Abstract
The valorization of wood waste as a sustainable bacterial feedstock for the production of Polyhydroxybutyrate (PHB) is explored in this study, aiming to provide an environmentally friendly alternative to conventional plastics. Wood waste, treated with 4% sulfuric acid, served as the carbon source for isolating bacteria from Jalandhar waste streams, with the strain Klebsiella sp. MK3 identified as the most effective in PHB production after 16s rRNA sequencing. Analytical methods including the Molisch test, DNS, and sugar utilization tests confirmed sugar presence and consumption by the bacterial isolate. Media optimization using Design Expert 12.0 utilized a quadratic model, achieving a robust fit with an R² value of 98.6%. Optimization via Plackett-Burman design and response surface methodology enhanced PHB yield to 4.37 mg/mL, a significant increase over previous benchmarks. This yield was achieved under optimal conditions of 1.7% carbon concentration, 0.105% nitrogen concentration, and a constant temperature of 37 °C. Qualitative analysis of PHB by UV-Vis spectroscopy, FTIR, and NMR confirmed its purity and composition. The study highlights the potential of wood waste and wastewater as substrates for cost-effective PHB production, with significant applications in packaging, agriculture, medicine, and more, thus promoting reduced reliance on non-renewable resources and advancing sustainability goals.
Similar content being viewed by others
Introduction
Plastic pollution represents a severe environmental issue due to its persistent, non-degradable nature in the environment1. The extensive use of traditional plastics, derived from non-renewable petroleum resources, has led to widespread environmental degradation, contributing significantly to landfills and oceanic waste. The durability that makes plastics so useful also renders them a long-lasting pollutant, affecting wildlife and ecosystems globally1,2.
Traditional plastics, derived from fossil fuels, have contributed significantly to environmental pollution due to their non-biodegradable nature, resulting in microplastic accumulation and ecosystem disruption3,4. Polyhydroxybutyrate (PHB), a microbial biopolymer, offers a sustainable alternative, being fully biodegradable and biocompatible. Produced by strains like Cupriavidus necator and Bacillus megaterium under nutrient-limited conditions, PHB exhibits thermoplastic properties comparable to polypropylene5,6. It degrades naturally in the environment through microbial action, transforming into water, carbon dioxide, and biomass under aerobic conditions, and methane under anaerobic conditions4. Significant microbial strains capable of PHB production include Bacillus spp., Cupriavidus necator, and certain genetically engineered strains of E. coli, which are known for their efficiency in PHB synthesis5. PHB is not only comparable to conventional plastics in terms of physical properties such as thermoplasticity and biocompatibility but also features material characteristics that are favorable for medical applications, packaging, agricultural films, and even as a feedstock for 3D printing6.
Prior studies have demonstrated the effective utilization of wood waste as a substrate for the production of polyhydroxyalkanoates (PHAs), emphasizing its potential to enhance yield while promoting sustainability. For instance, the study by Li et al. (2021) highlighted the conversion of wood hydrolysates into PHAs using microbial fermentation. The hydrolysates provided essential carbon sources such as glucose and xylose, enhancing PHA production without requiring expensive purification steps. Additionally, wood waste utilization addresses dual objectives: reducing environmental waste and providing an economical alternative to refined substrates. Optimized hydrolysis conditions have been shown to increase sugar release, directly correlating to improved microbial growth and PHA accumulation7,8. However, research on using wood waste is less extensive despite its rich carbon content, predominantly composed of cellulose and hemicellulose, which can be hydrolyzed into fermentable sugars using acid treatments like sulfuric acid. This process enhances the accessibility of carbon, improving the substrate’s fermentability and, subsequently, the yield of PHB. Previous study demonstrated a significant enhancement in PHB yield when utilizing treated wood waste, underscoring its potential as an effective feedstock7. In India, wood like Sal and tea wood are commonly used in timber mills for furniture and various purposes. The ready availability of this wood makes it a suitable choice as feedstock for the proposed research.
This research aims to further explore the valorization of wood waste for PHB production by optimizing bioprocess conditions through factorial design and Response Surface Methodology (RSM). It seeks to address the challenges associated with the low fermentability of lignocellulosic materials and to achieve an efficient, cost-effective, and environmentally friendly production process. The novelty of this study lies in its approach to integrating wood waste valorization with microbial synthesis of PHB, aiming to contribute substantially to sustainable waste management and plastic production.
Materials and methods
Sample collection, isolation, and screening of PHB producing bacteria
Sewage water samples were collected using grab sampling technique from four locations of Jalandhar, Punjab (Sample 1 (L-1) location: latitude 31° 16.1126’ N, longitude 75° 35.7164’ E, Sample 2 (L-2) location: latitude 31° 20.2469’ N, and longitude 75° 40.1239 E, Sample 3 (L-3) location: latitude 31° 20.4499’ N, and longitude 75° 31.7189’ E, Sample 4 (L-4) location: latitude 31° 13.6414’ N, and longitude 75° 38.2179 E)9. All the sample collected were inoculated into sterile nutrient broth and labeled as mother samples and subjected to serial dilution, and aliquot of 0.1 mL was spread on MSM agar along with 10 g/L sucrose, 0.225 mg/L Nile blue, and 15 g/L agar. Incubation at 30 °C for 48 h facilitated primary screening, where Nile blue incorporated into bacterial cytoplasm during growth. Under U.V. light, plates exhibited fluorescence, allowing identification of fluorescent colonies as potential PHB producers10. Sudan Black staining was conducted on heat-fixed samples using a solution made by dissolving 0.3 g of Sudan Black B in 75 mL of 95% ethanol, then diluting it to 100 mL with distilled water. The samples were stained with this solution for 10 min, dried using filter paper, and clarified with drops of xylene. After another round of drying, they were counterstained with a 0.5% aqueous safranine solution for 5 s. Blue black colonies were observed and picked as confirmation of PHB producers11.
PHA production, extraction, and identification
Production of PHB using MSM media
The isolates were inoculated in test tubes containing 10 mL of mineral salts medium (MSM) for PHB production. The inoculated tubes were incubated at 37 °C and 150 rpm for a duration of 72 h. The MSM composition for PHB production included ammonium chloride (1.5 g/L), yeast extract (0.16 g/L), KH2PO4 (1.52 g/L), Na2HPO4 (4.0 g/L), MgSO4·7H2O (0.52 g/L), CaCl2 (0.02 g/L), glucose (20 g/L), and a trace element solution (0.1 mL). The trace element solution contained ZnSO4·7H2O (0.13 g/L), FeSO4·7H2O (0.02 g/L), (NH4)6MO7O24. 4H2O (0.06 g/L), and H3BO3 (0.06 g/L). Glucose and trace element solutions were autoclaved separately and reconstituted before inoculation12,13.
Extraction process of PHB using hypochlorite method
Following incubation at 37 °C for 72 h, the production media was centrifuged at 12,000 rpm for 15 min. Pellets obtained were treated with 13% sodium hypochlorite for cell digestion, followed by incubation at 50 °C for 2 h. The resulting cell extract was centrifuged at 8000 RPM for 15–20 min, followed by separate washes with distilled water, acetone, and methanol. The residual white-colored precipitates were identified as PHB granules14. The heated chloroform was carefully placed in a sterile petri plate or watch glass, with PHB granules as chloroform has ability to dissolve PHB. After some hours of incubation at room temperature, chloroform eventually evaporates and a thin, light-colored PHB film became visible in the Petri plate15.
The dry biomass of the extracted PHB was quantified in g/L. The residual biomass was determined as the difference between the dry cell biomass and the dry biomass of PHB. The percentage of intracellular PHB accumulation was computed as the proportion of PHB in the dry cell weight. (DCW = Total dry cell weight)16.
Genotypic characterization Pf the potent strain by 16s rRNA sequencing
The PCR process began with the extraction and purification of DNA from an overnight bacterial culture using a series of steps involving centrifugation and enzymatic treatments. Universal primers (U5F and U4R) targeting conserved regions of the 16 S rRNA gene were employed for PCR amplification, ensuring amplification from any bacterial species. Each PCR reaction contained 100 ng of purified DNA in a 20 µl reaction mixture consisting of 10X Buffer, deoxy-nucleoside tri-phosphates, primers, and Taq polymerase. PCR cycles included initial denaturation at 95 °C for 3 min, followed by 32 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min. A final extension cycle at 72 °C for 10 min concluded the amplification. Analysis of the PCR products was performed by electrophoresis on 1.5% agarose gel containing ethidium bromide, followed by visualization under UV illumination. This comprehensive PCR protocol facilitated the amplification of a segment (~ 1200 bp) of the 16 S rRNA gene, enabling the identification and characterization of bacterial species present in the initial culture17.
Hydrolysis of wood waste
Sal and teak wood waste obtained from Phagwara and Jalandhar was subjected to collection and sieving. Subsequently, 10% w/v of the wood waste was blended with waste-water water (100 mL) and 4% v/v sulphuric acid. The resulting mixture was incubated at 120 degrees for 1 h. Following incubation, the mixture was filtered using muslin cloth, separating the supernatant. Quantitative and qualitative assessments, including the Molisch test, DNS spectrophotometry, and sugar utilization test, were then conducted on the supernatant to determine sugar concentration after adjusting pH at 712,13.
Optimization using statistical experimental design
The utilization of Design of Experiments (DoE) or Statistical Experimental Design (SED), particularly through the Plackett and Burman (P.B.) orthogonal arrays, offers an efficient method for screening influential factors in PHB production. By systematically organizing experiments and minimizing the number of variables tested, this approach provides accurate estimations of direct effects. Employing PBD, the fundamental values of independent variables were coded into High (+ 1) and Low (– 1) levels, and the resulted response was analysed relatively at 95% significance level. Overall, this strategy aids in selecting relevant factors, defining appropriate ranges, and preventing experimental failures, thus facilitating the development of sustainable PHB production processes18.
Biopolymer synthesis was conducted in a 100 mL Erlenmeyer flask with a 50 mL working volume for the minimal medium. The formulation of the production media, as shown in Table 1, about parameters such as carbon and nitrogen concentrations, pH, temperature, ferric citrate, incubation period, inoculum size, KH2PO4, Na2HPO4, MgSO4.7H2O, and trace elements, was determined utilizing Design Expert 12.0 software. To ensure reproducibility, each step of the experiment in the flask was carried out in triplicates. Further optimization, considering the most influential factors, was conducted using Response Surface Methodology (RSM)19. RSM, coupled with a well-designed experiment, has become a prevalent approach for formulation optimization. Carbon concentration (1–4%), nitrogen concentration (0.01–0.2%), and temperature (28 °C to 40 °C) were identified as the three primary factors significantly affecting the production rate of PHB. 6 center points,2 axil (star) point replications were accounted for this design. DoE of these factors is as mentioned in the Table 1.
Qualitative analysis of PHB using UV-Vis spectroscopy
The granules or PHB films were blended in 10 mL of concentrated sulfuric acid and subjected to a water bath for 15 min at 100 °C. This process transformed the polymer into brown-colored crotonic acid, which was subsequently cooled and analyzed using a UV spectrophotometer at 235 nm, comparing it to the sulfuric acid blank. The UV spectrophotometer quantified the PHB content in the extracted samples at 235 nm, referencing the standard graph of 3-hydroxybutyric acid20,21.
Fourier transform-infrared spectroscopy (FT-IR analysis)
The PHB extracted from the organism was analysed by Fourier-transform infrared spectroscopy (FT-IR) analysis using JASCO FT/IR instrumentation. The analysis was conducted within a spectral range of 4000 –400 cm⁻¹ to validate the functional groups present in the extracted polymer22.
1H and 13C NMR
To conduct carbon-13 (C-NMR) analysis, we followed the method outlined by23 This involves preparing a solution of PHB by dissolving it in a deuterated solvent, typically deuterated chloroform, and then transferring it into an NMR tube. The spectrum is then obtained using an appropriate NMR spectrometer, and chemical shifts are compared to the solvent peak. Proton (1H-NMR) analysis follows a similar process, where the sample is dissolved in a deuterated solvent like deuterated chloroform. Both 13C and 1H-NMR spectra provide valuable insights into the polymer’s structure, with 13C-NMR revealing carbon connections and 1H-NMR offering information on hydrogen environments.
Results and discussion
Isolation of PHB producer
Following the Nile blue staining isolation process, 26 isolates were identified on agar plates, labelled as samples 1 to 26 or S-1 to S-26. Subsequent Sudan black staining verified 19 isolates, all of which exhibited positive indications of being potential PHB producers, as detailed in Table 2.
Extraction and quantification of PHB
After the extraction process and washing, a white precipitate remained, which was estimated as the pure PHB content. It was measured for further study of the production rate of PHB as shown in Table 3.
After overnight incubation of chloroform and PHB mixture, chloroform evaporates and a distinct, light white-coloured, thin layer of PHB film became visible in the Petri plate, upon meticulous examination of the DCW, it was evident that isolate no. 3 exhibited the highest production of PHB. Consequently, isolate no. 3 was identified as the optimal strain for subsequent production, utilizing wood waste as the substrate medium, and subjected to statistical analysis. To identify the isolate, 16 S sequencing was carried out.
16S rRNA sequencing
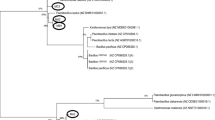
After observing the peak production rate of around 67% demonstrated by isolate M3, we proceeded with further optimization. A 16s sequencing analysis was performed to characterize the bacterial isolate and generate a phylogenetic tree using the acquired sequences. The analysis of Blast results and the phylogenetic tree indicated a significant similarity between the M3 strain and three specific Klebsiella strains, as illustrated in Fig. 1. As a result, the taxonomic classification of the isolate was determined as Klebsiella sp. MK3. The same has been submitted to NCBI and accession number is PP109354.
Qualitative and quantitative on wood waste supernatant
The Molisch test confirmed the presence of carbohydrates through a purple ring. Absorbance values recorded for sal wood, teak wood, and their mixture were 0.722, 0.741, and 0.903, respectively, at 540 nm post one-third dilution for the mixture. Comparison with a standard DNS assay graph (Fig. 2) indicated that hydrolyzed sal and teak wood contained approximately 36 mg/mL of sugar25. During fermentation, the selected isolates demonstrated the ability to utilize sugars from the wood hydrolysate efficiently. The color change from purple to light brown after 24 h indicates active fermentation and successful substrate utilization26. The availability of diverse carbon sources can significantly impact microbial metabolism, influencing the yield and efficiency of fermentation. The observed color change suggests successful metabolic activity, but quantitative assessments, such as measuring biomass accumulation, pH changes, and gas production, would offer a more robust understanding of microbial growth dynamics.
Plackett and Burman design (PBD)
Design Expert 12 software optimized PHB production using two phases: Plackett-Burman Design (PBD) and Response Surface Methodology (RSM). PBD assessed 11 factors across 12 runs for each of the 19 isolates, focusing on Carbon, Nitrogen, and other key parameters. Isolate no. 3 excelled, surpassing a 60% production rate. Experiments were conducted in triplicate to ensure accuracy, using specific concentrations of wastewater and wood waste substrate, with the significance of factors determined by a t-test27. Table 4 shows the dataruns of PBD and the experimental data of PHB yield.
The presented data indicates a significant Model F-value of 1099.30 and a p-value of 0.0235, confirming the model’s effectiveness in explaining the variance in the response variable (Table 5). Key factors like A-Carbon, B-Nitrogen, and D-temperature significantly impact the system, evidenced by their low p-values and high F-values. Other factors like E-PH and G-MgSO4.7H2O also contribute significantly but less so than Carbon and Nitrogen. The Predicted R2 of 0.9869 closely aligns with the Adjusted R2 of 0.9990, suggesting robust model fit and predictive accuracy without overfitting. The model’s low Standard Deviation and Coefficient of Variation further confirm its precision, while an Adequate Precision of 110.353 indicates a reliable signal for navigating design space.
A Pareto chart, depicted in Fig. 3, displays the relative significance of eleven factors in a Plackett-Burman design on PHB yield. Three factors (Carbon, Nitrogen, and Temperature) exceed the t-value limit of 12.70, indicating their substantial impact on the PHB response. The height of bars on the chart reflects each factor’s impact magnitude, allowing for easy comparisons. This chart is a valuable tool for identifying the most influential factors affecting the response variable and highlighting those needing further investigation or optimization. Additionally, it provides a concise and clear way to communicate the outcomes of the P.B. design18.
The analysis highlights A-Carbon as the most influential factor in the study, accounting for 51.16% of the effect with a substantial sum of squares at 1875. B-Nitrogen follows with a 22.74% contribution, while D-Temperature also plays a significant role at 9.91%. Other factors like E-PH, F-Inoculum Size, and G-MgSO4.7H2O have smaller impacts, contributing 6.15%, 1.10%, and 2.33% respectively. H-Na2HPO4 and L-Trace Elements also impact outcomes, contributing 1.54% and 4.01%. C-Ferric Citrate and J-K2HPO4 show minimal influence, suggesting their irrelevance in further analysis. This data assists in prioritizing factors for optimization and decision-making based on their contributions and effects28.
According to the results of the Pareto chart and numeric percentage contribution stats, carbon, nitrogen, and Temperature plays a vital role in PHB production yield. These three factors are considered for further optimization techniques using response surface methodology (RSM).
Response surface methodology
Design Expert 12’s Response Surface Methodology (RSM) proves invaluable for result optimization, delving into intricate variable and response relationships through mathematical modeling. By predicting and optimizing response variables with a focus on interactive effects among factors, RSM aids in making informed decisions. Design Expert 12 facilitates this process with its user-friendly interface and statistical tools for experimental design, model fitting, response optimization, and result visualization. In this study, three optimization factors—Carbon, Nitrogen, and Temperature—are considered. The experimental and projected PHB values are presented in Table 6, demonstrating the efficiency of RSM in exploring the design space and determining optimal factor settings for achieving superior outcomes.
Predicted vs. actual value
The importance of the graph comparing predicted versus actual values lies in its capacity to assess the model’s goodness of fit. The model is deemed precise and dependable when the predicted values closely correspond to the actual values. Conversely, substantial deviations between the predicted and actual values suggest that the model might necessitate further refinement or investigation. As illustrated in Fig. 4, the data from 12 runs involving nine factors align along a straight line, indicating the accuracy of the data and the absence of outliers29.
The Model F-value of 29.89 confirms the statistical significance of the model, with significant terms including A, B, AB, A2, B2, and C2, indicating strong model terms. Non-significant terms can be pruned to enhance model efficiency. The Lack of Fit F-value at 0.25, with an 85.68% probability of being noise, suggests an excellent fit, as non-significant lack of fit is ideal Table 7). The Predicted R² of 0.9393 closely matches the Adjusted R² of 0.9909, underscoring model consistency. An Adequacy Precision of 34.659 indicates a robust signal, making this model highly reliable for navigating the design space11,30.
ANOVA for quadratic model
Final equation
Y = − 164.016 + 45.2164 * carbon + 333.684 * nitrogen + 10.6934 * temperature + 56.1404 * carbon * nitrogen + − 0.277778 * carbon * temperature + − 3.50877 * nitrogen * temperature + − 7.06667 * carbon^2 + -1263.16 * nitrogen^2 + − 0.136111 * temperature^2.
Model graphical representation
The contour and 3D surface plots (A–F), depicted in Fig. 5, utilize Response Surface Methodology (RSM) to explore the interactions between key factors—Carbon, Nitrogen, and Temperature—on PHA production. These visualizations effectively demonstrate how varying combinations of these factors influence PHA yield, providing insights into optimal operational conditions.
Plots A and B focus on the interaction between Carbon (X1) and Nitrogen (X2) percentages, with Temperature held constant at 34 °C. These plots reveal that the highest PHA production occurs at a Carbon level of approximately 2% and a Nitrogen level close to 0.1%. The red regions in both plots highlight areas of maximum PHA yield, suggesting that these are the most favorable conditions for PHA production within the tested range. Plots C and D examine the effects of Carbon percentage (X1) and Temperature (X2), keeping Nitrogen percentage fixed at 0.1%. The data shows that optimal PHA production is achieved when Carbon is around 2% and Temperature is near 37 °C. The contour plot (C) illustrates those moderate increases in both temperature and carbon percentage lead to higher PHA yields, indicated by the expanding red zone, which marks the region of peak production. Lastly, plots E and F analyze the interaction between Nitrogen percentage (X1) and Temperature (X2), with Carbon set at 2%. These plots indicate that increasing both Nitrogen and Temperature to their optimal values—approximately 0.1% for Nitrogen and 37 °C for Temperature—maximizes PHA yield. The 3D plot (F) vividly demonstrates this relationship, with a clear peak in the response surface at these conditions, confirming their effectiveness for enhancing PHA production.
Illustrating contour plots and 3D-Surface response prepared using Design Expert (version 12.0) by StatEase: interactive effects of (A) and (B) varied the intensity of the carbon and nitrogen at temperature 34; (C) and (D) varied the intensity of the carbon and Temperature at nitrogen 0.105; (E) and (F) varied the intensity of the nitrogen and Temperature at carbon 1.7.
Point prediction values
In the context of this study, the predicted values for carbon, nitrogen, and temperature are specified as 1.74, 0.105, and 37 °C, respectively, as outlined in Table 8. These values imply that maximizing PHB yield can be achieved under these specified conditions.
Final production
Using Response Surface Methodology (RSM), the optimal conditions for the production of Polyhydroxybutyrate (PHB) by Klebsiella pneumoniae Mk1 were scaled down for a smaller volume. The ideal carbon concentration was determined to be 1.7% (17 g/L), nitrogen concentration 0.1% (1 g/L), and the incubation temperature was set at 37 °C. For a 500 mL substrate, the medium was prepared by mixing 236 mL of hydrolyzed wood waste water with 264 mL of distilled water. The hydrolyzed wood waste contained approximately 36 mg/mL of sugar content, determined by the DNS test standard graph. To this solution, 1 g of ammonium chloride was added as the nitrogen source.
The medium was inoculated with the microbial isolate and incubated at 37 °C for 72 h. After the incubation period, the culture was centrifuged to separate the cells from the supernatant. The cells were then processed for PHB extraction. After adjusting for the smaller volume, the final yield of PHB (Fig. 6) obtained was approximately 2185.625 mg per 500 mL of the medium, equivalent to 4.37 mg/mL.
The availability and type of carbon source play a pivotal role in PHB production24. Pineapple peel, an inexpensive agro-waste, was successfully utilized as a carbon source for Bacillus drentensis BP17, yielding enhanced PHB accumulation through the optimization of pineapple peel concentration and fermentation conditions31. Similarly, Sachan et al. (2024) highlighted glucose as a key carbon source for Bacillus paranthracis, where its concentration was fine-tuned to achieve improved PHB synthesis32. For Bacillus megaterium, agricultural hydrolysates containing glucose and xylose were optimized for fermentation, demonstrating the impact of balanced carbon source utilization33. The nitrogen source and its concentration are critical for influencing microbial metabolism toward PHB accumulation. The C/N ratio was optimized in Bacillus megaterium PNCM 1890, revealing that a low nitrogen concentration promotes PHB33. Additionally, Kojuri et al. (2021) utilized ammonium sulfate as the primary nitrogen source and achieved enhanced PHB production through its optimized concentration in petrochemical wastewater34. pH and temperature optimization are fundamental for maintaining microbial activity and PHB yield. Trakunjae et al. (2021) reported that pH levels between 6.5 and 7.5 and temperatures of 30–35 °C were optimal for Rhodococcus sp. BSRT1-1, indicating that these factors significantly influence enzymatic activity and metabolic pathways35.
The global attention on sustainable and biodegradable polymers has spurred important advancement in the microbial synthesis of polyhydroxybutyrate (PHB) from lignocellulosic waste products. The present work shows that Klebsiella sp. MK3 effectively valorized an affordable substrate and presented a competitive yield relative to past literature by using sulfuric acid-treated wood waste to create 4.2 g/L of PHB. Under batch fermentation conditions, Abu-Thabit et al. (2022) investigated PHB generation from different lignocellulosic and plastic waste sources, noticing generally between 0.5 and 3.5 g/L, often requiring detoxification due to inhibitory molecules36. Attaining a yield of 4.2 g/L, the present work essentially avoids significant detoxification and shows the durability of Klebsiella sp. MK3 as well as the efficacy of 4% sulfuric acid pretreatment. Using Paraburkholderia sacchari to metabolize degradation monomers formed from PHB-based polymers, Zoghbi et al. (2023) produced PHB yields of about 2.3 g/L. Though different in approach, their reliance on bioplastic-derived carbon sources limits wider applicability in comparison to this work, which makes use of abundant, non-bioplastic wood waste37. With Burkholderia ambifaria E5-3, Arai et al. (2024) recorded PHB generation approaching 3.7 g/L using hydrolysates obtained from oil palm trunks. Although an inhibitor-tolerant strain was used, yields still fell short of those obtained in the current study38, thereby supporting once more the effectiveness of Klebsiella sp. MK3 and its substrate compatibility. Using acid-pretreated biomass, Saratale et al. (2022) developed a co-culture method producing PHB titers almost equal 3.5 g/L. Using the monoculture system of Klebsiella sp. MK3 used in this work avoids the complexity of co-culture systems that causes challenges in scaling and process control.
From ambient-alkaline-treated agricultural wastes39, Hossain et al. (2022) achieved PHB concentrations of 2.9 g/L; Davaritouchaee et al. (2023) reported just 1.1 g/L PHB from orange peel hydrolysates using a one-step oxidation process40,41.
This study highlighted the importance of maintaining substrate availability without causing inhibitory effects from substrate overload. The application of RSM has demonstrated significant potential in optimizing PHB production by systematically analyzing the effects of key parameters such as carbon and nitrogen sources, pH, temperature, and many more.
Qualitative analysis of PHB using UV-Vis spectroscopy
The UV spectroscopy result is significant in identifying and characterizing the presence of Polyhydroxybutyrate (PHB), a key type of PHA, by comparing it to the known crotonic acid standards42. Here are the key aspects of the result:
The similarity in peaks is crucial for confirming the presence of PHB, as crotonic acid is a known degradation product of PHB during polymer breakdown shown in Fig. 7.
The slight variations in peak intensities between the bacterial strains and the crotonic acid standard could be due to differences in the yield or purity of PHB extracted from the bacterial strain.
Fourier transform-infrared spectroscopy (FTIR)
For PHB, the FTIR spectrum typically exhibits absorption peaks corresponding to the carbonyl (C=O) and ester (C-O) functional groups, which are distinctive features of the polymer backbone. These peaks offer insights into the polymer’s properties. In Fig. 8, specific absorption peaks include stretching of the OH. carboxyl group, C.H. bending, halogen compound, C=O ester, and CH, C-O, CH3, and OH groups of the polymer, occurring at wave numbers 725, 1018, 1106, 1270, 1461, 1724, and 2938 cm–143,44. The analysis of these peaks provides crucial information about the chemical composition and structure of PHB. The presence of the ester group and methyl group indicates that the microbial bioprocessed polyester is a PHB polymer from the PHB family. PHB, within the PHB family, is distinguished by its unique chemical composition, featuring a methyl functional group (CH3) and an ester linkage group (-COOR). These specific chemical groups contribute to PHB’s thermoplastic nature, hydrophobic properties, high crystalline structure, and brittleness.
1H and 13C NMR analysis
The carbon-13 (C-13) NMR peaks as shown in Fig. 9a indicating various structural elements within the polymer. Peaks around 173.313, 169.617, and 165.739 ppm likely signify carbonyl (C=O) groups in the ester linkages of PHB’s backbone. Peaks at 134.075 and 129.556 ppm suggest aromatic or alkenyl carbons, potentially reflecting aromatic or unsaturated regions in PHB. Peaks around 77 ppm may represent carbons adjacent to ester groups. Peaks at 69 and 64 ppm could relate to the polymer backbone or side chains, while those below 33.866 ppm likely denote alkyl or alkyl halide groups from PHB’s side chains. In the proton nuclear magnetic resonance (1 H-NMR) spectrum, as Shown in Fig. 9b peaks at 8.095 and 7.268 ppm suggest aromatic or alkenyl hydrogen environments, while peaks between 5.186 and 4.090 ppm indicate hydrogen atoms near double bonds or ring structures, possibly from the polymer backbone or side groups. Peaks around 4 ppm likely correspond to methylene (CH2) groups, common in PHB’s polymer structure44.
Conclusion
This study delves into the innovative use of wood waste and wastewater mixture as substrate media for the production of polyhydroxybutyrate (PHB) facilitated by Klebsiella sp. Worldwide, concerted efforts are underway to promote Polyhydroxybutyrate (PHB) accumulation as a sustainable alternative to conventional plastics. Researchers, industries, and policymakers collaborate to advance PHB production technologies, exploring its diverse applications. This collective endeavor aims to mitigate plastic pollution and foster a greener, more environmentally friendly future. This research offers promising benefits, including cost reduction and decreased dependence on petroleum-based plastics, aligning with environmental sustainability goals. It finds utility in packaging, agricultural mulch films, biomedical applications, disposable items like cutlery, textiles, and 3D printing. Its eco-friendly nature aids in reducing environmental pollution and dependence on non-renewable resources, offering sustainable solutions across diverse sectors. Leveraging statistical methods such as the Plackett-Burman design (PBD) and response surface methodology (RSM) has proven instrumental in optimizing key factors influencing PHB production. The PBD highlighted significant effects of temperature, carbon concentration, and nitrogen concentration on PHB production. Subsequently, RSM pinpointed the optimal conditions for maximal PHB production, revealing that a carbon concentration of 1.7%, nitrogen concentration of 0.105%, and a temperature of 37 °C resulted in a maximum production rate of 4.37 mg/mL using a substrate media recomposed of wood waste and wastewater that is approx. two-folds. However, furthermore, kinetic modeling of sugar uptake and metabolite production could help predict the efficiency of different carbon sources in promoting microbial growth. The UV-Vis spectroscopy, FTIR & NMR confirms the produced product is PHB. This optimized approach holds promise for substantial cost savings in PHB production, fostering a more sustainable and eco-friendly manufacturing process.
Data availability
The Klebsiella sp. isolate has been submitted to NCBI under the accession number PP109354, ensuring accessibility for further research and reference.
Change history
29 January 2026
The original online version of this Article was revised: In the original version of this Article, Mukesh Kumar was incorrectly affiliated with ‘Department of Medical Laboratory Sciences, GNA University, Phagwara 144401, Punjab, India’. The correct affiliation is ‘Department of Microbiology, School of Bioengineering and Biosciences, Lovely Professional University, Phagwara 144411, Punjab, India’. In addition, affiliation ‘Department of Microbiology, Graphic Era (Deemed to be University), Dehradun, Uttarakhand, India’ was omitted for Arun Karnwal.
References
Sin, M. et al. Viscoelastic, spectroscopic, and microscopic characterization of novel bio-based plasticized poly (vinyl chloride) compound. hindawi.comMC Sin. SN Ganint. J. Polymer Sci. (2014). https://www.hindawi.com/journals/ijps/2014/846189/abs/.
Kumar, M. & reports, I. T. B. technology, Municipal secondary sludge as carbon source for production and characterization of biodiesel from oleaginous bacteria. Elsevier (2018). https://www.sciencedirect.com/science/article/pii/S2589014X18300975.
Saravanan, K., Umesh, M. & Kathirvel, P. Microbial polyhydroxyalkanoates (PHAs): a review on biosynthesis, properties, fermentation strategies and its prospective applications for sustainable future. J. Polymers Env. 30 (12), 4903–4935 (2022). https://link.springer.com/article/https://doi.org/10.1007/s10924-022-02562-7.
Laumeyer, C. M., Zimmer, J. & Steinmetz, H. From fruit juice wastewater to biopolymer—how the mixed microbial culture and PHA content develop over time. Chem. Eng. J. 503, 158314 (2025).
Saravanan, K., Subramaniam, Y. & Kathirvel, P. Valorization of custard apple (Annona squamosa) waste through polyhydroxybutyrate (PHB) production by Bacillus megaterium MAPCS4: optimization, characterization, and biodegradation studies. Biomass Convers. Biorefin. 14 (20), 26121–26137 (2023). https://link.springer.com/article/10.1007/s13399-023-04691-y.
Song, Y. T., Sitthikitpanya, N., Usmanbaha, N., Reungsang, A. & Chu, C. Y. Optimization of polyhydroxyalkanoate (PHA) production from biohythane pilot plant effluent by Cupriavidus necator TISTR 1335. Biodegradation 36 (1), 1–14 (2025). https://link.springer.com/article/10.1007/s10532-025-10110-y.
Li, D., Ma, X., Li, J. & Sun, B. Insights into enhanced polyhydroxyalkanoate production by the synergistic use of waste wood hydrolysate and volatile fatty acids by mixed microbial cultures. Bioresour Technol. 337, 125488 (2021).
Ben Abdallah, M. et al. Advances in polyhydroxyalkanoate (PHA) production from renewable waste materials using halophilic microorganisms: A comprehensive review. Sci. Total Environ. 963, 178452 (2025).
Valenzuela, E. F., Menezes, H. C. & Cardeal, Z. L. Passive and grab sampling methods to assess pesticide residues in water. A review. Environ. Chem. Lett. 18 (4), 1019–1048 (2020).
Spiekermann, P., Rehm, B. H. A., Kalscheuer, R., Baumeister, D. & Steinbüchel, A. A sensitive, viable-colony staining method using nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 171 (2), 73–80 (1999).
Gao, H. et al. Medium optimization for the production of avermectin B1a by Streptomyces avermitilis 14-12A using response surface methodology. Bioresource Technol. (2009). https://www.sciencedirect.com/science/article/pii/S0960852409002466.
Jiang, Y., Marang, L., Tamis, J., research, M. & van, L. W. Waste to resource: converting paper mill wastewater to bioplastic. Elsevier (2012). https://www.sciencedirect.com/science/article/pii/S0043135412005222.
Morgan-Sagastume, F. Characterisation of open, mixed microbial cultures for polyhydroxyalkanoate (PHA) production. Rev. Environ. Sci. Biotechnol. 15 (4), 593–625 (2016).
Singh, G. et al.Optimization of poly-B-hydroxybutyrate production from Bacillus species. Eur. J. Biol. Sci. (2011). https://www.academia.edu/download/81689187/3.pdf.
Pereira, J. et al. Demonstration of the adhesive properties of the medium-chain-length polyhydroxyalkanoate produced by Pseudomonas chlororaphis subsp. aurantiaca from glycerol. Fortunato Int. J. Biol. Macromol. (2019). https://www.sciencedirect.com/science/article/pii/S014181301833650X.
Zakaria, M. et al. Biosynthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer from wild-type Comamonas sp. EB172. Elsevier (2024). https://www.sciencedirect.com/science/article/pii/S0141391010000303.
Janda, J. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. Am. Soc. Microbiol. (2007). https://doi.org/10.1128/JCM.01228-07.
Shaikh, S. S., Wani, S. J. & Sayyed, R. Z. Statistical-based optimization and scale-up of siderophore production process on laboratory bioreactor. 3 Biotech. 6 (1), 1–10 (2016).
Tesfaye, T. & Sithole, B. Valorisation of waste chicken feathers: optimisation of decontamination and pre-treatment with bleaching agents using response surface methodology. Elsevier (2018). https://www.sciencedirect.com/science/article/pii/S2352554117300827.
Law, J. Assay of poly-β-hydroxybutyric acid. Am. Soc. Microbiol. (1961). https://doi.org/10.1128/jb.82.1.33-36.1961.
Lee, I., Chang, H. & of, Y. P. J. M, A simple method for recovery of microbial by alkaline solution treatment. Koreascience (1995). https://koreascience.kr/article/JAKO199511922233685.pdf.
Porras, M. A., Cubitto, M. A. & Villar, M. A. A new way of quantifying the production of poly(hydroxyalkanoate)s using FTIR. J. Chem. Technol. Biotechnol. 91 (5), 1240–1249 (2016).
Jiang, G., Johnston, B., Townrow, D., Radecka, I. & Polymers, M. K. Biomass extraction using non-chlorinated solvents for biocompatibility improvement of polyhydroxyalkanoates. Polymers (2018). https://www.mdpi.com/2073-4360/10/7/731.
biochemistry MSTJ of 1938 Biochemical studies on carbohydrates. J. Biochem. (1938). http://jlc.jst.go.jp/JST.Journalarchive/biochemistry1922/28.479?from=Google.
Saqib, A. & Bioenergy PWB. Differential behaviour of the dinitrosalicylic acid (DNS) reagent towards mono-and di-saccharide sugars. Biomass Bioenergy (2011). https://www.sciencedirect.com/science/article/pii/S0961953411004673.
Bergman, H. C. & Drury, D. R. Effect of feeding and fasting on sugar utilization of eviscerated rabbits. Proc. Soc. Exp. Biol. Med. 37(2), 414–417 (1937).
enzymology, S. P. M. Detection, isolation, and characterization of siderophores. Elsevier (1994). https://www.sciencedirect.com/science/article/pii/0076687994351511.
Darani, K. K. Application of the Plackett-Burman statistical design to optimize poly (Î2-hydroxybutyrate) production by Ralstonia eutropha in Batch culture (2024). https://www.ijbiotech.com/article_6901_8e3fe7fd844be84b124390b3876a3688.pdf.
Hong, C., Hao, H., Bioenergy, W. H. B. & Hao, H. Process optimization for PHA production by activated sludge using response surface methodology. Biomass and Bioenergy (2009). https://www.sciencedirect.com/science/article/pii/S096195340800247X.
Mohite, B. & Polymers, S. P. C. Physical, structural, mechanical and thermal characterization of bacterial cellulose by G. hansenii NCIM 2529. Elsevier (2014). https://www.sciencedirect.com/science/article/pii/S0144861714001325.
Penkhrue, W. et al. Response surface method for polyhydroxybutyrate (PHB) bioplastic accumulation in Bacillus drentensis BP17 using pineapple peel. PLoS One 15(3), e0230443 (2020). https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0230443.
Sachan, R. S. K. et al. Investigating chemical pre-treatment methods: valorization of wheat straw to enhance polyhydroxyalkanoate (PHA) production with novel isolate Bacillus paranthracis RSKS-3. Heliyon 10, 11 (2024).
Dañez, J. C. A. et al. Optimization of fermentation factors for polyhydroxybutyrate (PHB) production using Bacillus megaterium PNCM 1890 in simulated Glucose-Xylose hydrolysates from agricultural residues. Philipp J. Sci. 149 (1), 163–175 (2020).
Kojuri, S. A., Issazadeh, K., Heshmatipour, Z., Mirpour, M. & Zarrabi, S. Production of bioplastic (polyhydroxybutyrate) with local bacillus megaterium isolated from petrochemical wastewater. Iran J. Biotechnol. 19 (3), 1–9 (2021). https://pubmed.ncbi.nlm.nih.gov/34825017/.
Trakunjae, C. et al. Enhanced polyhydroxybutyrate (PHB) production by newly isolated rare actinomycetes Rhodococcus sp. strain BSRT1-1 using response surface methodology. Sci. Rep. 11 (1), 1–14 (2023). https://www.nature.com/articles/s41598-021-81386-2.
Abu-Thabit, N. Y., Pérez-Rivero, C., Uwaezuoke, O. J. & Ngwuluka, N. C. From waste to wealth: upcycling of plastic and lignocellulosic wastes to PHAs. J. Chem. Technol. Biotechnol. 97 (12), 3217–3240 (2022).
Zoghbi, L. et al. Circular PHB production via paraburkholderia Sacchari cultures using degradation monomers from PHB-based post-consumer bioplastics as carbon sources. Biochem. Eng. J. 191, 108808 (2023).
Arai, T. et al. Efficient production of polyhydroxybutyrate using lignocellulosic biomass derived from oil palm trunks by the inhibitor-tolerant strain Burkholderia ambifaria E5-3. World J. Microbiol. Biotechnol. 40, 8 (2024). https://pubmed.ncbi.nlm.nih.gov/38869634/.
Saratale, R. G. et al. Developing microbial co-culture system for enhanced polyhydroxyalkanoates (pha) production using acid pretreated lignocellulosic biomass. Polymers , Vol 14, 726 (2022). https://www.mdpi.com/2073-4360/14/4/726/htm.
Davaritouchaee, M., Mosleh, I., Dadmohammadi, Y. & Abbaspourrad, A. One-step oxidation of orange peel waste to carbon feedstock for bacterial production of polyhydroxybutyrate. Polymers (Basel) 15, 3. https://doi.org/10.3390/polym15030697 (2023).
Hossain, M. A. et al. Upcycling agricultural waste to biodegradable polyhydroxyalkanoates by combined ambient alkaline pretreatment and bacterial fermentation. Ind. Crops Prod. 185, 56 (2022).
Sachan, R. S. K. et al. Polyhydroxyalkanoate production using sulfuric acid Pre-Treated wheat straw (SAPWS) as a feedstock: RSM (Response surface Methodology)-Based optimization using Bacillus paranthracis RSKS-3. Indian J. Microbiol. (2024).
Sachan, R. S. K., Devgon, I., Karnwal, A. & Mahmoud, A. E. D. Valorization of sugar extracted from wheat straw for eco-friendly polyhydroxyalkanoate (PHA) production by Bacillus megaterium MTCC 453. Bioresour. Technol. Rep. 2024, 25 (2024).
Kumar, M., Sachan, R. S. K., Kauts, S., Karnwal, A. & Mahmoud, A. E. D. Unlocking sustainable solutions: wood waste as a novel substrate for polyhydroxybutyrate (PHB) production to combat plastic pollution. Environ. Technol. https://doi.org/10.1080/09593330.2024.2409994 (2024).
Acknowledgements
I am obliged to Lovely Professional University, Phagwara, to conduct my research. The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-33).
Funding
No funding was received during the conduct of research.
Author information
Authors and Affiliations
Contributions
MK, RSKS: conceptualization, methodology, data curation, writing-original draft. AK, DS: writing-review and editing, resources, Former Analysis.RT, AK, HA: writing-review and editing, methodology, Fund acquisition.SM, AGA: Project Administration, supervision, Validation, Software.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, M., Sachan, R.S.K., Karnwal, A. et al. Valorization of wood waste for enhanced polyhydroxybutyrate production by Klebsiella sp. MK3. Sci Rep 15, 18205 (2025). https://doi.org/10.1038/s41598-025-01305-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01305-7