Abstract
Sleep disturbance is common but often overlooked after stroke. Regular sleep is increasingly recognised as important for overall health, yet little is known about how sleep regularity changes after stroke. This study examined differences in the Sleep Regularity Index (SRI) between stroke survivors and healthy controls using actigraphy data from an existing dataset (~ 1 week per participant). Data were analysed for 162 stroke survivors (mean age 61 ± 14 years, 5 ± 5 years post-stroke, 89 males) and 60 controls (mean age 57 ± 17 years, 32 males). Stroke survivors had significantly lower SRI scores than controls (p = 0.001), indicating less regular sleep. In the stroke group, higher SRI correlated with longer total sleep time (p = 0.003) and better self-reported sleep quality (p = 0.001) but not with other sleep metrics. Lower SRI was associated with worse depressive symptoms (p = 0.006) and lower quality of life (p = 0.001) but not with disability (p = 0.886) or time since stroke (p = 0.646). These findings suggest that sleep regularity is disrupted post-stroke and may influence well-being. Future research should explore interventions to improve sleep regularity and related health outcomes in stroke survivors.
Similar content being viewed by others
Introduction
Stroke is a neurological condition caused by interrupted blood supply to the brain and is one of the leading causes of mortality and disability worldwide1,2. Sleep-related impairments are a prevalent, yet often overlooked, complication following stroke3. Diminished subjective (self-reported) and objective (polysomnography) sleep quality has been commonly reported in comparison with control groups4,5. This is important as disruptions in sleep are thought to exacerbate coexisting symptoms, such as cognitive deficits, fatigue, and depression6,7,8. Moreover, these sleep disturbances may impede rehabilitation trajectories and hinder an individual’s capacity to reintegrate into society6,9.
To investigate sleep in stroke survivors, multiple approaches can be taken. Polysomnography (PSG), the gold standard for sleep monitoring, records physiological changes that occur during sleep. However, PSG is typically conducted in sleep clinics utilising specialised equipment and trained personnel, rendering it costly and difficult to access10. In comparison, actigraphy, which is used to infer sleep based on movement recorded from a sensor on a limb, has demonstrated good sensitivity and accuracy similar to PSG11,12. High sensitivity refers to the ability of actigraphy to accurately detect periods of sleep, while its lower specificity indicates a reduced capacity to correctly identify periods of wakefulness. Despite lower specificity than PSG, actigraphy benefits from its capability to be worn continuously in the home environment over multiple days/nights, allowing for the investigation of longer-term sleep patterns4,10,13. In addition to assessing sleep, by leveraging around-the-clock data, actigraphy can also be used to investigate circadian rhythm alterations. This is important as both sleep disturbances and circadian rhythm dysfunctions can be risk factors for, and consequences of, stroke14. The circadian rhythm, responsible for controlling the sleep-wake cycle and regulating physiological processes, is vital for overall health15. Laboratory studies have demonstrated that misalignment between the timing of the sleep-wake cycle and endogenous circadian rhythmicity can impair attention, neurobehavioral performance, mood, and cognition16. These findings highlight the potential impact of sleep regularity on various aspects of health and well-being of relevance to stroke recovery.
In 2017, Phillips and colleagues17 introduced a new metric to measure sleep regularity with actigraphy, named the Sleep Regularity Index (SRI). The SRI is defined as the probability of an individual being in the same state (asleep or awake) at any two time points 24 h apart. Unlike other actigraphy measures, the SRI does not rely on a single main sleep period, making it suitable for individuals with multiple sleep periods throughout the day17. Lunsford-Avery and colleagues18 used the SRI in older adults, finding that less regular sleep was linked to increased cardiometabolic risk, delayed sleep timing, increased daytime sleep and sleepiness, and reduced light exposure, however the SRI was independent of sleep duration. Additionally, they established a link between sleep irregularity and worse depression symptoms in older adults. Furthermore, it has been established that sleep regularity, as measured by the SRI, is more strongly associated with all-cause mortality risk than sleep duration19. Taken together, these results underscore the importance of circadian rhythm continuity to overall health: however, the SRI has not been investigated in stroke survivors. Exploring the impact of stroke on sleep regularity and its association with other post-stroke effects could enhance our understanding and management of sleep-related issues in stroke patients. We therefore aimed to investigate the SRI in stroke survivors to better understand the differences in comparison to healthy controls, the relationship to self-reported sleep and actigraphy sleep metrics, as well as explore the potential correlations with disability, depression, and quality of life. Our primary hypothesis was that there would be a significant difference in SRI between stroke survivors and healthy controls, with a lower SRI (i.e., less regular sleep) for stroke survivors. We also hypothesised that SRI would correlate significantly with self-reported sleep and actigraphy sleep metrics (sleep fragmentation, sleep efficiency, Wake After Sleep Onset) but not with the Total Sleep Time in line with previous findings showing no association between TST and SRI in older adults18.
Methods
Sample
We utilised previously acquired datasets at the Wellcome Centre for Integrative Neuroimaging (WIN), University of Oxford. Stroke patients and healthy controls were recruited for several studies spanning from 2017 to April 20244,20,21,22. All participants included in the analysis provided informed consent at the time of their initial involvement. All five studies received ethical approval from either the National Research Ethics Service or by the University of Oxford Central University Research Ethics Committee (11/H0605/12, R40803, 22/EM/0080, R85306, 22/LO/0353). All research was performed in accordance with relevant guidelines and regulations, and was performed in accordance with the declaration of Helsinki. We only included participants living in the community, as sleep can be significantly affected by the hospital environment23. All studies included stroke survivors aged 18 years or older who were able and willing to provide informed consent. Participants ranged from the sub-acute to chronic phase of stroke recovery, with several studies requiring residual upper limb impairment or an expressed interest in improving sleep. Common exclusion criteria across studies included the presence of other neurological or psychiatric conditions unrelated to stroke, uncontrolled seizures, pregnancy, and current engagement in psychological therapy for insomnia. An overview of all the studies and their respective inclusion/exclusion criteria and any additional study-specific criteria (e.g., untreated or diagnosed sleep disorders prior to stroke or participation in motor tasks) are detailed in Supplementary Table S1. Moreover, the control group was recruited as part of the first included stroke study and consisted of individuals over the age of 18 who provided informed consent and had no history of stroke. Controls were age- and sex-matched to stroke participants in the original study, and recruitment aimed to capture a representative range of sleep experiences—from individuals who reported good sleep to those with self-perceived sleep difficulties. Additionally, there are criteria for the actigraphy that should be satisfied to guarantee reliable calculation of the sleep-wake metric, these are detailed under SRI calculation.
As SRI has not previously been investigated in this population, there are no existing studies to guide sample size estimations. Thus, a sensitivity power analysis was performed (calculation of effect size given alpha, power, and available sample size). The data available at the start of the analysis included 184 stroke patients and 70 controls. Therefore, to perform an analysis of covariance (ANCOVA) for the primary outcome to detect the difference in SRI for stroke survivors compared to controls with covariates age and sex, the smallest effect size detectable was f = 0.17 (G*Power v.3.1; alpha = 0.05, power = 80%, n = 254 analysis of covariance).
Measures
Actigraphy
Across all studies we used a Motionwatch 8 device24, with participants instructed to wear it for approximately one week. If able, participants were directed to press the event marker both when initiating sleep in the evening and upon waking in the morning. Participants were instructed to wear the waterproof actigraphy device continuously, including during bathing and all waking and sleeping hours throughout the recording period. They were also asked to complete a 7-night sleep diary, recording their attempted sleep and final wake times. Stroke survivors were instructed to wear the monitor on their less-affected wrist, controls and non-motor impaired participants were instructed to wear the monitor on their non-dominant wrist. In the current study, the single axis algorithm and peak detection recording mode of the MotionWatch 8 is used, as recommended for sleep recording with this device.
Actigraphy data were automatically classified as sleep or wake for each 30 s interval by the MotionWare programme. The start and end of the sleep period was based on a combination of information from the sleep diary, activity, ambient light, and the utilisation of the event marker. These data were used to calculate the following sleep metrics: Total Sleep Time (TST), sleep fragmentation index, Wake After Sleep Onset (WASO), sleep efficiency and average Sleep Time per 24 h (a full breakdown of how these metrics were calculated can be found in the supplementary materials).
Calculation of SRI
The Sleep Regularity Index (SRI) used epoch data from actigraphy to calculate the percentage probability of an individual being in the same state (asleep vs. awake) at any two time-points 24 h apart, averaged across the study17. The practical scale of the index ranges from 0 (completely random sleep/wake patterns) to 100 (perfectly consistent sleep/wake patterns)25 and can then be used to classify individuals as Regular (top quintile) or Irregular (bottom quintile) sleepers17. The current computation utilizes the 30-second epochs extracted from MotionWare and is in accordance with the calculations from Lunsford Avery et al.18. Considering N days of recording segmented into M daily epochs, \(\:{s}_{i,j}\) = 1 indicates the participant’s sleep status on day i during epoch j, and \(\:{s}_{i,j}\) = 0 indicates wakefulness. The SRI is then computed using the following Eq. (1), where \(\:\delta\:\left({s}_{i,j},{s}_{i+1,j}\right)\) = 1 if \(\:{s}_{i,j}\) = \(\:{s}_{i+1,j}\) and 0 otherwise.
To guarantee a reliable calculation of the SRI, several criteria have been reported25. First, to guarantee sufficient data, participants must have at least five valid wear days (i.e. 5 full rounded days). Second, previous studies report that each participant should ideally have a weekend day included in the recording. Third, participants should not have > 2 h of no recording (i.e. device off or not worn) in their duration of wearing the device18.
These criteria were visually inspected per participant. Where these criteria were not satisfied, the recording was ruled invalid and excluded from analysis. 36 participants (26 patients/10 controls) were excluded based on these criteria, resulting in a final sample size of 222 (162 patients/60 controls).
Actigraphy demonstrates greater sensitivity compared to specificity, indicating proficiency in identifying periods of sleep, but relative ineffectiveness in accurately detecting wakefulness26. The SRI is unique in that it considers both daytime and night-time activity, necessitating a resolution to this inaccuracy while maintaining the distinctive capability to detect periods of sleep during the day, e.g. naps. To address this issue and enhance the reliability of recordings for SRI calculations, specialised algorithms can be implemented; the current study used the UCSD algorithm (with a scaling factor of 0.10) and applied Webster’s rescoring rules. To ensure transparency and accurate comparison between research studies, our procedure and refinements are detailed in supplementary materials (S2).
Questionnaires
Self-reported sleep in both the healthy control and stroke cohort was assessed with the Sleep Condition Indicator (SCI; range 0–32), a valid and reliable method to diagnose DSM-5 insomnia disorder and symptoms post-stroke27,28. For solely the stroke cohort, depression scores were assessed with the Patient Health Questionnaire (PHQ-8; range 0–24, higher score indicates more severe depressive symptoms)29. Post-stroke disability was assessed with the modified Rankin Scale (mRS; score range 0= “no symptoms at all” to 6=“death”)30. Finally, quality of life was assessed with short-form Stroke Impact Scale (SF-SIS; max score 100, higher score indicates better quality of life)31.
Statistical analysis
Analyses were conducted with Python (v.3.10.12), RStudio (R v.4.3.2) and MotionWare (v.1.3.33). Plots were created with GraphPad Prism (v.10.2.3). To compare age between groups, a Welch Two Sample t-test was performed, as it accommodates unequal variances and different sample sizes. To test the relationship between pairs of categorical variables (e.g. sex and group) a chi-square test was performed. To test the primary hypothesis that stroke survivors have a lower SRI than controls, an analysis of covariance (ANCOVA) was planned, with age and sex as covariates. However, the assumption of non-interaction in an ANCOVA model was violated. Therefore, a linear regression model was used instead. Testing of assumptions and further details can be found in supplementary materials (S3). To test whether the SRI relates to standard questionnaire and actigraphy-derived sleep metrics Pearson’s correlations were used (adjusted for age and sex) with Bonferroni correction for the multiple comparisons of the sleep metrics (α = 0.01; analysis details in supplementary S3). To ascertain if the strength of these correlations differs between groups, a Fisher’s R to Z-test was performed. Interpretation of Pearson’s correlations coefficients are reported according to the following guidelines: r < 0.4, weak; 0.4 ≤ r ≤ 0.7, moderate; and r > 0.7, strong32, all confidence Intervals (CIs) are reported at the 95% level and p-values were computed using a Wald t-distribution approximation.
Results
Demographics and descriptive statistics SRI
The final dataset included 162 stroke survivors (mean (± sd) 61 (± 14) years of age, 5 (± 5) years post-stroke, 89 males) and 60 healthy controls (57 (± 17) years of age, 32 males; Table 1). There was no significant difference in age (t(89.06) = 1.51, p = 0.135) or sex distribution (χ2 = 3.78e-03, p = 0.951) between the groups.
The mean (± sd) SRI for the stroke cohort was 41.21 (± 12.58) and values ranged from 16.03 to 74.00. For the healthy controls, the mean (± sd) was 47.80 (± 11.74) and the values ranged from 21.72 to 72.94 (Fig. 1).
(A) Relationship between sleep regularity (SRI) and total sleep time (TST) for the stroke cohort (dashed line) and healthy controls (solid line). Plots that show the sleep/wake cycle according to the SRI calculation for four participants, two classified as irregular sleepers (B = stroke, C = control), with SRI values in the first quintile, and two regular sleepers (D = stroke, E = control), with SRI values in the fifth quintile. The colours reflect ‘irregular’ (pink), ‘normal’ (orange) and ‘regular’ (teal) sleepers. The figure displays results from the current analysis, though the design is based on similar figures created by other studies17,18.
Difference in sleep regularity between stroke survivors and healthy controls
SRI values are shown in Fig. 2. To test for differences between groups, a linear regression model employing Ordinary Least Squares (OLS) estimation was utilised to predict SRI based on the factors Group (0 = Control, 1 = Stroke), Age, and Sex. The linear regression model accounted for a significant but weak proportion of the variance in SRI (R2 = 0.12, F(3, 218) = 10.00, p < 0.001, adj. R2 = 0.11). Within this model, the effect of Group was significant and negative (β = -6.07, 95% CI [-9.65, -2.48], t(218) = -3.33, p = 0.001; Std. β = -0.48, 95% CI [-0.76, -0.20]), indicating that stroke survivors had a lower SRI, and thus less regular sleep compared to controls, as hypothesised. The effect of Age was significant and negative (β = -0.12, 95% CI [-0.23, -0.01], t(218) = -2.16, p = 0.032; Std. β = -0.14, 95% CI [-0.27, -0.01]) demonstrating that increased age is associated with less regular sleep. The effect of Sex was significant and positive (β = 5.07, 95% CI [1.86, 8.29], t(218) = 3.11, p = 0.002; Std. β = 0.20, 95% CI [0.07, 0.33]), demonstrating that, overall, women have a higher SRI (more regular sleep) compared to men. Permutation testing (i.e., randomly subsampling the stroke group to account for the unequal sample sizes) revealed similar results (supplementary S4).
As the current research incorporates data from five distinct stroke studies, each with individualised objectives, there is an inherent sampling bias. In particular, several studies20,21 recruited stroke survivors based on their subjective experience of sleep issues and interest in receiving sleep treatment. We found a significant correlation between SCI and the SRI (with stroke and control combined; r = 0.25, p < 0.001; Fig. 3), indicating the SRI is higher for participants with better self-reported sleep. We therefore repeated the linear regression with SCI as a predictor, to control for any differences between groups. The effect of Group is negative but no longer significant (β = -2.22, 95% CI [-6.29, 1.85], t(217) = -1.07, p = 0.284; standardized β = -0.18, 95% CI [-0.50, 0.15]).
Investigating correlations between SRI and other sleep metrics
Stroke survivors
Counter to our hypothesis, there was a weak but statistically significant correlation between SRI and Total Sleep Time (r = 0.22, p = 0.004), indicating that when stroke survivors have more regular sleep, they have longer overall nighttime sleep. There was a weak but significant correlation between SRI and Sleep Condition Indicator score (r = 0.25, p = 0.001), suggesting that better self-reported sleep after stroke is associated with more regular sleep-wake patterns. However, there were no significant correlations between SRI and Wake After Sleep Onset (r = -0.05, p = 0.56), Fragmentation Index (r = -0.13, p = 0.12) nor Sleep Efficiency (r = 0.18, p = 0.03; ns with Bonferroni correction), Fig. 4. Interestingly, when the Average Sleep Time per 24 h is used, there is a significant correlation with the SRI, whereby more regular sleep is associated with shorter average sleep time per 24 h. Details and figures can be found in Supplementary materials (figure S8 part A).
Plotted residuals accounted for age and sex to demonstrate partial correlations for the stroke cohort between the sleep regularity index (SRI) and (A) total sleep time (TST), (B) wake after sleep onset (WASO), (C) fragmentation index, (D) sleep efficiency, (E) sleep condition indicator (SCI), α = 0.01 (Bonferroni corrected).
Healthy controls
There were no correlations between SRI and TST (r = 0.07, p = 0.616), Fragmentation Index (r = 0.04, p = 0.792), Sleep efficiency (r = -0.06, p = 0.659), wake after sleep onset (r = 0.01, p = 0.943), nor the sleep condition indicator (r = 0.28, p = 0.040; ns with Bonferroni correction) in the control group (Fig. 5). Notably, when the Average Sleep Time per 24 h is used, there is a significant correlation with the SRI, whereby more regular sleep is associated with shorter average sleep time per 24 h. Details figures can be found in Supplementary materials (figure S8 part B). Comparisons of the strength of correlations between the groups revealed no statistically significant differences (supplementary S4).
Plotted residuals accounted for age and sex to demonstrate partial correlations for the healthy control cohort between the sleep regularity index (SRI) and (A) and total sleep time (TST), (B) wake after sleep onset (WASO), (C) fragmentation index, (D) sleep efficiency, (E) sleep condition indicator (SCI), α = 0.01 (Bonferroni correction for multiple comparisons).
Stroke group analyses
To test the secondary hypothesis that stroke survivors with less regular sleep would have higher (worse) depression scores, a Pearson’s correlation (adjusted for age and sex) was performed on available data (n = 113). There was a significant correlation between the SRI and PHQ-8 (r = -0.26, p = 0.006; Fig. 6A) indicating that less regular sleep is associated with worse depression scores.
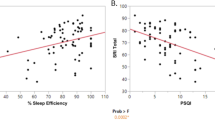
An exploratory objective was to investigate the correlation between SRI and quality of life (SF-SIS, n = 54). There was a significant correlation (adjusted for age and sex; r = 0.40, p = 0.002), revealing that when stroke survivors have more regular sleep, their reported quality of life is higher (Fig. 6B). However, there was no significant correlation between SRI and disability (mRS) or Time Since Stroke (supplementary S4).
Discussion
The relationship between sleep and stroke is intricate, as sleep-wake disturbances are both a risk factor and potential outcome of stroke. In the present study we utilised a pre-existing actigraphy dataset from community-dwelling stroke survivors and established a significant disparity in sleep regularity between stroke survivors and healthy controls. We also found, for the stroke group alone, that more regular sleep was linked to longer overall nighttime sleep time, better self-reported sleep quality, less severe depression scores and better quality of life.
The group difference suggests that the stroke cohort exhibited greater irregularity in their sleep patterns. This result aligns with a growing body of literature suggesting that many aspects of sleep are negatively affected after stroke4,33,34 — which could result from several interrelated factors. Neurological damage resulting from a stroke can impair regions of the brain responsible for sleep regulation, leading to disruptions in the sleep-wake cycle. Indeed, the neuroanatomical basis of sleep control is complex and involves multiple structures such as the thalamus, brainstem, hippocampus, and striatum —regions that can be affected by stroke35. However, the relationship between stroke lesion characteristics and sleep disruption remains poorly understood. The impact of stroke lesions on the microstructure of sleep, including brain oscillations such as slow waves and sleep spindles, is also an area requiring further exploration, given their role in memory consolidation and neuroplasticity processes that are vital for recovery36,37. Additionally, psychological factors such as anxiety, depression, and stress, which are prevalent among stroke survivors, could also contribute to sleep irregularities38. These psychological factors have specifically been linked to post-stroke fatigue, a prevalent phenomenon that affects patients’ quality of life39. Post-stroke fatigue, as well as environmental factors and treatment schedules during hospitalisation and rehabilitation disrupt normal sleep routines, further complicating sleep regularity. Sleep irregularity has been a largely overlooked feature that may have clinical relevance. Specifically, the circadian system is crucial for maintaining overall health as it regulates numerous physiological functions, including the sleep-wake cycle15, which are instrumental in post-stroke recovery. Indeed, it has been shown that poor sleep can have a negative effect on rehabilitation outcomes40. Poor sleep following a stroke has further been associated with notable cognitive and attentional impairments, along with negative health and clinical outcomes41. However, when our analysis was repeated with subjective sleep quality (SCI) included in the model, the effect of group was no longer significant. We cannot rule out the influence of a sampling bias given that several of the included studies recruited participants with the intention of accessing a programme to try to improve their sleep problems. Therefore, we have also conducted a sensitivity analysis that can be found in supplementary materials (Fig. S5). However, given that stroke survivors typically experience worse self-reported sleep than controls4 this result may reflect inherent differences between these populations5,42, irrespective of recruitment techniques. The finding that better self-reported sleep is associated with more regular sleep patterns in stroke survivors is of particular interest as many studies report that subjective and objective measures of sleep do not necessarily align well20,43. One possible explanation for this association is that patients may be more attuned to irregularities in their sleep patterns than to other metrics, such as sleep efficiency. For example, disruptions to regular sleep schedules may manifest in noticeable behaviours like the need for daytime naps to compensate for inadequate or inconsistent nighttime rest. This heightened awareness of irregular sleep could account for the stronger subjective reporting of poorer sleep quality when sleep patterns are less regular. Furthermore, aspects of insomnia treatment (cognitive behavioural therapy for insomnia) aim to regulate sleep and wake patterns to improve patient perceptions of sleep quality. Future research should thus test whether improvements in sleep regularity can impact sleep quality, or vice versa, after stroke.
We also found that being of older age and male was associated with lower sleep regularity. This is consistent with prior research, with males generally having less regular sleep than females44,45. With regards to age, it has been proposed that changes in sleep with aging may result from diminished circadian control of the sleep-wake cycle. Moreover, sleep disturbances are particularly pronounced in neurodegenerative conditions such as dementia, where they become increasingly severe with disease progression. Despite the high prevalence of these disturbances, evidence-based treatment options remain limited and underexplored. Adjusting the circadian timing system has been suggested as a potential strategy to mitigate age- and disease-related declines in sleep quality and daytime functioning46, which warrants further investigation. The SRI values observed in our study are lower than those reported in some larger population-based studies, which may be attributable to several factors. First, our study population is generally older than those used in many SRI studies, and as stated above, older adults may tend to exhibit lower SRI scores compared to younger, healthier individuals. Second, larger datasets, such as those from population cohorts like the UK Biobank, tend to capture a broader range of values, including more extreme scores, due to increased sample size and variability. Third, the specific type of actigraphy watch, smoothing algorithm and collection modality used (PIM in our study, versus ZCM in others) may also contribute to differences in SRI scores. While this difference in device sensitivity does not affect internal comparisons within our study, it should be considered when comparing our results to findings from studies using alternative actigraphy devices.
Within the stroke cohort only, a significant link was found between SRI and total sleep time (TST), where more regular sleep correlated with a longer actigraphy-derived total sleep time during the night. No associations were found within the healthy controls, but the strength of correlation did not differ significantly between the groups. Previous work has not found a correlation between TST and SRI in young healthy adults17 nor in a diverse sample of older adults18. Suboptimal sleep duration has been linked to an elevated risk of stroke occurrence47, and it remains to be seen whether sleep irregularity could also influence secondary stroke risk. We speculate that disruption to sleep after stroke could increase patients’ tendency to nap during the day, thereby diminishing sleep regularity. Furthermore, in our exploratory analysis, we observed that individuals with higher average total sleep across the 24-hour period, likely reflecting increased daytime napping, had lower sleep regularity, as indicated by a negative association between SRI and average daily sleep duration. This was evident in both the stroke and control cohorts, suggesting that greater fragmentation or redistribution of sleep across the 24-hour period may compromise the regularity of the sleep-wake cycle. These results align with the earlier observation that longer nighttime sleep was linked to higher SRI in the stroke cohort, likely due to reduced need for compensatory daytime naps for people who have longer nighttime sleep. Importantly, this metric is derived from the same actigraphy data used to calculate the SRI, making it an accessible and informative variable for future research on sleep regularity and recovery after stroke.
Within the stroke cohort, the correlation between depression scores and SRI was significant (controlling for age and sex). This is consistent with previous studies that have revealed an association between sleep regularity and depression scores18,48. As the relationship between sleep and depression is complex and bi-directional49, it may be that irregular sleep patterns contribute to higher depression scores and/or that more severe depressive symptoms adversely affect sleep-wake patterns. In addition to depression, apathy has also been reported as a common yet understudied neuropsychiatric consequence of stroke. Characterised by diminished goal-directed behaviour, apathy can negatively impact recovery and quality of life. Often overlapping with depression but also occurring independently, apathy may be relevant in understanding post-stroke sleep-wake disturbance and would be interesting to include in future research50.
We did not observe a significant relationship between SRI and time since stroke. This is consistent with previous findings that sleep problems can persist over time. Studies have shown that neither self-reported sleep nor actigraphy measures of sleep disruption improve significantly during subacute rehabilitation, and that there is no clear relationship between time since stroke and sleep disturbances4,9. However, some studies do report improvements in sleep from acute to chronic timepoints51. This underscores the importance of longitudinal research to better understand how sleep regularity evolves over time and in relation to stroke recovery trajectories. Similarly, we found no association between sleep regularity and level of disability after stroke. While this could indicate that functional impairment does not influence this specific aspect of sleep, it likely reflects limitations in sensitivity of this measure or cohort characteristics. These possibilities are further considered in the limitations section.
Finally, there was a significant relationship between sleep regularity and quality of life in stroke survivors, with higher sleep regularity indicating better quality of life. This finding aligns with previous work in other patient populations52, and is, to our knowledge, the first account reported in stroke. This is crucial, as earlier work has highlighted that sleep disorders can further reduce the quality of life in stroke patients, and diagnosing and treating sleep disturbance is vital to optimising functional outcomes and improving quality of life53. This finding presents an opportunity to investigate whether interventions targeting sleep regularity can enhance quality of life post-stroke.
Limitations and future research
Limitations of the present study should be mentioned. As the data were obtained from several studies investigating post-stroke sleep, each with distinct objectives, there was a potential sampling bias. All participants are community-dwelling stroke survivors, ranging from the sub-acute to the chronic phase (mostly in the chronic phase). Future research could attempt to replicate our findings, but in a less heterogeneous group and earlier post-stroke. Furthermore, a limitation of this study is the lack of data on participants’ occupational or academic status. Social factors, including work schedules and daily responsibilities, are known to influence sleep-wake rhythms. While our sample included both retired and working individuals in both groups, we were unable to formally account for these variables, which may differentially affect sleep-wake patterns—particularly when comparing stroke survivors, who may experience reduced social constraints post-stroke, with healthy controls. It should further be noted that the sample consists mainly of stroke survivors who range from no to moderate disability, as measured by the mRS. There are very few participants with severe functional disability in the entire dataset. A general limitation in stroke research is that stroke survivors with very severe disabilities may not meet specific study criteria and may lack the physical or cognitive ability to participate, limiting generalisability across stroke. Moreover, we did not have access to NIHSS scores to assess acute stroke severity, which may influence sleep patterns and offer a more sensitive measure than functional disability alone. While we included related metrics such as the mRS and SF-SIS to capture post-stroke disability and quality of life, future studies should incorporate formal measures of stroke severity like the NIHSS to better understand how stroke characteristics relate to sleep outcomes. Additionally, a limitation of the current study is the absence of cognitive assessments within the dataset, as this analysis was conducted using pre-existing data. While previous research suggests a potential link between sleep disturbances and cognitive impairment in acquired brain injury6,7, we were not able to explore this relationship directly. Future studies incorporating cognitive measures would be valuable in further elucidating the interplay between post-stroke sleep-wake disturbances and cognitive functioning. Finally, although we found no relationship between SRI and time since stroke, the study was cross-sectional. Future research should focus on examining changes in sleep regularity longitudinally, as this remains unexplored and the timescale by which sleep-wake problems occur post-stroke is unclear.
Conclusion
We have demonstrated that the sleep regularity index could be a useful metric for post-stroke sleep research. When combined with other sleep metrics, SRI could potentially help address unresolved questions and provide new insights, as it uniquely accounts for sleep and wake patterns over the entire day and night. To our knowledge, this study is the first to investigate the sleep regularity index in a stroke cohort. Stroke survivors exhibited greater irregularity in their sleep patterns than controls, and more irregular sleep was associated with shorter total sleep time and worse self-reported sleep. Most notably, there was evidence connecting sleep regularity with quality of life and depression scores after stroke. Sleep regularity could be a novel target to improve post-stroke quality of life, but research is first needed to study changes in sleep regularity longitudinally, particularly to test for changes alongside recovery, and to identify potential methods for addressing sleep regularity problems.
Data availability
The code for all processing is open access and can be found on GitHub (https://github.com/KatSchruers/RISES). The data of this study are available from the corresponding author, MKF, upon reasonable request.
References
Kim, A. S., Cahill, E. & Cheng, N. T. Global stroke belt: Geographic variation in stroke burden worldwide. Stroke 46, 3564–3570 (2015).
Bakhshaie, J. et al. Temporal precedence of the change in obsessive-compulsive symptoms and change in depressive symptoms during exposure and response prevention for pediatric obsessive-compulsive disorders. Behav. Res. Ther. 133, 103697 (2020).
Byun, E. et al. Stroke impact symptoms are associated with sleep-related impairment. Heart Lung 49, 117–122. https://doi.org/10.1016/j.hrtlng.2019.10.010 (2020).
Fleming, M. K. et al. Self-reported and objective sleep measures in stroke survivors with incomplete motor recovery at the chronic stage. Neurorehabilit. Neural Repair 35, 851–860 (2021).
Baglioni, C. et al. Polysomnographic characteristics of sleep in stroke: A systematic review and meta-analysis. PLoS One 11, e0148496 (2016).
Ouellet, M. C., Savard, J. & Morin, C. M. Book review: insomnia following traumatic brain injury: A review. Neurorehabilit. Neural Repair 18, 187–198 (2004).
Falck, R. S. et al. Sleep and cognitive function in chronic stroke: A comparative cross-sectional study. Sleep. https://doi.org/10.1093/sleep/zsz040 (2019).
Suh, M., Choi-Kwon, S. & Kim, J. S. Sleep disturbances after cerebral infarction: Role of depression and fatigue. J. Stroke Cerebrovasc. Dis. 23, 1949–1955. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.01.029 (2014).
Fleming, M. K. et al. Sleep disruption after brain injury is associated with worse motor outcomes and slower functional recovery. Neurorehabilit. Neural Repair 34, 661–671 (2020).
Marino, M. et al. Measuring sleep: Accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 36, 1747–1755. https://doi.org/10.5665/sleep.3142 (2013).
Lichstein, K. L. et al. Actigraphy validation with insomnia. Sleep 29, 232–239 (2006).
Sadeh, A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med. Rev. 15, 259–267 (2011).
Fekedulegn, D. et al. Actigraphy-Based assessment of sleep parameters. Ann. Work Expo Health 64, 350–367. https://doi.org/10.1093/annweh/wxaa007 (2020).
Gottlieb, E. et al. The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: A systematic review. Sleep Med. Rev. 45, 54–69 (2019).
Khan, S., Duan, P., Yao, L. & Hou, H. Shiftwork-Mediated disruptions of circadian rhythms and sleep homeostasis cause serious health problems. Int. J. Genomics 2018, 8576890. https://doi.org/10.1155/2018/8576890 (2018).
Czeisler, C. A. Duration, timing and quality of sleep are each vital for health, performance and safety. Sleep Health J. Natl. Sleep. Found. 1, 5–8 (2015).
Phillips, A. J. K. et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci. Rep. 7, 3216. https://doi.org/10.1038/s41598-017-03171-4 (2017).
Lunsford-Avery, J. R., Engelhard, M. M., Navar, A. M. & Kollins, S. H. Validation of the sleep regularity index in older adults and associations with cardiometabolic risk. Sci. Rep. 8, 14158. https://doi.org/10.1038/s41598-018-32402-5 (2018).
Windred, D. P. et al. Sleep regularity is a stronger predictor of mortality risk than sleep duration: A prospective cohort study. Sleep https://doi.org/10.1093/sleep/zsad253 (2023).
Fleming, M. K. et al. Improving sleep after stroke: a randomised controlled trial of digital cognitive behavioural therapy for insomnia. J. Sleep Res. e13971 (2023).
Weightman, M. et al. Improving sleep and learning in rehabilitation after stroke, part 2 (INSPIRES2): Study protocol for a home-based randomised control trial of digital cognitive behavioural therapy (dCBT) for insomnia. BMJ Open 13, e071764. https://doi.org/10.1136/bmjopen-2023-071764 (2023).
Weightman, M. et al. Sleep and motor learning in stroke (SMiLES): A longitudinal study investigating sleep-dependent consolidation of motor sequence learning in the context of recovery after stroke. BMJ Open 14, e077442 (2024).
Wesselius, H. M. et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern. Med. 178, 1201–1208 (2018).
CamNtechLtd. MotionWare Software. https://www.camntech.com/motionware-software/.
Fischer, D., Klerman, E. B. & Phillips, A. J. K. Measuring sleep regularity: Theoretical properties and practical usage of existing metrics. Sleep. https://doi.org/10.1093/sleep/zsab103 (2021).
de Zambotti, M. et al. State of the science and recommendations for using wearable technology in sleep and circadian research. Sleep zsad325 (2023).
McLaren, D. M. et al. Assessing insomnia after stroke: A diagnostic validation of the sleep condition Indicator in self-reported stroke survivors. BMJ Neurol. Open 6, e000768. https://doi.org/10.1136/bmjno-2024-000768 (2024).
Espie, C. A. et al. The sleep condition indicator: Reference values derived from a sample of 200 000 adults. J. Sleep Res. 27, e12643 (2018).
Shin, C., Lee, S. H., Han, K. M., Yoon, H. K. & Han, C. Comparison of the usefulness of the PHQ-8 and PHQ-9 for screening for major depressive disorder: Analysis of psychiatric outpatient data. Psychiatry Investig. 16, 300–305. https://doi.org/10.30773/pi.2019.02.01 (2019).
Banks, J. L. & Marotta, C. A. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke 38, 1091–1096 (2007).
Jenkinson, C., Fitzpatrick, R., Crocker, H. & Peters, M. The stroke impact scale. Stroke 44, 2532–2535. https://doi.org/10.1161/STROKEAHA.113.001847 (2013).
Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 18, 91–93. https://doi.org/10.1016/j.tjem.2018.08.001 (2018).
Cai, H., Wang, X. P. & Yang, G. Y. Sleep disorders in stroke: An update on management. Aging Dis. 12, 570 (2021).
Frange, C., Murray, B. J. & Coelho, F. M. S. The importance of sleep for successful neurorehabilitation after stroke. Sleep Sci. 16, e335–e343 (2023).
Gottlieb, E. et al. Regional neurodegeneration correlates with sleep–wake dysfunction after stroke. Sleep 43, zsaa054 (2020).
Diekelmann, S. & Born, J. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010).
Duss, S. B. et al. The role of sleep in recovery following ischemic stroke: A review of human and animal data. Neurobiol. Sleep. Circadian Rhythms 2, 94–105 (2017).
Bassetti, C. L. In Seminars in Neurology. 19–32 (Thieme Medical Publishers Inc., 2005).
Ponchel, A. et al. Influence of medication on fatigue six months after stroke. Stroke Res. Treat. 2016, 2410921. https://doi.org/10.1155/2016/2410921 (2016).
Iddagoda, M. T., Inderjeeth, C. A., Chan, K. & Raymond, W. D. Post-stroke sleep disturbances and rehabilitation outcomes: A prospective cohort study. Intern. Med. J. 50, 208–213. https://doi.org/10.1111/imj.14372 (2020).
Gudberg, C. & Johansen-Berg, H. Sleep and motor learning: Implications for physical rehabilitation after Strokegudberg. Front. Neurol. https://doi.org/10.3389/fneur.2015.00241 (2015).
Cavalcanti, P., Campos, T. & Araujo, J. Actigraphic analysis of the Sleep–Wake cycle and physical activity level in patients with stroke: Implications for clinical practice. Chronobiol. Int. 29, 1267–1272. https://doi.org/10.3109/07420528.2012.719960 (2012).
Unruh, M. L. et al. Subjective and objective sleep quality and aging in the sleep heart health study. J. Am. Geriatr. Soc. 56, 1218–1227. https://doi.org/10.1111/j.1532-5415.2008.01755.x (2008). https://doi.org:.
Guarnieri, B. et al. Multicenter study on sleep and circadian alterations as objective markers of mild cognitive impairment and Alzheimer’s disease reveals sex differences. J. Alzheimers Dis. 78, 1707–1719. https://doi.org/10.3233/JAD-200632 (2020).
Alves Facundo, L. et al. Sleep regularity in athletes: Comparing sex, competitive level and sport type. Chronobiol. Int. 39, 1381–1388. https://doi.org/10.1080/07420528.2022.2108716 (2022).
Cajochen, C., Münch, M., Knoblauch, V., Blatter, K. & Wirz-Justice, A. Age‐related changes in the circadian and homeostatic regulation of human sleep. Chronobiol. Int. 23, 461–474. https://doi.org/10.1080/07420520500545813 (2006).
He, Q. et al. Sleep duration and risk of stroke: A dose–response meta-analysis of prospective cohort studies. Sleep Med. 32, 66–74. https://doi.org/10.1016/j.sleep.2016.12.012 (2017).
Castiglione-Fontanellaz, C. E. G. et al. Sleep regularity in healthy adolescents: Associations with sleep duration, sleep quality, and mental health. J. Sleep Res. 32, e13865. https://doi.org/10.1111/jsr.13865 (2023).
Fang, H., Tu, S., Sheng, J. & Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 23, 2324–2332. https://doi.org/10.1111/jcmm.14170 (2019).
van Dalen, J. W., van Charante, E. P. M., Nederkoorn, P. J., van Gool, W. A. & Richard, E. Poststroke apathy. Stroke 44, 851–860 https://doi.org/10.1161/STROKEAHA.112.674614 (2013).
Bakken, L. N., Kim, H. S., Finset, A. & Lerdal, A. Subjective sleep quality in relation to objective sleep estimates: Comparison, gender differences and changes between the acute phase and the six-month follow‐up after stroke. J. Adv. Nurs. 70, 639–650 (2014).
Trivedi, R. et al. Irregular sleep/wake patterns are associated with reduced quality of life in Post-treatment Cancer patients: A study across three Cancer cohorts. Front. Neurosci. https://doi.org/10.3389/fnins.2021.700923 (2021).
Salah, S., Rekik, M. & Frih, Z. Association of quality of life and sleep quality in patients with ischemic stroke. J. Diabetes Treat. 10, 2574–7568 (2018).
Acknowledgements
Tom Smejka and Ellie Macey who were involved in original data collection.
Funding
This work is supported by the Wellcome Trust and the NIHR Oxford Health Biomedical Research Centre (NIHR203316). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. MKF is Funded by Guarantors of Brain and HJB is funded by the Wellcome Trust (222446/Z/21/Z). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z and 203139/A/16/Z).
Author information
Authors and Affiliations
Contributions
Conceptualization: K.B.S., M.K.F.; Methodology: K.B.S., M.K.F., A.á.V.G., M.W.; Investigation: M.W., B.R.; Data Curation: K.B.S., A.á.V.G., M.W., B.R.; Formal Analysis: K.B.S., A.á.V.G., M.W.; Writing – Original Draft: K.B.S.; Writing – Review & Editing: K.B.S., A.á.V.G., M.W., B.R., M.K.F., H.J.B.; Supervision: M.K.F.; Funding Acquisition: M.K.F., H.J.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schruers, K.B., Weightman, M., Guttesen, A.á.V. et al. Sleep regularity index as a novel indicator of sleep disturbance in stroke survivors: a secondary data analysis. Sci Rep 15, 17510 (2025). https://doi.org/10.1038/s41598-025-01332-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01332-4