Abstract
Infection following rhino-prosthesis surgery poses a significant challenge, with primary treatment strategies centered on identifying specific pathogenic bacteria through bacterial culture and administering effective antibiotics. This study aimed to investigate whether conducting bacterial cultures that specifically target the nasal prosthesis could provide more objective guidance for clinical decision-making. We included patients who developed infections subsequent to prosthesis rhinoplasty in this investigation. The clinical significance of bacterial cultures obtained from nasal prostheses was assessed by comparing the culture results with those derived from the nasal mucosa or secretions from disrupted wounds. Notably, the bacterial detection rate in samples taken from prosthetic devices was significantly higher than that observed in conventional specimens from the same patients, exhibiting a statistically significant difference. Within the prosthetic group, no statistically significant difference was identified in bacterial detection rates between patients who received antibiotics 3 days prior to surgery and those who did not. In contrast, in the conventional control group, there was a marked decrease in bacterial detection rates among patients following antibiotic administration. Among patients treated with antibiotics, the detection rate of bacteria cultured from prosthetic specimens was significantly higher compared to that in the control group; however, there were no statistical differences found regarding bacterial detection rates for those not treated with antibiotics. In analyzing materials within the prosthesis group: silicone implants showed a bacterial detection rate of 80%, expanded polytetrafluoroethylene materials had a rate of 79.2%, and other materials demonstrated a 90.9%rate. No statistically significant differences were noted among these three material types within this cohort. In contrast, within the control group: silicone implants exhibited a bacterial detection rate of 66.7%, expanded polytetrafluoroethylene materials registered at 73.6%, while other materials maintained a lower count at 63.6%. Similarly, no statistically significant differences prevailed among these groups. Culturing prosthetic specimens can enhance the detection rate of bacteria, particularly in patients who have received antibiotic therapy prior to surgery, offering distinct advantages. Therefore, we advocate for the implementation of prosthetic specimen cultures as an adjunctive measure for detecting infections post-nasal prosthesis reconstruction. Among patients treated with antibiotics, the rate of bacteria detected from cultured prosthetic specimens was significantly higher compared to that in the control group. However, no statistically significant differences were observed regarding bacterial detection rates in patients not receiving antibiotic treatment. When analyzing materials within the prosthesis group: silicone implants exhibited a bacterial detection rate of 80%, expanded polytetrafluoroethylene (ePTFE) materials had a detection rate of 79.2%, and other materials demonstrated a higher rate of 90.9%. Notably, there were no statistically significant differences among these three material types within this cohort. In contrast, within the control group: silicone implants displayed a bacterial detection rate of 66.7%, ePTFE materials showed a rate of 73.6%, while other materials recorded a lower count at 63.6%. Similarly, no statistically significant differences emerged among these groups. Culturing prosthetic specimens can enhance the detection rates of bacteria, particularly in patients who have undergone antibiotic therapy prior to surgery, thereby offering distinct advantages for infection diagnosis. Therefore, we advocate for incorporating cultures of prosthetic specimens as an adjunctive measure for detecting infections following nasal prosthesis reconstruction.
Level of evidence: Level 4.
Similar content being viewed by others
Introduction
With the increasing emphasis on aesthetic appeal, the plastic surgery industry has experienced swift growth, particularly within the domain of nasal prosthesis procedures. These surgical interventions are highly sought after for their effectiveness in enhancing facial contours and improving overall aesthetic appearance1,2,3. However, despite advancements in technology and a diversification of surgical techniques that have significantly improved both safety and efficacy, postoperative complications continue to be a critical factor influencing surgical outcomes and patient satisfaction4,5,6. Notably, postoperative infections are a common yet serious complication that can not only lead to prosthesis rejection but also give rise to a range of more complex health issues6,7. The overall prevalence of prosthesis rhinoplasty has been reported to be 3.7%8,9. This situation imposes substantial psychological and economic burdens on patients.
Despite the potential risk of complications, why are prostheses for rhinoplasty still frequently utilized in clinical practice? Currently, the primary techniques for rhinoplasty utilizing autologous tissue transplantation include auricular cartilage grafting, costal cartilage grafting, and autologous fascia grafting9,10. The quantity of autologous ear cartilage that can be harvested is significantly limited, which may result in morphological damage to the donor site and inadequate structural support. Despite providing excellent support, autologous rib cartilage is characterized by its relatively tough texture and a potential for long-term deformation. This limits its applicability in rhinoplasty, particularly concerning dorsal nasal grafts where the risk of long-term asymmetry is notably elevated10,11. While autologous fascia transplantation can effectively augment the nasal dorsum, it exhibits significant long-term resorption, and its inadequate support makes it challenging to elevate the nasal tip or extend the nasal length. Given the limitations associated with autologous materials, artificial materials remain an essential alternative in rhinoplasty and have gained popularity due to the advantage of avoiding donor site morbidity.
The mechanism of infection following nasal prosthesis surgery is intricate and may be influenced by a multitude of factors, including the surgical technique employed, postoperative care protocols, the patient’s immune status, and the invasion of pathogenic microorganisms7,12,13. Research indicates that the primary infectious pathogens are predominantly common skin strains such as Staphylococcus aureus and Staphylococcus epidermidis, alongside various opportunistic pathogens. If these bacteria are not effectively managed during the healing process of postoperative wounds, they can lead to infections that hinder recovery and potentially result in displacement or expulsion of the prosthesis7,13,14. Currently, research on bacterial cultures related to infections following nasal prosthesis plastic surgery remains relatively sparse13,15. Notably, there is a lack of systematic analysis pertaining to diverse patient backgrounds, surgical techniques utilized, and postoperative care strategies implemented. Therefore, conducting a clinical study focused on bacterial cultures associated with infections after nasal prosthesis surgery is essential not only to address this notable research gap but also to provide significant insights for clinical practice.
Previously, pathogen detection for infections following nasal prosthesis surgery primarily relied on bacterial cultures obtained from either the ruptured wound or the nasal mucosa14,15,16. However, this methodology may produce false positive results and could potentially decrease the true positive detection rate. Such inaccuracies can lead to misguided therapeutic interventions. Given that the nasal prosthesis is embedded within tissue, its associated false positive rate is likely to be lower; hence, it generally serves as a more accurate focal point of infection16,17,18. Consequently, conducting bacterial cultures specifically targeting the nasal prosthesis may offer more objective guidance in clinical decision-making.
In this study, we included patients with infections following prosthesis rhinoplasty. We investigated the clinical significance of bacterial cultures from nasal prostheses by comparing them to cultures from nasal mucosa or secretions from ruptured wounds. We hope our findings will provide an objective basis for clinical diagnosis and treatment.
Methods
Patients
This study adhered to the Declaration of Helsinki guidelines for human research. Informed consent was obtained from all participants, ensuring their privacy rights were maintained. We conducted a retrospective review of patients diagnosed with infections after prosthetic rhinoplasty between January 2013 and December 2023 at Jiangxi Provincial People’s Hospital affiliated with Nanchang Medical College in Jiangxi, China, and Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Patient inclusion criteria:
(1) Patients diagnosed with an infection following prosthetic rhinoplasty;
(2) Patients who underwent bacterial culture of wound or nasal mucosa;
(3) Patients who had their prosthesis removed and nasal debridement performed;
(4) Prosthetic implants extracted from the patient’s nose were subjected to bacterial culture;
(5) The patient’s medical history was complete and accurately documented;
(6) The patient was informed about the study and provided consent.
Patient Exclusion Criteria:
(1) The patient had a prior history of infection before undergoing nasal prosthesis surgery;
(2) The patient also presented with sinusitis or a respiratory tract infection;
(3) The patient’s medical history record was incomplete;
(4) The patient did not provide consent to participate in the study.
Bacterial cultivation
Upon admission, we began cleansing the nasal cavity with physiological saline. For patients with skin or mucosal lesions, secretions from these areas were collected for bacterial culture. When no lesions were present, a cotton swab was used to sample the nasal mucosa for bacterial culture. This collection process served as the control group for our study. This was also the traditional method of obtaining specimens and bacteria culture group.
In accordance with the laboratory department’s standard operating procedures, wound secretions were collected using sterile cotton swabs in a controlled environment and promptly sent for examination. Upon receipt, pathogenic bacteria were isolated using routine culture methods with disposable sterile blood culture bottles and blood agar plates. Initial bacterial counts were recorded only for patients from whom repeated strains had been consecutively isolated twice. For identification of pathogenic bacteria, individual colonies underwent characterization through a bacterial assay system.
For the implants, residual blood was carefully removed with saline, and the implants were placed in a sterile petri dish. The excised implants were transported to a lab for processing within two hours. Each implant’s surface was coated with cooled melted tryptone soybean agar (TSA) and cultured at 37 °C in an atmosphere of 5% CO2. Daily monitoring assessed bacterial growth, supplementing sterile TSA as needed to prevent desiccation. If bacterial colonies appeared, samples from three distinct locations were collected and sent for pathogen identification. This procedure was continued for 14 days or until evidence of colony proliferation emerged. Finally, the implants were disposed of according to medical waste regulations. We designated this cohort as the prosthesis group.
Comprehensive analysis of medical history data
The patient’s preoperative medical history was documented with a focus on prior nasal surgeries, nasal infections, antibiotic use, prosthetic materials, immunocompromised status, and other health conditions. Standardized photographs of the patient’s face were taken in specific positions before surgery. Postoperative assessment involved close monitoring of the wound for infection signs—such as redness, swelling, warmth, and pain—which were recorded comprehensively. Additionally, detailed information regarding medical history—including surgical records, post-infection treatment protocols, and patient recovery data—was meticulously collected and analyzed.
Statistical analysis
The Chi-square test was used for statistical analysis of count data. A P-value under 0.05 indicated a statistically significant difference.
Results
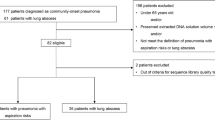
The design framework for this study is illustrated in Fig. 1.
In this study, a total of 120 eligible patients were enrolled. Among the participants, 89 were female and 31 were male. The mean age of the included patients was 32.5 ± 11.3 years. The duration of postoperative infection in these patients ranged from 1 week to 6 years following surgery. Patients exhibited signs of infection, including tissue redness, swelling, warmth, and pain. Of the total cohort, 92 patients experienced skin or mucosal rupture, while 28 patients did not present with such ruptures.
For the prosthetic group, 98 patients tested positive for bacterial cultures associated with the prosthesis, while 22 patients yielded negative results. In the control group, 83 patients exhibited positive outcomes for these cultures, whereas 37 patients tested negative (Fig. 2a). The identified bacterial species included Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Staphylococcus hominis, Enterobacter cloacae, Proteus mirabilis, Citrobacter spp., and Acinetobacter baumannii. The difference in bacterial detection rates between the two groups was found to be statistically significant (P = 0.0353; see Fig. 2b).
For preoperative antibiotic administration, 70 patients received antibiotics for three days prior to surgery, while 50 patients did not receive any antibiotics during the same period. Within the prosthesis group, the bacterial detection rates among patients who were administered antibiotics and those who were not were found to be 82.9% (58/70) and 80% (40/50), respectively; this difference was determined to be statistically insignificant (P = 0.8116; see Fig. 3a). In the control group, the rates of bacterial detection among patients who had received antibiotics compared to those who had not within the three days preceding surgery were 60% (42/70) and 82% (41/50), respectively; this difference was established as statistically significant (P = 0.0155; see Fig. 3b).
Comparison of bacterial detection rates within group with or without antibiotic use. (A) Within the prosthesis group, the bacterial detection rates among patients who were administered antibiotics and those who were not were found to be 82.9% and 80%, respectively; this difference was determined to be statistically insignificant. (B) In the control group, the rates of bacterial detection among patients who had received antibiotics compared to those who had not within the three days preceding surgery were 60%and 82%, respectively; this difference was established as statistically significant.
Patients in the prosthesis group who received antibiotics exhibited a higher rate of bacterial detection compared to those in the control group, with this difference achieving statistical significance (82.9% vs 60%, P = 0.0047, see Fig. 4a). There was no statistically significant difference in bacterial detection rates between patients who did not use antibiotics in the prosthetic group and those who did not use antibiotics in the control group (80% vs 82%, P > 0.05, see Fig. 4b).
Comparison of bacterial detection rates between groups with or without antibiotic use. (A) Patients in the prosthesis group who received antibiotics exhibited a higher rate of bacterial detection compared to those in the control group, with this difference achieving statistical significance. (B) There was no statistically significant difference in bacterial detection rates between patients who did not use antibiotics in the prosthetic group and those who did not use antibiotics in the control group.
Among the patients included in this study, 45 individuals received silicone implants, 53 were implanted with expanded polytetrafluoroethylene materials, and 22 had other types of implants, such as Medpor. In the prosthesis group, the bacterial detection rates for silicone implants, expanded polytetrafluoroethylene materials, and other materials were found to be 80% (36/45), 79.2% (42/53), and 90.9% (20/22), respectively. There was no statistically significant difference in the bacterial detection rates among the three materials within this group (P = 0.4616; see Fig. 5a). In the control group, the bacterial detection rates for silicone implants, expanded polytetrafluoroethylene materials, and other materials were recorded at 66.7% (30/45), 73.6% (39/53), and 63.6% (14/22), respectively. Similarly, there was no statistically significant difference in bacterial detection rates among these three materials within the control group (P = 0.6273; see Fig. 5b).
Comparison of bacterial detection rates between different prosthesis uses in the two groups. (A) There was no statistically significant difference in the bacterial detection rates among the three materials within the prosthesis group. (B) In the control group, there was no statistically significant difference in bacterial detection rates among these three materials.
Discussion
Bacterial culture of nasal prostheses and traditional bacterial culture of nasal wound secretions each offer unique advantages and limitations in clinical applications19,20. A comparative analysis and discussion of these two methodologies can not only assist clinicians in their diagnostic and therapeutic decisions but also enhance the optimization of postoperative management and infection control strategies.
From the perspective of sample sources, bacterial culture of nasal prostheses primarily concentrates on detecting bacteria present in the tissues surrounding the implant and on the surface of the prosthesis21,22,23. This methodology facilitates a direct assessment of the risk associated with bacterial infection linked to the prosthesis, particularly when overt symptoms of infection emerge post-surgery; it allows for expedited identification of the source of infection24,25. In contrast, traditional bacterial culture methods involve analyzing nasal wound secretions obtained from patients who have undergone trauma, surgery, or suffer from chronic rhinitis26,27. While this technique can also identify infections, its results may be influenced by external environmental factors impacting the wound as well as individual physiological conditions28,29. Consequently, this presents greater challenges in analyzing infections associated with prosthetic devices.
The sensitivity and specificity of culture techniques are influenced by standardized bacterial culture protocols; however, the combination of nasal prosthesis bacterial cultures with advanced molecular biology methods, such as PCR, can significantly improve the detection rates of certain pathogens that are difficult to isolate or culture30,31,32. In contrast, traditional cultures of nasal wound secretions often require prolonged incubation periods, which may lead to delayed pathogen identification—an aspect particularly critical during acute infections—and consequently result in missed opportunities for optimal treatment.
The timeliness of clinical application serves as a crucial comparative factor between the two approaches33,34. In the immediate postoperative period following nasal prosthesis surgery, there is an elevated risk of infection; therefore, the rapid acquisition of pathogen information through bacterial culture from the nasal prosthesis enables timely adjustments that can alleviate patient discomfort and decrease medical expenses35,36. Conversely, traditional bacterial cultures of nasal wound secretions require a substantial amount of time to yield results, potentially leading to delayed treatment and increased risks of complications for patients experiencing acute or severe infections37,38. From both economic and operational perspectives, collecting nasal wound secretions is relatively straightforward and cost-effective, making it suitable for routine outpatient care as well as emergency situations39,40,41,42. In contrast, bacterial culture from nasal prostheses typically necessitates specialized sampling techniques and culturing processes that may demand greater technical expertise and financial resources43,44,45. Nevertheless, with advancements in technology, some hospitals have progressively begun to implement rapid culture techniques to enhance early diagnosis of periprosthetic infections.
In this study, the bacterial detection rate in prosthesis specimens was significantly higher than that observed in traditional specimens from the same patients, with a statistically significant difference evident. This finding underscores the advantages of bacterial culture from prosthesis specimens concerning their detection rates. In the prosthetic group, there was no statistically significant difference in bacterial detection rates between patients who received antibiotics three days prior to surgery and those who did not. Conversely, in the conventional control group, following antibiotic administration, the bacterial detection rate among patients decreased markedly. This suggests that a protective biofilm of bacteria may form around the prosthesis, impeding antibiotic penetration and thereby reducing their bactericidal and bacteriostatic efficacy. Furthermore, this implies that early removal of prostheses may be an essential intervention for managing infections in patients with nasal infections involving implants.
Among patients treated with antibiotics, the detection rate of bacteria cultured from prosthetic specimens was significantly higher compared to that in the control group; however, there were no statistical differences found regarding bacterial detection rates for those not treated with antibiotics. Therefore, in patients using antibiotics, bacterial culture of prostheses can improve the detection rate of bacteria. In analyzing materials within the prosthesis group: silicone implants showed a bacterial detection rate of 80% (36/45), expanded polytetrafluoroethylene materials had a rate of 79.2% (42/53), and other materials demonstrated a 90.9% (20/22) rate. No statistically significant differences were noted among these three material types within this cohort. In contrast, within the control group: silicone implants exhibited a bacterial detection rate of 66.7% (30/45), expanded polytetrafluoroethylene materials registered at 73.6% (39/53), while other materials maintained a lower count at 63.6% (14/22). Similarly, no statistically significant differences prevailed among these groups. This shows that the incidence of infection may not be very different when different artificial materials are used for rhinoplasty.
Limitations
This study has several limitations. First, the sample size was relatively small, which may limit the generalizability of our findings. Second, the cross-sectional nature of the study design means that causality cannot be inferred from the observed associations. Additionally, the assessment of some variables relied on self-reported data, which is subject to information bias. Future research with larger sample sizes and longitudinal study designs would be beneficial to validate and extend our findings.
Conclusions
Infection following rhino-prosthesis surgery presents a significant challenge, with the primary treatment strategies focusing on the identification of specific pathogenic bacteria through bacterial culture and the administration of effective antibiotics. Culturing prosthetic specimens can enhance the detection rate of bacteria, particularly in patients who have received antibiotic therapy prior to surgery, offering distinct advantages. Consequently, we advocate for the implementation of prosthetic specimen cultures as an adjunctive measure for detecting infections post-nasal prosthesis reconstruction.
Data availability
The datasets used and/or analysed analyzed during the current study are available from the corresponding author on reasonable request.
References
Lawless, M., Swendseid, B., Windheim, N. V., Vankoevering, K., Seim, N., & Old, M. Review of cost and surgical time implications using virtual patient specific planning and patient specific implants in midface reconstruction. 整形与美容研究 (英文版). 9(4), 11–24 (2022).
Zeng, Q., Hu, Y. G., Tang, Y. X., Yu, B. F. & Li, X. Complications and treatments of pseudomonas aeruginosa infection after rhinoplasty with implants: A clinical study. J. Craniofac. Surg. 34(2), E104–E108 (2023).
Dermody, S. M., Lindsay, R. W. & Justicz, N. Considerations for optimal grafting in rhinoplasty. Facial Plast. Surg. 39(6), 625–629 (2023).
Ganga, K., & Mcmullen, C. State-of-the-art management for challenging complications in head and neck surgery. 整形与美容研究 (英文版). 10(12), 32–41 (2023).
Wei, J., Dai, C. & Li, S. Revision rhinoplasty in Asians. Clin. Plast. Surg. 50(1), 141–149 (2023).
Dm, T. et al. Evaluation Of postoperative infection rates in 3084 rhinoplasty cases using antibiotic soaks and/or irrigations. Facial Plast. Surg. Aesthet. Med. 23(5), 368–374 (2021).
Is, K. Augmentation rhinoplasty using silicone implants. Facial Plast. Surg. Clin. N. Am. 26(3), 285–293 (2018).
Jy, H., Sa, A. & Bf, A. Complications of the nasal dorsum reconstruction using autologous or alloplastic grafts: evidence from systematic review and meta-analysis. Braz. J. Otorhinolaryngol. 88, 406–420 (2022).
Bf, Yu. et al. An innovative stent consisting of expanded polytetrafluoroethylene and ear cartilage in rhinoplasty for Asians: Application I of Dai’s exogenous extension stent concept. J. Craniofac. Surg. 34, 2506–2509 (2023).
Yu, B. F., Wei, J. & Dai, C. C. A mortise and tenon framework composed of costal cartilage and expanded polytetrafluoroethylene in rhinoplasty for Asians: Application II of Dai exogenous extension stent concept. J. Craniofac. Surg. 1, 1 (2024).
Bf, Yu. et al. Designing a nasal lining-framework complex for reconstructing total nasal defects. Otolaryngol. Head Neck Surg. 172, 457–465 (2025).
Diaspro, A., Redaelli, A., Saban, Y., Sulamanidze, C., Pascali, M., & Bertossi, D. Medical nose reshaping: Current evidence behind techniques. 整形与美容研究 (英文版). 10(6), 30–48 (2023).
Radulesco, T. et al. A safe nonsurgical rhinoplasty procedure. Plast. Reconstr. Surg. 150(1), 83e–86e (2022).
Jalil, J., Bonanthaya, K. & Parmar, R. Nasoalveolar molding: Benefits and burdens. 整形与美容研究 (英文版) 10(4), 68–84 (2023).
Kn, T. & Yj, J. Incidence and predisposing factors of postoperative infection after rhinoplasty: A single surgeon’s 16-year experience with 2630 cases in an East Asian population. Plast. Reconstr. Surg. 150(1), 51e–59e (2022).
Cj, S. & Dt, G. Imaging features of rhinoplasty. AJNR Am. J. Neuroradiol. 35(2), 216–222 (2014).
Persson, M. Cutting edge-an innovative psychosocial training program for healthcare professionals who provide appearance-altering procedures. 整形与美容研究 (英文版). 10(4), 56–67 (2023).
Jy, C. Complications of alloplast rhinoplasty and their management: A comprehensive review. Facial Plast. Surg. 36(5), 517–527 (2020).
An, Y., Wang, G. & Zhen, Y. A perioperative disinfection and caring procedure to prevent infection after rhinoplasty. Aesthetic Plast. Surg. 47(5), 2217–2218 (2023).
Tb, W. & Hr, J. Complications of costal cartilage asian rhinoplasty and their management. Facial Plast. Surg. 36(5), 528–538 (2020).
Heilbronn, C., Cragun, D. & Wong, B. Complications in rhinoplasty: A literature review and comparison with a survey of consent forms. Facial Plast. Surg. Aesth. Med. 22(1), 50–56 (2020).
Yoo, Sh. & Yj, J. Rib cartilage in Asian rhinoplasty: New trends. Curr. Opin. Otolaryngol. Head Neck. Surg. 27(4), 261–266 (2019).
Chen, Y. et al. Complications following thread rhinoplasty. J. Cosmet. Dermatol. 21(10), 4722–4726 (2022).
Ls, B. History of rhinoplasty. Oral Maxillofac. Surg. Clin. N. Am. 24(1), 1–9 (2012).
Fanous, N. et al. Soft and firm alloplastic implants in rhinoplasty: Why, when and how to use them: A review of 311 cases. Aesth. Plast. Surg. 41(2), 397–412 (2017).
Challita, R., Shouman, M. & Ghanime, G. Rhinoplasty and external nasal splinting: Is it really a must. Plast. Reconstr. Surg. Glob Open. 7(8), E2374 (2019).
Rettinger, G. Risks and complications in rhinoplasty. Gms Curr. Top Otorhinolaryngol. Head Neck Surg. 6, 8 (2007).
Yj, J. & Dy, K. Treatment strategy for revision rhinoplasty in Asians. Facial Plast. Surg. 32(6), 615–619 (2016).
Berghaus, A. & Stelter, K. Alloplastic Materials In Rhinoplasty. Curr Opin Otolaryngol Head Neck Surg. 14(4), 270–277 (2006).
Rogal, J., Glasgold, A. & Glasgold, R. A. Safety and efficacy of non- and minimally irradiated homologous costal cartilage in primary and revision rhinoplasty. Facial Plast. Surg. Aesth. Med. 23(1), 25–30 (2021).
Zhao, R., Pan, B., Li, D. & An, Y. Application of paranasal augmentation rhinoplasty in asians with midfacial concavity. Ann. Plast. Surg. 90(Suppl 2), S147–S152 (2023).
Nguyen, T. A., Reddy, S. & Gharavi, N. Specific complications associated with non-surgical rhinoplasty. J. Cosmet. Laser Ther. 22(4–5), 171–173 (2020).
Sajjadian, A., Rubinstein, R. & Naghshineh, N. Current status of grafts and implants in rhinoplasty: Part I. Autologous Grafts. Plast. Recons. Surg. 125(2), 40e–49e (2010).
Tham, T. et al. Clinical outcomes in dorsal preservation rhinoplasty: a meta-analysis. Facial Plast. Surg. Aesth. Med. 24(3), 187–194 (2022).
Wang, X. et al. Predicting risk of infection after rhinoplasty with autogenous costal cartilage: A cohort study. Aesthetic Plast. Surg. 46(4), 1797–1805 (2022).
Aldosari, B. Lengthening short noses in rhinoplasty. Ear Nose Throat J. 1455613241288486 (2024).
Holt, G. R., Garner, E. T. & Mclarey, D. Postoperative Sequelae and complications of rhinoplasty. Otolaryngol. Clin. N. Am. 20(4), 853–876 (1987).
Datta, S. et al. Nasal tip deprojection in rhinoplasty. Plast. Reconstr. Surg. 1, 1 (2024).
Bussi, M., Palonta, F. & Toma, S. Grafting in revision rhinoplasty. Acta Otorhinolaryngol. Ital. 33(3), 183–189 (2013).
Li, J. et al. Long-term complications from diced cartilage in rhinoplasty: A meta-analysis. Facial Plast. Surg. Aesth. Med. 24(3), 221–227 (2022).
D’ascanio, L. et al. Endoscopic, “quick” septoplasty in preservation rhinoplasty. Ann. Plast. Surg. 86(2), 137–141 (2021).
Georgiou, I., Farber, N., Mendes, D. & Winkler, E. The role of antibiotics in rhinoplasty and septoplasty: A literature review. Rhinology 46(4), 267–270 (2008).
Mcgraw-Wall, B. & Macgregor, A. R. Concurrent functional endoscopic sinus surgery and rhinoplasty: Pros. Facial Plast. Surg. Clin. N. Am. 12(4), 425–429 (2004).
Dn, E., Grunebaum, Ld. & Be, H. Actinomyces: An under appreciated cause of postoperative infection in rhinoplasty. Laryngoscope. 133(11), 2948–2950 (2023).
Hoang, T. A., Lee, K. C., Dung, V. & Chuang, S. K. Augmentation rhinoplasty in cleft lip nasal deformity using alloplastic material and autologous cartilage. J. Craniofac. Surg. 33(8), E883–E886 (2022).
Acknowledgements
We would like to thank all the participants for participating in this study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The study was initiated and designed by Yu-Xi Tang and Qi Zeng. The data collections were made by Yu-Xi Tang and Bao-Fu Yu. Analysis of data were made by Yu-Xi Tang and Qi Zeng, and the final statistical analysis was performed by Bao-Fu Yu. The manuscript draft was made by Yu-Xi Tang, Qi Zeng and Bao-Fu Yu. Critical revision of the manuscript for key intellectual content were made by Yu-Xi Tang, Qi Zeng and Bao-Fu Yu. and all authors reviewed and approved the final version of the article submitted for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was in accordance with the Declaration of Helsinki and was approved by the IRB of Jiangxi Provincial People’s Hospital. All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeng, Q., Tang, YX. & Yu, BF. Prosthetic bacterial culture for bacterial identification of nasal infections after rhinoplasty. Sci Rep 15, 16572 (2025). https://doi.org/10.1038/s41598-025-01377-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01377-5