofilm layer formed around the stone. Furthermore, the HU value of the stone’s distal surface in the collecting system was found to be lower. This may be due to bacteria ascending through the upper urinary tract, initially colonizing the distal surface and forming a denser biofilm layer in this region, leading to lower HU values compared to the proximal surface. These results strongly support the hypothesis of the study.
It has been emphasized in the literature that urease-producing organisms were cultured in 71% of positive SCs and that bacteria were highly concentrated on the stone surface21. Previous studies have suggested that bacteria may play a potential role in lithogenesis due to the increased bacterial density on the surface of the stone30,31. Various bacterial species, particularly in calcium oxalate (CaOx) stones, have been shown to induce or exacerbate metabolic stone disease through ionic interactions between anions on the bacterial surface and cations (i.e., Ca²⁺) on CaOx crystals32. The presence of biofilm-coated microorganisms on the surface of urinary stones and the protective effect of antibacterial proteins within stone cores are also implicated in the composition of stones33,34. There is a clear relationship between the density and composition of stones and bacteria. However, uncertainties in stone formation, composition, and density underscore the need for further in vivo and in vitro studies.
Consistent with previous studies, the results of the current study demonstrated that SC was more sensitive than MSUC or RPUC for predicting infectious complications following stone surgery35. SC positivity correlates with a higher rate of infectious complications following endourological procedures; however, it exhibits significant disagreement with MSUC results obtained preoperatively36. While SC presents results within 24–48 h and offers clinical guidance in managing antibiotic therapy in cases of sepsis, it provides delayed insights into predicting which patients may develop infectious complications37. Preoperative modeling could serve as an alert mechanism to anticipate the risk of such complications38. Apart from HU parameters, factors identified in previous studies, such as prolonged operation time, high preoperative hydronephrosis grade, and the presence of preoperative stents or nephrostomy catheters, were also supported by this study as significant predictors of SC positivity36. In cases of USD, predicting infectious complications postoperatively without waiting for SC results can be achieved using the proposed model, which incorporates the radiological characteristics of stones.
The retrospective design of this study is susceptible to bias. Due to advancements in surgical instruments, the use of suction-enabled sheaths known to reduce infective complications, and the replacement of reusable instruments with disposable ones were significant sources of heterogeneity in this study. The study had limitations, such as not including variables with potential risk factors, such as diabetes or immunosuppression, and not examining other relevant variables in the patients. The inclusion of MSUC positive patients in the study and the fact that they underwent surgery under the influence of long-term antibiotic treatment may have affected the potential for SC positivity. In addition, infectious complications in the postoperative period can occur for reasons unrelated to infection, including cardiogenic events, atelectasis, hypovolemia, and pain. Despite its limitations, this study is the first to investigate the relationship between SC and HU parameters in stone surgery, paving the way for future prospective studies to establish clearer conclusions.
Conclusion
In predicting SC positivity, HU parameters identified to have significant cut-off values were RelativeHUdifference distal&proximal < 81.8, HUproximal < 807.0, and AbsoluteHUdifference core&periphery mean > 179.5. In addition, prolonged operation duration, high preoperative hydronephrosis grade, and the presence of preoperative stents or nephrostomy catheters were determined to be significant predictive factors. By incorporating these parameters into preoperative models, patients at risk for postoperative infectious complications can be predicted.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dai, X., Chang, Y. & Hou, Y. Associations between the conicity index and kidney stone disease prevalence and mortality in American adults. Sci. Rep. 15, 902. https://doi.org/10.1038/s41598-025-85292-9 (2025).
Zeng, G. et al. International alliance of urolithiasis (IAU) guidelines on the metabolic evaluation and medical management of urolithiasis. Urolithiasis 51, 4. https://doi.org/10.1007/s00240-022-01387-2 (2022).
Grosso, A. A. et al. Intraoperative and postoperative surgical complications after ureteroscopy, retrograde intrarenal surgery, and percutaneous nephrolithotomy: a systematic review. Minerva Urol. Nephrol. 73, 309–332. https://doi.org/10.23736/s2724-6051.21.04294-4 (2021).
Danilovic, A. et al. High-risk patients for septic shock after percutaneous nephrolithotomy. Int. Braz. J. Urol. 50, 561–571. https://doi.org/10.1590/s1677-5538.Ibju.2024.0154 (2024).
Zhou, G. et al. The influencing factors of infectious complications after percutaneous nephrolithotomy: a systematic review and meta-analysis. Urolithiasis 51, 17. https://doi.org/10.1007/s00240-022-01376-5 (2022).
Corrales, M., Sierra, A., Doizi, S. & Traxer, O. Risk of Sepsis in retrograde intrarenal surgery: A systematic review of the literature. Eur. Urol. Open. Sci. 44, 84–91. https://doi.org/10.1016/j.euros.2022.08.008 (2022).
Mariappan, P., Smith, G., Bariol, S. V., Moussa, S. A. & Tolley, D. A. Stone and pelvic urine culture and sensitivity are better than bladder urine as predictors of Urosepsis following percutaneous nephrolithotomy: a prospective clinical study. J. Urol. 173, 1610–1614. https://doi.org/10.1097/01.ju.0000154350.78826.96 (2005).
Singh, I., Shah, S., Gupta, S. & Singh, N. P. Efficacy of intraoperative renal stone culture in predicting postpercutaneous nephrolithotomy urosepsis/systemic inflammatory response syndrome: A prospective analytical study with review of literature. J. Endourol. 33, 84–92. https://doi.org/10.1089/end.2018.0842 (2019).
De Lorenzis, E. et al. Feasibility and relevance of urine culture during stone fragmentation in patients undergoing percutaneous nephrolithotomy and retrograde intrarenal surgery: a prospective study. World J. Urol. 39, 1725–1732. https://doi.org/10.1007/s00345-020-03387-6 (2021).
Ripa, F. et al. Clinical significance of stone culture during endourological procedures in predicting post-operative urinary sepsis: should it be a standard of care-evidence from a systematic review and meta-analysis from EAU section of urolithiasis (EULIS). World J. Urol. 42, 614. https://doi.org/10.1007/s00345-024-05319-0 (2024).
Akram, M. et al. Urological guidelines for kidney stones: overview and comprehensive update. J. Clin. Med. 13 https://doi.org/10.3390/jcm13041114 (2024).
Castellani, D. et al. The significance of stone culture in the incidence of sepsis: results from a prospective, multicenter study on infections post flexible ureterorenescopy (I-FUN) and laser lithotripsy for renal stones. J. Endourol. 38, 948–955. https://doi.org/10.1089/end.2024.0182 (2024).
Deshmukh, S., Kambadakone, A., Sahani, D. V. & Eisner, B. H. Hounsfield density of renal papillae in stone formers: analysis based on stone composition. J. Urol. 193, 1560–1563. https://doi.org/10.1016/j.juro.2014.10.089 (2015).
Celik, S. et al. A novel method for prediction of stone composition: the average and difference of Hounsfield units and their cut-off values. Int. Urol. Nephrol. 50, 1397–1405. https://doi.org/10.1007/s11255-018-1929-3 (2018).
Yuruk, E. et al. Computerized tomography Attenuation values can be used to differentiate hydronephrosis from pyonephrosis. World J. Urol. 35, 437–442. https://doi.org/10.1007/s00345-016-1888-1 (2017).
Boeri, L. et al. Hounsfield unit Attenuation value can differentiate pyonephrosis from hydronephrosis and predict septic complications in patients with obstructive uropathy. Sci. Rep. 10, 18546. https://doi.org/10.1038/s41598-020-75672-8 (2020).
Basmaci, I. & Sefik, E. A novel use of Attenuation value (Hounsfield unit) in non-contrast CT: diagnosis of pyonephrosis in obstructed systems. Int. Urol. Nephrol. 52, 9–14. https://doi.org/10.1007/s11255-019-02283-2 (2020).
Silvani, C. et al. The clinical role of bacteremia and bacterial spread into the irrigation fluid during percutaneous nephrolithotomy: a prospective study. World J. Urol. 41, 135–142. https://doi.org/10.1007/s00345-022-04217-7 (2023).
Lin, J. et al. A novel infrared spectroscopy marker for assessing the postoperative infection risk in patients with upper urinary tract calculus. Sci. Rep. 14, 19398. https://doi.org/10.1038/s41598-024-69720-w (2024).
McAleer, I. M., Kaplan, G. W., Bradley, J. S., Carroll, S. F. & Griffith, D. P. Endotoxin content in renal calculi. J. Urol. 169, 1813–1814. https://doi.org/10.1097/01.ju.0000061965.51478.79 (2003).
Englert, K. M., McAteer, J. A., Lingeman, J. E. & Williams, J. C. Jr. High carbonate level of apatite in kidney stones implies infection, but is it predictive? Urolithiasis 41, 389–394. https://doi.org/10.1007/s00240-013-0591-6 (2013).
Grases, F. et al. Evidence of bacterial imprints in different types of non-struvite kidney stones. BMC Urol. 25 https://doi.org/10.1186/s12894-025-01755-1 (2025).
Agudelo, J. et al. Cefazolin shifts the kidney microbiota to promote a lithogenic environment. Nat. Commun. 15, 10509. https://doi.org/10.1038/s41467-024-54432-6 (2024).
Kim, A. et al. What is the cause of recurrent urinary tract infection?? Contemporary microscopic concepts of pathophysiology. Int. Neurourol. J. 25, 192–201. https://doi.org/10.5213/inj.2040472.236 (2021).
Türk, C. et al. EAU guidelines on interventional treatment for urolithiasis. Eur. Urol. 69, 475–482. https://doi.org/10.1016/j.eururo.2015.07.041 (2016).
Ganesan, V., De, S., Shkumat, N., Marchini, G. & Monga, M. Accurately diagnosing uric acid stones from conventional computerized tomography imaging: development and preliminary assessment of a pixel mapping software. J. Urol. 199, 487–494. https://doi.org/10.1016/j.juro.2017.09.069 (2018).
Torricelli, F. C. et al. Predicting urinary stone composition based on single-energy Noncontrast computed tomography: the challenge of cystine. Urology 83, 1258–1263. https://doi.org/10.1016/j.urology.2013.12.066 (2014).
Qin, L. et al. The combination of mean and maximum Hounsfield unit allows more accurate prediction of uric acid stones. Urolithiasis 50, 589–597. https://doi.org/10.1007/s00240-022-01333-2 (2022).
Marchini, G. S. et al. Stone characteristics on Noncontrast computed tomography: Establishing definitive patterns to discriminate calcium and uric acid compositions. Urology 82, 539–546. https://doi.org/10.1016/j.urology.2013.03.092 (2013).
Dornbier, R. A. et al. The Microbiome of calcium-based urinary stones. Urolithiasis 48, 191–199. https://doi.org/10.1007/s00240-019-01146-w (2020).
Ahmed, A. E. et al. Metabolic stone workup abnormalities are not as important as stone culture in patients with recurrent stones undergoing percutaneous nephrolithotomy. Urolithiasis 51, 47. https://doi.org/10.1007/s00240-023-01422-w (2023).
Mushtaq, S. et al. Identification of myeloperoxidase, alpha-defensin and Calgranulin in calcium oxalate renal stones. Clin. Chim. Acta. 384, 41–47. https://doi.org/10.1016/j.cca.2007.05.015 (2007).
Chutipongtanate, S., Sutthimethakorn, S., Chiangjong, W. & Thongboonkerd, V. Bacteria can promote calcium oxalate crystal growth and aggregation. J. Biol. Inorg. Chemistry: JBIC : Publication Soc. Biol. Inorg. Chem. 18, 299–308. https://doi.org/10.1007/s00775-012-0974-0 (2013).
Jongjitaree, K. et al. The application of Next-Generation sequencing in preoperative evaluation for urologic stone surgery. J. Endourol. 38, 908–915. https://doi.org/10.1089/end.2024.0167 (2024).
Li, Y., Xie, L. & Liu, C. Prediction of systemic inflammatory response syndrome and Urosepsis after percutaneous nephrolithotomy by urine culture, stone culture, and renal pelvis urine culture: systematic review and meta-analysis. Heliyon 10, e33155. https://doi.org/10.1016/j.heliyon.2024.e33155 (2024).
Castellani, D. et al. Assessing the optimal urine culture for predicting systemic inflammatory response syndrome after percutaneous nephrolithotomy and retrograde intrarenal surgery: results from a systematic review and Meta-Analysis. J. Endourol. 36, 158–168. https://doi.org/10.1089/end.2021.0386 (2022).
Talizin, T. B. et al. Postoperative antibiotic prophylaxis for percutaneous nephrolithotomy and risk of infection: a systematic review and meta-analysis. Int. Braz. J. Urol. 50, 152–163. https://doi.org/10.1590/s1677-5538.Ibju.2023.0626 (2024).
Danilovic, A. et al. One week pre-operative oral antibiotics for percutaneous nephrolithotomy reduce risk of infection: a systematic review and meta-analysis. Int. Braz. J. Urol. 49, 184–193. https://doi.org/10.1590/s1677-5538.Ibju.2022.0544 (2023).
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
Conseption and design: B.K., A.B. A., Data acquisition: B.K., A.B.A., A. Ç., M.Ö.Y. Data analysis and interpretation: B.K., A.B. A., H.S., C.B. Drafting the manuscript: B.K., A.B.A., A.Ç., M.Ö.Y. Critical revision of the manuscript for scientific and factual content; A.Ç., B.K. Statistical analysis: B.K., A.B.A., A.Ç. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Statement
This study received approval from the Non-Interventional Clinical Research Ethics Committee of Adıyaman University (decision date: January 24, 2023, meeting number: 1, decision number: 2023/1–5).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kalyenci, B., Çift, A., Aydemir, A.B. et al. Investigation of the predictive value of Hounsfield units in predicting stone culture results in urinary stone disease. Sci Rep 15, 16249 (2025). https://doi.org/10.1038/s41598-025-01214-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01214-9
Keywords
This article is cited by
-
Recognizing uric acid type of urinary stones by deep learning
Urolithiasis (2025)