Abstract
The increased risk of recurrence and metastasis are obstacles to treating TNBC. Propionate-related genes play an important role in tumor development and immune cell infiltration. The study was to identify the association between propionate-related genes and the prognosis of TNBC patients. Propionate-related genes were collected and analyzed to establish propionate-related gene characteristics. Then, the survival analysis was performed, and the responses to immunotherapies were evaluated. Furthermore, the drug sensitivity of some traditional chemotherapeutic drugs was evaluated. Finally, the hub genes were discovered and validated by in vitro experiments. Based on the five-propionate-related gene signature, TNBC patients were divided into high and low-risk groups. In addition, DEGs between the different risk groups were enriched in the biological activities associated with immunity. TNBC patients in the high-risk groups were suggested to have worse responses to immunotherapies and a poorer prognosis. SRD5A3 was finally found to be a hub gene, and in vitro experiments revealed that silencing SRD5A3 inhibited tumor cell proliferation, invasion, and migration. The five-propionate-related risk model presented novel insights into the efficacy of immunotherapy. It was found that down-regulation of SRD5A3 inhibited the growth and invasion of tumor cells, thereby affecting the prognosis of TNBC.
Similar content being viewed by others
Introduction
BC is the most prevalent malignant tumor in women. As shown in the recent statistics, BC accounted for 31% of cancer cases in 2023, and its fatality rate has risen to 15%1. According to additional studies, the number of newly diagnosed BC cases is anticipated to increase by more than 40% by 20402. Based on multiple histological characteristics and molecular biology techniques, BC can be divided into five distinct subgroups: (1) Luminal A; (2) Luminal B; (3) human epidermal growth factor receptor 2 (HER2) overexpression; (4) basal-like, also known as TNBC; and (5) normal breast-like tumors3,4. BC is heterogeneous, mainly reflecting different types of metabolism, histologic progression, metastasis, prognosis, and response to therapy5. Despite the high recurrence and metastasis rates, great advances have been made in the diagnosis and treatment of TNBC as medical technology continues to progress, which has led to an increase in the survival rate of TNBC6,7,8. However, it is still essential to screen for novel, reliable prognostic markers, and therapeutic targets for individualized and accurate treatment.
Metabolic reprogramming is a hallmark of malignancy, in which tumor cells can adapt their metabolic pathways and activity profiles to the constantly changing microenvironment and the demands of growth. Reprogrammed metabolic processes may be used in certain situations to detect, track, and manage carcinoma. Some metabolic processes were identified with vital roles in tumor cell cultivation, including aerobic glycolysis, amino acid metabolism, glutamine catabolism, and lipid metabolism9. Certain metabolites produced by metabolic reprogramming of tumors are capable of driving cancer progression and metastasis in autocrine, paracrine, and juxtacrine signaling pathways10. These metabolites can contribute to cancer progression not only by activating cellular signaling pathways but also by restructuring metabolic networks. In the past decade, tumor metabolism has received increasing attention in the diagnosis of tumors and the development of intervention therapies11. Numerous metabolic inhibitors that aim to interfere with these pathways have progressed to clinical studies9,12,13. Additionally, one potential variable influencing the antitumor immune response in cancer is the overlapping metabolic reprogramming of immune cells and cancer cells10. Increased evidence suggests that metabolites produced by metabolic reprogramming of tumors may influence immune cell infiltration and metabolism14,15,16. Consequently, it is important to investigate the mechanisms underlying the specific role of metabolic reprogramming in tumor progression and the tumor immune microenvironment.
Propionate metabolism varies among species. The growth of propionate can inhibit the fatty acid synthesis but does not affect cholesterol metabolism17. In addition, increased evidence suggests that propionate metabolism is associated with amino acid and protein degradation18. Recently, dysfunction of propionate metabolism has been found to play an important role in cancer and to provide a potential target for therapeutic strategies for recurrent and metastatic cancers. According to the study conducted by Gomes and colleagues, an imbalance in propionate metabolism increased the risk of metastasis in breast malignancies via downregulating methylmalonyl-CoA epimerase (MCEE) and stimulating the SP1/EGR1 switch, which was activated by ERK217,19. Besides regulating the invasion of BC cells, propionate could inhibit the proliferation of hepatocellular carcinoma cells via GPR43 signaling pathway activation20,21. Sara Daniela Gomes et al. found that fermentation of propionate in the colon could inhibit proliferation and induce apoptosis in colon cancer cells to further prevent cancer development22. Although propionate metabolism is closely associated with tumor development, the role that propionate metabolism-related genes play in the mechanism of TNBC has remained unclear.
Steroid 5 alpha-reductase 3 (SRD5A3) is a protein-coding gene known as a member of the SRD5A family that is localized primarily in the cytoplasm. These enzymes are involved in the conversion of steroid hormones and are typically found in the cytosolic compartment rather than being associated with the cell membrane or nucleus23. It can catalyze the androgen24. Hence, SRD5A3 is identified as a regulator of steroid hormone production and male sexual development. It has been observed that SRD5A3 is highly expressed in a variety of tumor tissues, including breast cancer, prostate cancer, endometrial cancer, and hepatocellular carcinoma, and participates in various malignant biological processes of tumors, serving as a key regulator of tumorigenesis and development25,26,27. Recent studies have revealed that high expression of SRD5A3 promoted tumor growth, and down-regulation of SRD5A3 may affect the androgen metabolism28,29. In castration-resistant prostate cancer, knockdown of SRD5A3 can reduce the proliferative vigor of prostate cancer cells30. However, there are few reports describing the expression of SRD5A3 in TNBC, and the role of SRD5A3 in TNBC is poorly understood.
In this study, the relationship between propionate metabolism-related genes and TNBC prognosis was analyzed on the basis of public databases. The specific public databases include The Cancer Genome Atlas Program (TCGA) and GeneCards databases. SRD5A3 was identified as a critical gene, and its molecular mechanism in TNBC tumor proliferation, metastasis, drug resistance, and metabolism were further elucidated by subsequent experiments.
Materials and methods
Workflow
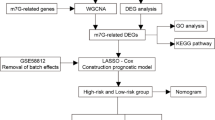
A sequential technique spanning a few phases was utilized to create a risk model for five genes related to propionate and study potential mechanisms by which these genes affect TNBC prognosis (Fig. 1).
Data acquisition
From the TCGA database, the clinical characteristics and RNA-seq expression profiles of TNBC patients were taken out to use as the training cohort. Similarly, the GEO database was used to gather the relevant data for the GSE58812 dataset, which was then used as an external validation cohort. The criteria for inclusion in the samples were as follows: (1): samples that were removed from the original tumor; (2) samples that included transcriptome expression data in addition to comprehensive prognostic information. The criterial for exclusion from the samples were as follows: The exclusion criteria for samples are as follows: (1) samples with clinical data incomplete; (2) sample with an overall survival (OS) under 60 days. As a result, for the purpose of further analysis, a total of 145 TNBC patients from the TCGA database and 105 TNBC patients from the GEO database were included in this study. Additionally, Supplemental Table 1 presents 302 propionate-related genes that were gathered from GeneCards (https://www.genecards.org/).
Construction and validation of propionate-related gene signature
A propionate-related gene signature was created using the training cohort’s RNA-seq expression data. The “edgeR” package was utilized to determine the genes associated with propionate that were expressed differently in tumor cells compared to normal breast tissues, and the results were displayed using a volcano plot. The cutoff criteria for differential expression genes (DEGs) were set as |log2fold change (FC)|> 1 and false discovery rate (FDR)-adjusted p < 0.05. After that, the univariate Cox regression analysis was used to select the prognosis-related genes and the Venn diagram was drawn to identify the intersection of DEGs and prognostic genes. Subsequently, the least absolute shrinkage and selection operator (LASSO) Cox regression analysis was applied to avoid over-fitting the model by reducing redundant genes. Consequently, the prognostic risk model was built on the basis of 5 prognostic DEGs associated with propionate. The expression levels of propionate-related independent prognostic genes and the corresponding coefficients were used to calculate the individual risk score of the signature. The formula was established as follows: \(Risk\;score = \sum\nolimits_{i = 1}^{n} {\left( {Exp_{i} {*}Coe_{i} } \right)}\) (N = 5, \(Exp_{i}\) represents the expression level of each gene, and \({Coe}_{i}\) denotes the corresponding coefficient). Notably, the expression level of each gene was extracted from the RNA-seq expression data for each sample of TCGA and GEO databases. A log2 transformation was performed on the RNA-seq data in the database, and the values obtained after the transformation were substituted into the risk score formula for the operation. As a result, the patients with TNBC in the TCGA and GEO sets were divided into low-risk and high-risk groups respectively based on the median risk scores. Afterwards, the survival analysis was conducted between the different risk groups with “survminer” package in R software. The time-dependent receiver operating characteristic (ROC) curve was employed to evaluate the predictive accuracy of the risk model. Subsequently, the principal component analysis (PCA) was performed according to the signature via the “prcomp” function of the “stats” R package. Finally, the univariate and multivariate Cox regression analyses were employed to identify the independent prognostic risk factors among the propionate-related signature as well as clinicopathological characteristics. Bilateral P < 0.05 were regarded significant, and the 95% confidence intervals were determined by calculating the hazard ratio (HR).
Construction of functional enrichment analysis
Using the R package "clusterProfiler," gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes31,32,33 (KEGG) pathway analyses were carried out on the DEGs between various risk categories in the training and validation cohorts, respectively. P < 0.05 was used to classify KEGG pathways and GO keywords as statistically significant. Moreover, gene set enrichment analysis (GSEA), a knowledge-based approach for interpreting genome-wide expression profiles, was employed on the common differentially expressed genes (co-DEGs) using the “GSEA” R package.
Evaluation of tumor immune microenvironment (TIME) and immune cell infiltration
To examine the percentage of the immune-stromal component in TIME, the “estimate” package in R software was used to estimate the number of stromal and immunological cells in malignant tumor tissues using the expression (ESTIMATE) method. Based on the propionate-related gene signature, the Stromal Score, Immune Score, and ESTIMATE Score were calculated respectively in TCGA and GEO cohorts to show the proportion of the respective compositions in the TIME. Additionally, the “GSEAbase” R package was used to perform a single-sample gene set enrichment analysis (ssGSEA) to demonstrate the enrichment of gene sets associated with immune function.
Prediction of response to immunotherapy and drug sensitivity
It has been reported that the expression levels of immune checkpoint genes may correlate with the therapeutic efficacy of immune checkpoint inhibitors. The R package “GGPUBR”, “ggplot2”, and “ggExtra” were adopted to identify the relevance of propionate-related risk score and the expression levels of the gens associated with immune checkpoint. In addition, the sensitivity to immunotherapies (anti-PD-1 and anti-CTLA-4 treatments) was examined by the tumor immune dysfunction and exclusion (TIDE) algorithm (http://tide.dfci.harvard.edu/), and the results were displayed using the TIDE score. It was considered statistically significant when P < 0.05. Utilizing information from the Genomics of Drug Sensitivity in Cancer 2 (GDSC2) database (https://www.cancerrxgene.org/), the drug sensitivity study was carried out. Using the “oncoPredict” R package, the association between the drug sensitivity and the propionate-related risk score was examined.
Cell culture and regents
All cell lines used in this study were obtained from the American Type Culture Collection (ATCC, VA, USA). The cells were authenticated through short tandem repeat profiling (STR) at the beginning of this study. Cells were maintained in culture medium (MFM-223 cells in RPMI-1640, SUM-159PT, MDA-MB-231 and MDA-MB-468 cells in DMEM) supplemented with 10% FBS (Gibco, CA, USA) and 100 units/ml penicillin/streptomycin (Beyotime, Shanghai, China), MCF10A and HS578T cells were cultured in specific expansion media from Pricella (Wuhan, China), All cells cultured in a humidified incubator with 5% CO2 at 37 °C, and were routinely tested for mycoplasma contamination.
siRNA transfection
Cells were plated in 6-well dishes and underwent transfection with either specific or Negative Control (NC) siRNAs via Lipofectamine 3000 (Invitrogen, USA) following the guidelines provided by the manufacturer. The cells were collected 48 h after transfection for additional analysis. The siRNAs were custom synthesized by GenePharma (Suzhou, China), with the sequence details provided below:
-
SRD5A3-1 sense: 5′-CCAAUGUCAUGAUUCACGUTT-3′
-
Anti-sense: 5′-ACGUGAAUCAUGACAUUGGTT-3′
-
SRD5A3-2 sense: 5′-GAGACUGGUUUGAAUAUGUTT-3′
-
Anti-sense: 5′-ACAUAUUCAAACCAGUCUCTT-3′
-
SRD5A3-3 sense: 5′-CUAGUGGUGACAAAUGUCUTT-3′
-
Anti-sense: 5′-AGACAUUUGUCACCACUAGTT-3′
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
RNA was isolated from triple-negative breast cancer cell lines using NucleoZOL Reagent (Macherey–Nagel, Düren Germany) according to the manufacturer’s instructions. The isolated RNA was reverse into cDNA by reverse transcription kit (Takara,Jiangsu, China). Gene expression levels were analyzed by qRT-PCR using SYBR Mix (Vazyme, Jiangsu, China) in the QuantStudio™ 5 Real-Time PCR system (Thermo Fisher, MA, USA). The relative expression levels of target genes were normalized to b-actin and assessed by the delta-delta-Ct (ΔΔCT) method (expressed as 2−ΔΔCT). The primers used in this study were provided below:
SRD5A3:
-
5′-GTGCCTCTACGTCAGTGTCTTCTC-3′ (Forward)
-
5′-GACCACTCCATTCCTGCCATCC-3′ (Reverse)
b-actin:
-
5′-CATGTACGTTGCTATCCAGGC-3′ (Forward)
-
5-CTCCTTAATGTCACGCACGAT-3′ (Reverse)
Cells grown curve
Cells were plated in 96-well for overnight. Cells were fixed and stained by using methanol with 0.5% crystal violet, the rest cells were changed fresh medium for 5 days. The bound crystal violet was released using a 50% acetic acid solution. Optical absorbance (OD) of bound crystals. Bound crystal violet’s optical absorbance (OD) was calculated at 570 nm.
Colony-forming assays
SUM-159PT cells and SUM-159T cells subjected to siRNAs (si-SRD5A3-1, si-SRD5A3-2, and si-SRD5A3-3) were planted in 12-well plates and cultivated for the appropriate number of days, respectively. Every other day, fresh media was changed. All cells were fixed and stained with 0.5% crystal violet in methanol solution. Images of stained plates were captured using iBright 1500 (Thermo Fisher Scientific, US).
Wound-healing assay
In brief, Cells were sown in 12-well plates and grown until they became confluent. Using sterile pipette tips, the cells were scraped to create an artificial wound. To eliminate cell debris, the cell monolayer was washed three times with PBS. Fresh culture media was added. A phase-contrast microscope (Leica, Wetzlar, Germany) was used to obtain images of the wound at 0 and 48 h.
Migration and invasion assays
In the invasion experiments, Transwell with 8 mm pore filter inserts (Corning, USA) were utilized. Cells were resuspended in serum-free media and allowed to infiltrate via none or pre-coated Matrigel inserts. At the same time, 500 ml of 10% FBS-supplemented medium was added to the lower chamber. The cells were stained with 0.5% crystal violet solution after 48 h. Cotton swabs were used to remove cells that remained in the Matrigel or were stuck to the upper surface of the filter. A light microscope was used to observe cells on the bottom of the filter.
Statistical analysis
R software (version 4.2.0) was used for all statistical studies (https://www.r-project.org/). The Chi-square test was used to determine the relationship between clinicopathological characteristics in TNBC patients in the training and validation cohorts. To evaluate the survival data, the Kaplan–Meier curve was used. Through the use of univariate and multivariate Cox regression models, independent prognostic variables were assessed. The Wilcoxon rank sum test was involved in the ESTIMATE algorithm to evaluate the infiltrating immune cells. Statistics were judged significant at P < 0.05. All procedures were carried out in accordance with the applicable rules and regulations.
Results
Identification of propionate-related prognostic DEGs
A total of 302 propionate-related genes were gathered from the Gene Cards online database, and 255 of them were found in the TCGA database. Subsequently, 112 differentially expressed genes were identified between normal and tumor tissues, comprising 69 up-regulated and 43 down-regulated genes (Fig. 2A). According to the univariate Cox regression analysis, 11 propionate-related prognostic genes were discovered and visualized by the forest plot (Fig. 2B). Then, survival analysis was performed on 11 propionate-related prognostic genes (Supplemental Fig. 1). Afterwards, seven prognosis-related DEGs were screened out from the intersection of DEGs and prognosis-related genes via the Venn diagram (Fig. 2C).
Identification of prognostic differential expression of propionate-related genes in TNBC patients. (A) Volcano plot of differential expression genes. Upregulated genes were shown by red spots, and the downregulated ones were shown by green spots. (B) The forest plot showed the HR (95% CI) and p-value of selected prognostic genes by univariate Cox regression analysis. (C) The seven overlapping genes of differentially expressed prognostic genes and prognosis-related genes were shown by a Venn diagram. (D) LASSO coefficient profiles of seven genes with p < 0.05. (E) Five-fold cross-validation result, which identified optimal values of the penalty parameter λ.
Construction and validation of a propionate related gene prognostic model
As shown in Fig. 2D and E, LASSO regression analysis was performed on 7 overlapping genes to prevent overfitting of the prediction model, and fivefold cross-validation data were generated to confirm the optimal value of the penalty parameter (λ = 0.02128192). Therefore, 5 propionate-related prognostic genes were chosen for risk model establishment: PLAU, IFNG, GLDC, SRD5A3, and SRD5A2. As a result, a risk model for predicting the clinical outcomes of TNBC patients was developed based on the expression of five core genes and their regression coefficients: Risk score = (0.1377 × expression level of PLAU) + (− 0.1086 × expression level of IFNG) + (− 0.2110 × expression level of GLDC) + (0.3126 × expression level of SRD5A3) + (− 0.1242 × expression level of SRD5A2). The training set and validation set are then split into high-risk and low-risk groups, respectively, based on the median value of the risk score. In the training cohort, there were 72 patients in the high-risk group and 73 in the low-risk group, with the median risk score being 2.37952. In the validation cohort, there were 52 patients in the high-risk group and 53 in the low-risk group, with the median risk score being 1.524054. According to the Kaplan–Meier survival analysis, TNBC patients with high risk had a worse OS than patients with low risk (Fig. 3A and B). Subsequently, the time-dependent ROC analysis was conducted at 1, 2, and 3 years to further evaluate the predictive value of this propionate-related signature. As a result, the risk model had been validated to be robust efficient by the area under the curve (AUC) in predicting the OS for TNBC patients (training set: AUC = 0.802, 0.906, and 0.845 at 1, 2, and 3 years, respectively; validation set: AUC = 0.875, 0.747, 0.640 at 1, 2, and 3 years, respectively, Fig. 3C and C. Following that, PCA demonstrated that patients were grouped in the opposing manner based on different risk groupings. The PCA results are comparable between the training set and the validation set (Fig. 3E and F).
Assessment of the predictive effectiveness of the propionate-related gene risk model in the training cohort and validation cohort. (A, B) Kaplan–Meier survival curves for TNBC patients in the training cohort (A) and validation cohort (B). (C, D) ROC curve analysis for the accuracy of the signature to predict the prognosis of TNBC patients at 1, 2, and 3 years in the training (C) and validation (D) cohorts. (E, F) PCA analysis plot of the training cohort (E) and the validation cohort (F).
In addition, univariate and multivariate Cox regression analyses were carried out to assess the independent predictive capability of 5 propionate-related gene signature. The result of univariate Cox regression analysis revealed that risk score, T (tumor sizes), N (lymph node status), and Stage (AJCC stage) were associated with the prognosis of TNBC patients (Supplemental Fig. 2A, p< 0.001, HR 3.760, 95% CI 2.232–6.332; p = 0.011, HR 3.116, 95% CI 1.301–7.462; p < 0.001, HR 4.249, 95% CI 2.473–7.300). After that, multivariate Cox regression analysis demonstrated that the characteristic risk score and Stage may be employed as independent predictive markers for OS rate in patients with TNBC (Supplemental Fig. 2B, p < 0.001, HR 5.784, 95% CI 2.630–12.721; p = 0.008, HR 7.883, 95% CI 1.734–35.830).
GO and KEGG analysis of 5-propionate-related-gene signature
In the high-risk group and the low-risk group, GO enrichment analysis and KEGG31,32,33 pathway analysis were carried out to investigate relevant biological processes and signaling pathways. Figure 4A and B depict the outcomes of the GO enrichment terms, including biological process (BP), molecular function (MF), and cellular component (CC). In addition, according to KEGG analysis, numerous immune-related signaling pathways were considerably enriched, associated with immune cell activation, immunological regulation, and immune response (Fig. 4C and D). Furthermore, we assessed the Hallmark pathway using GSEA. The GSEA data showed that the high-risk group had a lot more activity in fatty acid metabolism, glycolysis, xenobiotic metabolism, and other processes (Supplemental Fig. 3).
Representative results of GO and KEGG enrichment analysis in the training and validation sets. (A, B) GO analysis showed that many immune-related biological processes were enriched in the training (A) and validation (B) cohorts. (C, D) KEGG analysis showed that some immune-related pathways and cancer proliferation pathways were enriched in the training (C) and validation (D) cohorts. The gene ratio refers to the ratio of DEGs between high- and low-risk groups to the set of genes for each GO or KEGG pathway.
The relevance of ESTIMATE scores and 5-propionate-related-gene signature
The ESTIMATE algorithm was used to calculate the stromal score, immune score, and ESTIMATE score of each sample, and the Wilcoxon rank sum test was used to determine the difference of infiltrating immune cells between the high-risk group and the low-risk group. As a result, the immune score in the low-risk group in the TCGA cohort was considerably higher (p < 0.05, Fig. 5B). However, the stromal and ESTIMATE scores were not statistically different between the high- and low-risk groups (Fig. 5A and C). In the GEO cohort, there were significant differences between the immune score and ESTIMATE score in the high and low risk categories (p < 0.05, Fig. 5E and F), but no statistical difference in the stromal score (Fig. 5D).
Features of the tumor immune microenvironment (TIME)
The difference of immune characteristic was investigated between low-risk and high-risk by ssGSEA in training and validation cohorts. The infiltrations of some immune cells were higher in the lower-risk cohort, for instance aDCs, B cells, CD8 + T cells, follicular helper T cells (Tfh cells), T helper 1 cells (Th1 cells), tumor infiltrating lymphocytes (TILs), and regulatory T cells (Tregs) (Fig. 6A and B). Although, the levels of some of these changes were not highly significant, this difference was statistically significant (Wilcoxon rank sum test, * p < 0.05, ** p < 0.01, *** p < 0.001). Additionally, numbers of immune signatures were activated in the low-risk groups, including APC co-inhibition, APC co-stimulation, checkpoint, HLA, inflammation promotion, MHC class I, T cell co-inhibition, T cell co-stimulation, and IFN response type I (Fig. 6C and D).
The association between TIME and the propionate-related risk score in TNBC. (A and B) The difference in immune cell infiltration between the high-risk group and the low-risk group of training (A) and validation (B) sets. (C and D) Distinguishing of enrichment of immune-associated signatures between different risk groups of training (C) and validation (D) sets. *p < 0.05; **p < 0.01; ***p < 0.001 (Wilcoxon rank sum test).
Differences in expression of immune checkpoint related genes
We evaluated the expression of immune checkpoint markers in various risk populations in light of the crucial role that immune checkpoint inhibitor-based immunotherapy plays. In the study, the expression of CD274, CTLA4, and LAG3 were further examined. In both the training set and the validation set, the expression of the three immune checkpoint related genes were remarkably higher in the low-risk groups (P < 0.05, Fig. 7A–C,E–F). Besides, the higher TIDE scores were identified in the high-risk groups (P < 0.05, Fig. 7D and H).
Immune checkpoint-related genes and drug-sensitivity prediction in TNBC patients. (A–H) The different expression levels of immune checkpoint genes and the different TIDE scores between high-risk and low-risk groups in the training (A, B, C, and D) and validation (E, F, G, and H) cohorts, p < 0.05 was regarded statistically significant. (I and J) The difference in the estimated IC50 of the six representative chemotherapeutic drugs between the two risk groups (p < 0.001 was represented as *** and p < 0.05 was represented as *).
The relationship between propionate-related signature and chemotherapeutic drugs
We also assessed how TNBC patients in the high-risk and low-risk groups responded to some chemotherapeutic treatments. The relationship between the risk score and the IC50 of six chemotherapy medicines was analyzed and presented in Fig. 7I and J, including Palbociclib, Paclitaxel, Epirubicin, Docetaxel, Cyclophosphamide, and Cisplatin. According to the findings, TNBC patients in the low-risk groups were more sensitive to Cyclophosphamide and Cisplatin.
SRD5A3 was identified as a targeted gene associated with the progression of TNBC
The random forest analysis was conducted to rank the associated genes and screen out the targeted gene for further study (Fig. 8A). In conjunction with LASSO regression analysis, SRD5A3 was discovered to be the targeted gene. As shown in Fig. 8B and C, based on the propionate-related signature, the expression of SRD5A3 was positively correlated with the risk score (P < 0.05).
SRD5A3 is associated with the proliferation and migration of TNBC
GEPIA (Gene Expression Profiling Interactive Analysis) database illustrated that SRD5A3 was upregulated in most cancers, including breast cancer (Supplemental Fig. 4). At the same time, univariate analysis indicated that SRD5A3 was associated with survival status, N (lymph node status), and Stage (AJCC stage) (Supplemental Table 2). Thereafter, the expression of SRD5A3 was validated in TNBC cell lines by qPCR analysis. As a result, SRD5A3 was considerably overexpressed in SUM-159PT compared with other TNBC cell lines (Fig. 9A). As shown in Fig. 9B, SRD5A3 was suppressed successfully in the SUM-159PT cell line by specific siRNAs (si-SRD5A3-1, si-SRD5A3-2, and si-SRD5A3-3). Cells grown curve and colony formation assays demonstrated that in SUM-159PT cells with or without siSRD5A3 (Fig. 9B), inhibition SRD5A3 decreased the proliferation of SUM-159PT cell lines (Fig. 9C and D). Following that, wound healing experiments revealed that SRD5A3 downregulation decreased tumor cell growth (Fig. 9E). Furthermore, Lower SRD5A3 expression also limited the SUM-159PT cells invasion ability (Fig. 9F). Together, these data suggest that SRD5A3 is essential for the proliferation and migration in TNBC.
Knockdown of SRD5A3 inhibits cell migration and proliferation. (A) The qPCR analysis of SRD5A3 expression in the normal mammary epithelial cell lines (MCF10A) and TNBC cell lines (SUM-159PT, MDA-MB-468, MFM-223, MDA-MB-231 and HS578T). (B) The inhibition efficiency of SRD5A3 by specific siRNAs (si-SRD5A3-1, si-SRD5A3-2, and si-SRD5A3-3) was verified by qPCR analysis in SUM-059PT cell lines. (C and D) The cell grown curve and a colony-forming test were performed to evaluate the proliferation of SUM-159PT cells after silencing SRD5A3. (E) Wound-healing assays in SUM-159PT cells transfected with si-SRD5A3-1, si-SRD5A3-2, and si-SRD5A3-3 (40 × magnification). (F) Migration and invasion assays in SUM-159PT cells transfected with si-SRD5A3-1, si-SRD5A3-2, and si-SRD5A3-3 (40 × magnification).
Discussion
As the most frequent malignant tumor in women, BC is closely correlated with alterations in the microenvironment34. Recent research has sought to identify molecular characteristics and prospective treatment targets for BC patients, as well as to develop unique and efficient gene model risk models for malignant tumors35,36. Given the evidence, propionate metabolism is critical for tumor invasion and progression19. However, the potential prognostic value of propionate-related genes has not been clarified in TNBC.
In the current study, a propionate-associated gene risk model was established from TCGA data involving five relevant genes: PLAU, IFNG, GLDC, SRD5A3, and SRD5A2. According to the median risk score of the training set, the TNBC patients were divided into high-risk and low-risk groups. After that, TNBC patients in the low-risk group showed a better prognosis according to the Kaplan–Meier curve. Due to the intricacy of the tumor microenvironment (TME), we subsequently performed GO analysis and KEGG31,32,33 pathway analysis and found that numerous immune regulatory pathways were enriched. Based on the aforementioned findings, the ssGSEA and ESTIMATE algorithms were further used to investigate the correlation between immune cell infiltration characteristics and the propionate-related risk model. Besides, the TIDE algorithm was employed to predict the treatment response to ICBs in TNBC patients with different risk scores. In our study, the high-risk groups had much higher TIDE scores and significantly lower levels of some immune checkpoint-related genes. Additionally, we found that TNBC patients in low-risk groups were identified as more sensitive to cyclophosphamide and cisplatin. According to our findings, the propionate-related gene risk model may be used to predict immunotherapeutic outcomes for TNBC patients and may pave the path for further investigation into cutting-edge therapeutic modalities.
As a member of the SRD5A family, SRD5A3 can catalyze the conversion of testosterone to dihydrotestosterone and control the production of steroid hormones27,37,38 Given the evidence, the high expression level of SRD5A3 has been identified in several cancers, including hepatocellular carcinoma, endometrial cancer, and prostate cancer26,39. According to Zhang et al.24 the mRNA and protein of SRD5A3 were highly expressed in the BC tissues and associated with tumor proliferation and lymph node metastasis, which was consistent with our findings. SRD5A3 may serve as an oncogene and further become a prognostic biomarker as well as a therapeutic target for TNBC. Furthermore, upregulation of SRD5A3 expression was observed to be correlated with resistance to PARP inhibitors. The PARP1-SRD5A3 pair, a mutation and copy number-driven gene pair, is significantly related to poorer prognostic outcomes through regulating androgen receptors and induce BC cell proliferation40. Notably, SRD5A3 can suppress apoptosis and induces cell proliferation by converting testosterone to dihydrotestosterone41. Consequently, not only in TNBC but also in other malignant tumors, SRD5A was identified as associated with tumor proliferation and metastasis. Li and colleagues found that knockdown of SRD5A3 expression could inhibit the growth and cell proliferation of prostate cancer29,40. In addition, the study on multiple myeloma conducted by Dou et al.41 revealed that suppression of SRD5A1 could promote cellular apoptosis and autophagy through the PI3K/Akt/mTOR signaling pathway. Besides, SRD5A2 polymorphism has been linked to prostate, breast, and liver cancers, according to numerous studies to date42,43,44.
In our study, SRD5A3 mRNA expression was significantly elevated in SUM-159PT cells compared to normal breast cells (MCF10A). This indicates that SRD5A3 is overexpressed in tumor cells and may be associated with tumorigenesis and progression. However, in other TNBC cell lines, SRD5A3 expression was not markedly increased and was significantly lower than in SUM-159PT cells. This suggests that SRD5A3 expression may be related to the heterogeneity of TNBC tumors. Further investigation into the expression patterns and mechanisms of SRD5A3 across different TNBC subtypes could provide valuable insights. Elucidating the role of SRD5A3 may offer new strategies for the classification and treatment of TNBC. In addition, in SUM-159PT cells treated with si-SRD5A3-1, SRD5A3 mRNA expression was reduced to the lowest levels observed. However, this treatment group did not show the lowest proliferation rate in the proliferation assays. Despite this discrepancy, we consider these findings to be meaningful. SiRNAs target distinct sites on the SRD5A3 mRNA, thereby modulating gene expression by interfering with different regions of the mRNA. This partial interference does not result in complete gene inactivation. Consequently, the decrease in SRD5A3 expression induced by these siRNAs does not fully represent the overall impact of SRD5A3 on cell proliferation. This observation illustrated an inherent limitation of small interfering RNAs. Nonetheless, it is evident that alterations in SRD5A3 expression can significantly affect the proliferation and other phenotypes of TNBC cells. Future studies should aim for a complete knockout of SRD5A3 to address this limitation and ensure more robust and accurate results.
This study aims to establish a propionate-related gene signature to predict the prognosis of TNBC patients. Notably, based on the signature, SRD5A3 was identified as a biomarker gene associated with the growth and invasion of TNBC. However, it still has some limitations that are worth discussing. First, the prognostic risk model was constructed on the basis of the online public database. A limitation of this study is the lack of external validation of the risk model using large, prospective clinical cohorts. Second, it is essential to conduct further experimental investigations to elucidate the role of SRD5A3 in propionate metabolism and other tumor-associated metabolic pathways. Third, SRD5A3 expression may vary across different TNBC cell lines, which could have important implications for TNBC classification and merits additional exploration. Finally, it is of significant importance to further explore the specific function of SRD5A3 in TNBC and the regulatory mechanisms of associated signaling pathways.
Conclusion
The 5-propionate-related gene risk model was established to predict the prognosis of TNBC patients. Upregulation of SRD5A3 was revealed in TNBC, and its suppression inhibited tumor cell growth and invasion, which in turn affected the prognosis of TNBC.
Data availability
The datasets analyzed during the current study are available in the TCGA database (http://cancergenome.nih.gov/), GEO database (https://www.ncbi.nlm.nih.gov/geo/), and GEPIA database (http://gepia.cancer-pku.cn/).
References
Siegel, R. L. et al. Cancer statistics, 2023. CA Cancer J. Clin. 73(1), 17–48 (2023).
Arnold, M. et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 66, 15–23 (2022).
Nagini, S. Breast cancer: Current molecular therapeutic targets and new players. Anti-Cancer Agents Med. Chem. 17(2), 152–163 (2017).
Choi, J., Jung, W.-H., Koo, J. S. Clinicopathologic features of molecular subtypes of triple negative breast cancer based on immunohistochemical markers. (2012).
Ossovskaya, V. et al. Exploring molecular pathways of triple-negative breast cancer. Genes Cancer 2(9), 870–879 (2011).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6), 394–424 (2018).
Goff, S. L. & Danforth, D. N. The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin Breast Cancer 21(1), e63–e73 (2021).
Park, M. et al. Breast cancer metastasis: Mechanisms and therapeutic implications. Int. J. Mol. Sci. 23(12), 6806 (2022).
Faubert, B., Solmonson, A. & Deberardinis, R. J. Metabolic reprogramming and cancer progression. Science 368(6487), eaaw5473 (2020).
Xia, L. et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 20, 1–21 (2021).
Gandhi, N. & Das, G. M. Metabolic reprogramming in breast cancer and its therapeutic implications. Cells 8(2), 89 (2019).
García-Castillo, V. et al. Targeting metabolic remodeling in triple negative breast cancer in a murine model. J. Cancer 8(2), 178 (2017).
Lapa, B. et al. Acute myeloid leukemia sensitivity to metabolic inhibitors: Glycolysis showed to be a better therapeutic target. Med. Oncol. 37(8), 72 (2020).
Hurley, H. J. et al. Frontline Science: AMPK regulates metabolic reprogramming necessary for interferon production in human plasmacytoid dendritic cells. J. Leucocyte Biol. 109(2), 299–308 (2021).
Guerra, L., Bonetti, L. & Brenner, D. Metabolic modulation of immunity: A new concept in cancer immunotherapy. Cell Rep. 32(1), 107848 (2020).
Jia, Y. et al. Hyperactive PI3Kδ predisposes naive T cells to activation via aerobic glycolysis programs. Cell Mol. Immunol. 18(7), 1783–1797 (2021).
Hosseini, E. et al. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 69(5), 245–258 (2011).
Santos, L. P. A. et al. Propionate metabolism in a human pathogenic fungus: Proteomic and biochemical analyses. IMA Fungus 11(1), 1–16 (2020).
Gomes, A. P. et al. Altered propionate metabolism contributes to tumour progression and aggressiveness. Nat. Metab. 4(4), 435–443 (2022).
Yoshida, H., Ishii, M. & Akagawa, M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch. Biochem. Biophys. 672, 108057 (2019).
Lu, X. et al. Bornlisy attenuates colitis-associated colorectal cancer via inhibiting GPR43-mediated glycolysis. Front. Nutr. 8, 706382 (2021).
Gomes, S. D. et al. The role of diet related short-chain fatty acids in colorectal cancer metabolism and survival: Prevention and therapeutic implications. Curr. Med. Chem. 27(24), 4087–4108 (2020).
Zhang, Y.-P. et al. Over-expression of SRD5A3 and its prognostic significance in breast cancer. World J. Surg. Oncol. 19, 1–11 (2021).
Zhang, Y.-P. et al. Over-expression of SRD5A3 and its prognostic significance in breast cancer. World J. Surg. Oncol. 19(1), 1–11 (2021).
Uemura, M. et al. Novel 5α-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 99(1), 81–86 (2008).
Wang, Z.-H. et al. Identification of novel cell glycolysis related gene signature predicting survival in patients with endometrial cancer. Cancer Cell Int. 19(1), 1–13 (2019).
Mai, Q. et al. Steroid 5 alpha-reductase 3 (SRD5A3) promotes tumor growth and predicts poor survival of human hepatocellular carcinoma (HCC). Aging (Albany NY) 12(24), 25395 (2020).
Chen, E. et al. Overexpression of SRD5A3 in hepatocellular carcinoma and its molecular mechanism: A study of bioinformatics exploration analysis with experimental verification. Evid. Based Complement. Altern. Med. eCAM 2022(1), 7853168 (2022).
Li, J. et al. Androgen regulation of 5α-reductase isoenzymes in prostate cancer: Implications for prostate cancer prevention. PLoS ONE 6(12), e28840 (2011).
Kong, P. et al. Emerging proteins in CRPC: Functional roles and clinical implications. Front. Oncol. 12, 873876 (2022).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28(11), 1947–1951 (2019).
Kanehisa, M. et al. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 53(D1), D672–D677 (2025).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1), 27–30 (2000).
Harbeck, N. et al. Breast cancer. Nat. Rev. Dis. Primers. 5(1), 66 (2019).
Marchese, F., Raimondi, I. & Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 18(1), 206 (2017).
Chi, Y. et al. Long non-coding RNA in the pathogenesis of cancers. Cells 8(9), 1015 (2019).
Makridakis, N. et al. Association of mis-sense substitution in SRD5A2 gene with prostate cancer in African-American and Hispanic men in Los Angeles, USA. Lancet (London, England) 354(9183), 975–978 (1999).
Luo, J. et al. Decreased gene expression of steroid 5 alpha-reductase 2 in human prostate cancer: Implications for finasteride therapy of prostate carcinoma. Prostate 57(2), 134–139 (2003).
Pchejetski, D. et al. Circulating chromosome conformation signatures significantly enhance PSA positive predicting value and overall accuracy for prostate cancer detection. Cancers (Basel) 15(3), 821 (2023).
Wang, L. et al. Functional regulations between genetic alteration-driven genes and drug target genes acting as prognostic biomarkers in breast cancer. Sci. Rep. 12(1), 10641 (2022).
Dou, R. et al. Suppression of steroid 5α-reductase type I promotes cellular apoptosis and autophagy via PI3K/Akt/mTOR pathway in multiple myeloma. Cell Death Dis. 12(2), 206 (2021).
Ding, Y. et al. THRSP identified as a potential hepatocellular carcinoma marker by integrated bioinformatics analysis and experimental validation. Aging (Milano) 14(4), 1743–1766 (2022).
Shiota, M. et al. The prognostic impact of serum testosterone during androgen-deprivation therapy in patients with metastatic prostate cancer and the SRD5A2 polymorphism. Prostate Cancer Prostat. Dis. 19(2), 191–196 (2016).
Francis, A. et al. SRD5A2 gene polymorphisms affect the risk of breast cancer. Breast (Edinburgh, Scotland) 23(2), 137–141 (2014).
Funding
This study was supported by Natural Science Foundation of Fujian Province (2023J01746), Fujian Provincial Health Commission Science and Technology Plan Project (2022GGA037) and Natural Science Foundation of Fujian Province (2023J01729).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Kelun Pan, Pengjun Qiu, Huikai Liang, Ningning Wan, Zundong Liu, and Qiaonan Guo. The first draft of the original draft was written by Kelun Pan, Pengjun Qiu, Zundong Liu and Qiaonan Guo and all authors commented on previous versions of the manuscript. Kelun Pan and Pengjun Qiu contributed equally to this work and shared the co-first authorship. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors agreed to publish.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pan, K., Qiu, P., Liang, H. et al. Machine learning identifies SRD5A3 as a propionate-related prognostic biomarker in triple-negative breast cancer. Sci Rep 15, 18911 (2025). https://doi.org/10.1038/s41598-025-01274-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01274-x