Abstract
Global warming, produced by the accumulation of greenhouse gases in the atmosphere, is causing alterations in the life cycle of flora and fauna species and consequently threatening food security. The objective of this research was to study the relationship between climatic variables and the incidence and presence of Perkinsiella saccharicida populations, a sucking insect considered a pest, that attacks sugarcane crops depending on the climatic season. This study was carried out in sugarcane plantations of Ingenio San Carlos, located in Marcelino Maridueña, Ecuador. We analyzed climatic data and Perkinsiella population records from 2006 to 2023, applying various parametric (KS, ANOVA, Bartlett, Dickey-Fuller) and non-parametric (Spearman, Shapiro, Kruskall-Wallis, Levene) statistical tests, along with CCF and causality analysis. The findings revealed that the current summer averages of atmospheric variables have increased and are now comparable to winter averages from ten years ago. High temperatures and Solar_R values were found to precede Perkinsiella population spikes by 6 months. A causal relationship was established between specific atmospheric variables and Perkinsiella. Initial outbreaks were observed during the summer, with population sizes increasing over time. Currently, there is a tendency for the pest to persist throughout the year (winter-summer), necessitating greater biological and chemical control measures to manage populations.
Similar content being viewed by others
Introduction
Sugarcane is the main source of raw material for Ecuador’s sugar agroindustry and represents the country’s second largest permanent crop in terms of production volume. In 2022, a total of 116,515 ha were planted nationwide, of which 113,148 ha were harvested, yielding 7,740,492 Tm of production. By 2023, these figures declined to 79,580 ha planted and 75,222 ha harvested - a 33.5% decrease – resulting in a 6,253,732 Tm of production. Sugarcane plantations are primarily concentrated in Guayas1. 70% of the cultivated area is located in the lower Guayas Basin, encompassing regions such as Milagro, Naranjito, Marcelino Maridueña (Ingenio San Carlos), El Triunfo and Playas.
In Ecuador, Perkinsiella saccharicida (Homoptera, Delphacidae) is a sucking insect that particularly affects sugarcane crops and is present across all sugarcane-growing regions of the country. It causes substantial yield losses ranging from12 to 36.68 tons per hectare2. However, the direct damage caused by the leafhopper through its bites on the sugarcane foliage is not considered the most significant threat. The primary concern is that this insect is a vector of the Fiji disease virus, a highly dangerous disease that is not currently present in the Americas, is difficult to control, and can cause severe crop losses. Therefore, preventive control of P. saccharicida3 is of critical importance.
According to Pulido 1980, earlier studies in Mauritius in 1903 and 1975 found that the pest’s phenological stage and duration are influenced by temperature and relative humidity. At temperatures of 22ºC and 25ºC, the life cycle of Perkinisella lasted 48 days and 56 days, respectively. The egg incubation stage can last 14 days, but at temperatures below 20ºC it can last 35 to 40 days4.
Scientific studies conducted worldwide indicate that climate change is a reality5,6,7,8. This phenomenon has contributed to rising temperatures, resulting in brief but abundant rainy seasons in some areas and prolonged dry seasons in others9. These changes affect the dissemination and distribution mechanisms of insects, and Perkinsiella is no exception. Temperature is the environmental factor that exerts the most significant influence on insect development10,11,12,13,14, as it affects development rates, survival, fecundity, and dispersion of several species15,16. According to Challinor et al.17, by 2030, crop yields are expected to decline by up to 70%, with losses ranging from 10 to 50% in approximately half of all cases due to climate change. Similarly, Savary et al.18, estimates that between 10% and 28% of global crop production is lost due to pests. This study aimed to evaluate the presence of P. saccharicida in sugarcane crops and analyze its relationship with environmental variables to determine whether climatic variations during both winter and summer influence its population dynamics.
Materials and methods
Study area
Ecuador is located in the southwest of South America. Its climate is influenced by oceanographic factors, atmospheric circulation and marine currents attributable to the Intertropical Convergence Zone (ITCZ)19. The country’s climate variability is attributed to the El Niño-Southern Oscillation (ENSO), with El Niño events characterized by abnormally warm ocean temperatures and increased cloudiness, and precipitation, while La Niña events are associated with abnormally cold ocean temperatures, leading to reduced cloudiness and precipitation. This allows the country to have four different climate regions. The coastal region characterizes for having a tropical climate20,21,22.
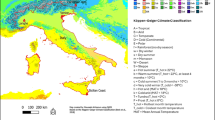
This study was conducted in Marcelino Maridueña, located 65 km away from Guayaquil, at a geographical position of 02°15’52’’ South Latitude and 79°28’00’’ West Longitude, and an altitude of 80 m.a.s.l. (Fig. 1). The area experiences a maximum temperature of 30.8 °C and a minimum of 22.3 °C, with a thermal oscillation of 8.5 °C. The potential evaporation is 1,027 mm, with 784 h per year of sunshine and 2,699 MJ*m2*year of solar radiation23.
Sugarcane population and sample size
Sugar cane belongs to the grass family and to the species Saccharum officinarum L. The size of the sugarcane population was of 12,169.53 hectares, distributed across 890 lots ranging from 3 to 40 hectares. Out of these, 96 lots (totaling 1,246 hectares) were evaluated. Observations were conducted annually from 2006 to September 2023, following the recommendations of the Entomology and the Sugar Cane Research Center of Ecuador (CINCAE).
The evaluation involved randomly selecting 50 sugarcane shoots per lot, each with up to 90 days of growth. For each shoot, the number of adult insects, as well as large and small nymphs (nymphs close to adulthood), were counted. It should be noted though, that the values presented in the following section were calculated as the average number of insects per shoot. To extrapolate them to hectares, they were multiplied by 70,000 (\(\:70\:rows*\left(10\:shoots*100\:mt\right),\) which corresponds to the number of shoots per hectare.
The lots with the highest insect infestation were monitored over a ten-year period (2006–2016). The results showed an annual average yield of 79.84 tons of sugarcane per hectare (ha), compared to a production potential of 97.1792 tons per hectare (based on the average of five harvests). This resulted in 17.33 tons of losses (sugarcane that was not harvested) per ha, resulting in an annual average of 17.83% of tons lost.
Description of variables
The study was based on the analysis of Perkinsiella abundance in relation to climatic variables. The data used in this study was obtained from the records made by officials from the Entomology and Statistics (Perkinsiella) and Meteorological (climate variables obtained from the “Casa Blanca” weather station located within San Carlos) Departments of Ingenio San Carlos. The insect population data was compared with the climate variables. Table 1 provides a detailed overview of these variables.
Source: Meteorological (Agronomy department) and insect data (Entomology and Statistics department) from Ingenio San Carlos.
Climatology
To demonstrate that considerable climatic variations have occurred in the study area, climatological analyses were performed. The current Technical Regulation No. 49 of the World Meteorological Organization (WMO)24 states that climatological normals correspond to the mean of the climatological data over a 30-year period, which starts in a year ending in one and finishes in a year ending in zero. These normals should be updated every ten years.
To calculate the current climatological normal, historical records of the climatic parameters from the past 30 years (a retrospective series starting from the most recent year ending in zero − 2020) were obtained from the Casa Blanca weather station. This period corresponded to the years 1991 to 2020, in accordance with WMO24 guidelines. As a first step, for each atmospheric parameter, the climatological normal (1991–2020) was calculated as the monthly average across the 30-year period, along with the corresponding monthly standard deviations. Subsequently, for the 2006–2023 data series, anomalies were calculated as the difference between the actual recorded value in a given month and the corresponding monthly mean value for that same month (climatological normal, 1991–2020). This produced a new series of anomalies for the period 2006–2023.
For this study, these anomalies were then normalized by dividing each monthly anomaly by the standard deviation corresponding to that month (calculated similarly to the climatological normal 1991–2020). This method of normalizing anomalies is used to reduce variability due to the long-term climatic fluctuations that has been observed. According to Trewin25, “for the most part, large-scale climatic fluctuations consist of nonlinear variations that oscillate, in the long term, irregularly around a climatological mean value.“. On the other hand, Perkinsiella anomalies (2006–2023), were calculated as the difference between the recorded values and the corresponding monthly means and subsequently normalized by dividing each value by the monthly standard deviation for the same period. These anomalies, were then used for analyses between Perkinsiella and atmospheric parameters.
Weather seasons
The analysis considered two typical weather seasons of the coastal region: the dry-summer season, which includes the months of low precipitation (June to November), and the rainy-winter season (December to May)19,20.
Statistical methods
Interpolation of data
In the case of Perkinsiella, atypical values were found in the months of July and August 2011.
The data series exhibited a sinusoidal pattern (behavior similar to the sine-cosine functions due to their association with the climatic cycles). Hence, the shape preserving interpolant method for curves known as Piecewise Cubic Hermite Interpolating Polynomial (PCHIP) was employed for interpolation to preserve the monotonicity and convexity, present in the data26. This technique uses cubic polynomials defined by segments, with the objective of estimating-interpolating values between known data points. The process begins by dividing the data into intervals and then calculating the derivatives at the data points so that the smoothness of the curve is maintained. Subsequently, cubic polynomials are constructed for each interval, so that the curve passes through the points while preserving consistent derivatives. This approach, which fits a specific cubic polynomial between each pair of points, was found to provide the most accurate interpolation for the weather parameters used in this study27.
Statistical analysis
Descriptive statistical analyses were conducted, including measures of central tendency (mean and median) and dispersion (standard deviation, range, coefficient of variation, and standard error of the mean).
Boxplot graphs were used to identify outliers, and time series graphs were employed to reveal behavioral patterns. A convolution method filter, MA (3), was applied to enhance and highlight the presence of intense events in the time series. Non-parametric hypothesis tests were employed, considering factors such as non-normality, small sample sizes, and the presence of outliers, with a significance level of \(\:\alpha\:=0.05\). Lilliefors28 was used to test for normality in big samples (n > = 50), and Shapiro29 for small samples (n < 50). To test for homogeneity of variance, Bartlett’s test was applied when assuming normality, whereas Levene’s test was employed in cases of non-normality.
For comparing means across more than two independent groups (e.g., year–season combinations), ANOVA was applied along with TukeyHSD when variances were homogeneous.When variances were not homogeneous, equality of medians Kruskall Wallis test30 and Wilcoxon’s pairwise31 were applied. Spearman test32 was used to study the linear relationship between the insect population and climate. Cross-correlation function (CCF) was used to identify out-of-phase correlations. To test for causality in the time series, the Granger test33,34 was applied to determine if one time series can forecast another time series. Stationarity was evaluated using the Dickey–Fuller test35. Cointegration analysis was conducted using the Johansen test, and a Vector Error Correction Model (VECM) was applied, given that the series were cointegrated and exhibited a long-term equilibrium relationship. The statistical analyses and map were performed using RStudio 2024.12.1 and QGIS 3.40.1 software.
Results
Analysis of atmospheric parameters anomalies based in the current climatological normal, the most recent atmospheric behavior in the coastal region (Ecuadorian coastal region), severity of pest infestation, and the relationship between climate and Perkinsiella abundance are presented. The period 2006–2023 was considered for all analyses throughout this research, prioritizing the continuity of complete data in the variables of interest.
Atmospheric conditions of the study area
The atmospheric behavior from January 2006 to September 2023 showed different trends depending on the climatic season (Table 2).
Period: 2006–2023, VC = variation coefficient, s.e.=standard error.
Rainy season
During this season, climatic variables exhibited the highest values for mean, median, minimum, and maximum. Ther_O, Evap and Helio showed greater variability, as indicated by their higher ranges and coefficients of variation. Standard errors (s.e.) were also higher for Acum_P, Evap, Helio, and Solar_R, indicating greater uncertainty in the mean values recorded during this time of the year (Table 2).
Dry season
Ther_O showed a similar mean to that of the rainy season, Evap and Helio reached higher minimum values, Acum_P was more variable (CV = 379.4%), as well as Helio (Table 2).
During the analysis period, the study area experienced notable climatic events, whose effects on atmospheric parameters are evident in the time series of anomalies. Starting in 2010, an upward trend was observed in temperature, Acum_P, and Solar_R (Fig. 2a, b, c, d, h), with the anomaly series (orange line) fluctuating above the central baseline (0). In contrast, Evap, Ther_O, and Helio (Fig. 2e, f, g) showed a downward trend, indicating a general decrease in these parameters over time (Fig. 2).
Notable positive peaks were observed across all analyzed climatic parameters, most prominently in temperature and Acum_P. These peaks were especially evident during the periods 2014–2016, 2018–2019, and 2023, indicating that during these years, the recorded values in the study area exceeded the expected climatological normals based on the most recent 30-year period. Similarly, negative anomalies with pronounced troughs were detected during 2007–2008, 2010–2012, 2016–2018, and 2020–2023, reflecting periods when climatic values fell significantly below the long-term average.
Severity of Perkinsiella infestation
Initially, Perkinsiella was scarcely present, with several months showing no recorded individuals. Thereafter, it exhibited both high and low abundances. The highest peaks occurred in July 2011 and 2020, with average insect counts per outbreak reaching 58.51 and 56.40, respectively (Fig. 3a). Seasonal changes in Perkinsiella behavior were evident. The insect was more abundant during the rainy season; however, since 2019, a decrease in median counts was observed, and the combined values of nymphs and adults became more consistent, reflecting reduced variability. The dry season was characterized by low counts, but since 2011, an increase in population size was noted along with greater variability and the presence of outliers, registering higher maximum values (Fig. 3b).
The seasonal change in patterns in both the insect and climate was analyzed in greater detail. Between 2006 and 2010, the insect exhibited distinct seasonal behavior, showing its highest abundance only during the rainy season (Fig. 4a). However, from 2011 to 2023, this pattern shifted, with a higher average abundance from March to October, in both rainy and dry seasons (Fig. 4b).
Perkinsiella-climate relationship
The standardized anomalies for the period 2006–2023 were analyzed to study the insect-climate relationship, with an emphasis on differences between weather seasons.
Rainy season
climatic variables exhibited higher mean, median, minimum and maximum values, particularly in temperature, Acum_P, and Solar_R. During this season, Perkinsiella reached a peak average abundance of 28.36 insects per outbreak (Table 2).
Dry Season: Perkinsiella abundance peaked at 58.51 insects per outbreak on average, the highest values observed across all sample statistics. Min_T and Acum_P showed higher coefficients of variation during this season (Table 2).
Across both seasons, fluctuations were observed in most climatic variables, except for Solar_R, where increases occurred during the rainy season and decreases during the dry season. To analyze the relationship between Perkinsiella and atmospheric parameters, Spearman correlation coefficients were calculated due to the presence of outliers (evident as peaks in the anomaly series, Fig. 2) and non-normal distributions—except for Max_T, Ther_O, and Solar_R, which met normality assumptions (Table 3).
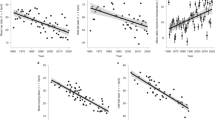
Despite the observed changes in both Perkinsiella and climatic conditions, statistically significant linear correlations were identified by weather season (Figs. 5 and 6). Significant correlation coefficients (p-value < 0.05) were obtained for the rainy season between Perkinsiella and Min_T (coef. correlation=−0.36) and Helio (coef. correlation = 0.24). Additionally, Med_T (coef. correlation = −0.19) and Ther_O (coef. correlation = 0.16) were significant at the α = 0.10 level. Positive correlations were observed with Ther_O and Helio, while negative correlations were observed with Min_T and Med_T, suggesting that Perkinsiella is particularly sensitive to increases in minimum temperatures (Fig. 5).
Temperature-related parameters exhibited significant positive linear correlations with the other atmospheric parameters (p-values < 0.05) in both climatic seasons, although stronger correlations occurred during the rainy season (Table 3; Figs. 5 and 6).
Figures 5 and 6 showed a weak linear trend between the climatic parameters and Perkinsiella, consistent with the low correlation coefficients, some of which were statistically significant.
The significant relationship between Perkinsiella and atmospheric parameters was further analyzed using the CCF, applied to the complete time series to fulfill the continuity condition required for a time series analysis. As shown in Fig. 7, it is evident that the strongest significant correlation (above the confidence limit) between the insect and climatic parameters occurred at different time lags. These correlations were positive in all atmospheric parameters except for Acum_P, indicating that above-average values in atmospheric variables are associated with a subsequent increase in Perkinsiella abundance. high Perkinsiella abundance was observed approximately six months after elevated values of Max_T, Med_T, and Solar_R. In contrast, for Evap, Ther_O, and Helio, high insect abundance preceded the increase in these climatic parameters by one month. In the case of Min_T and Acum_P, the strongest associations with Perkinsiella occurred at lags greater than 12 months.
To assess whether the insect–climate relationships exhibited causality, the Granger causality test (for stationary time series) and the Toda–Yamamoto approach (for cointegration analysis in non-stationary time series) were applied.
To perform the bivariate Granger test, time series for Perkinsiella and the atmospheric variables Min_T, Acum_P, Evap, Ther_O, and Helio were used. These series satisfied the stationarity condition, as confirmed by the Dickey–Fuller test (p-values < 0.05). None of the atmospheric parameters showed statistically significant Granger causality on Perkinsiella (p-values > 0.05); however, it should be noted that Min_T showed a p-value of 0.12, which is only slightly above the significance threshold of α = 0.10 (Table 4).
To further investigate potential causality, we explored whether joint dynamic interactions among multiple atmospheric variables could explain Perkinsiella dynamics.
Table 5 shows that only one relationship failed to demonstrate cointegration among the variables, as the null hypothesis was not rejected (9.88 < 11.65) at a 1% significance level. However, eight relationships exhibited cointegration, justifying the application of a Vector Error Correction Model (VECM). The model was implemented with a cointegration rank of 6, indicating that up to six cointegration relationships were estimated.
The VECM results revealed that the first lag ((values from the previous month) of Perkinsiella, Max_T, Evap, Helio, and Solar_R were statistically significant predictors of Perkinsiella abundance, with the model explaining 34.3% of the variance. The error correction term (Ect1) was negative, indicating that the system tends to return to equilibrium following a disturbance (Table 6).
Based on the VECM model results (Table 6), it is estimated that a 1 °C increase in Max_T leads to an increase of approximately 1.17 Perkinsiella individuals. Similarly, a one-unit increase in Helio is associated with an increase of 0.34 individuals. In contrast, a one-unit increase in Evap and Solar_R is associated with decreases of 0.34 and 0.27 Perkinsiella individuals, respectively.
The forecasts revealed the typical upward trend in Perkinsiella abundance during the winter months, with the highest predicted values occurring between April and August (as shown in the abundance forecast column). The VECM model, calculated the forecast in the form of standardized anomalies; these values were converted back to abundance values by applying the inverse of the standardization process (Table 7).
To explain the observed changes in Perkinsiella abundance and assess the influence of climatic variables, the non-parametric Kruskal–Wallis test was applied. This test was chosen due to small sample sizes (n = 6, months per season-year), non-normal data distribution, heterogeneity of variances, and the presence of outliers in some variables. The objective was to determine whether significant differences existed between seasons in recent years.
The results showed that Min_T, Max_T, Mean_T, Evap, Ther_O, and Helio during the dry seasons of 2011–2022 (a complete seasonal series) did not differ significantly from the median values of the rainy seasons during 2006–2010 (p > 0.05). This indicates that the current medians (2011–2022) of the dry season register statistically equal values to the medians that occurred in the rainy season of 2006 to 2010 (Table 8). Acum_P and Solar_R were characterized by showing significant differences between the climatic seasons.
Perkinsiella-climate relationship can be observed in Fig. 8, where Perkinsiella series (weather season-standardized and smoothed MA (3)) and temperature environmental parameters are compared.
Figure 9 shows how the peaks (amplitudes of positive and negative series) coincide between Perkinsiella series and atmospheric parameters. This suggests that during periods of negative temperature anomalies (i.e., temperatures below the monthly average), positive anomalies in Perkinsiella abundance (i.e., values above the monthly average) were also recorded (negative correlations in rainy season). A similar pattern was observed for other climatic parameters as well.
The results of this study demonstrate a progressive increase in temperature over time, which is associated with a higher incidence of Perkinsiella outbreaks. Furthermore, the data indicates that insect outbreaks not only occur during the dry season but also exhibit a marked tendency to persist throughout the year.
Discussion
In 2023, the values of Min_T, Max_T, and Mean_T from January to September recorded significantly higher positive anomalies compared to recent years. This is attributed to the high temperatures (Mean_T average of 27.0 °C) associated with the El Niño event and anthropogenic emissions. Additionally, there were locations where temperatures exceeded pre-industrial average temperatures by 1.5 °C on several days (during the summer solstice)5.
During this analysis period, the study area experienced ENSO events, and their effect on atmospheric parameters was evident through time series of anomalies, calculated relative to 1991–2020 period. The impact caused by the El Niño events of 2014–2016, 2018–2019 and 202336 generated considerable positive anomalies in all analyzed climatic parameters, particularly in temperature and Acum_P. Conversely, considerable amplitudes of negative anomalies, in the aforementioned variables, were observed due to the climatological phenomenon known as La Niña in the years 2007–2008, 2010–2012, 2016–2018, and 2020–202336. The period 2006–2023 did not record major ENSO events like those in the other periods, for example 1997–1998. However, in the period 2006–2023, the annual accumulated of Acum_P showed an increasing trend. This increase aligned with the expected behavior for Central and South America37.
The irregular seasonal occurrence pattern of Perkinsiella was also reported by Flores et al.38 at three study sites. Their findings indicated that infestations tipically begin during the dry season, but the highest population peaks occur during the rainy season (April and July in Valdez; November and March in Ecudos; and the first months of the rainy season at Ingenio San Carlos), demonstrating that the increase in temperature and relative humidity favor the development of the pest. Similarly Mendoza et al.39 observed significant fluctuations in population abundance throughout the year in Ecuador, noting that infestations generally begin during the dry season, persist through the rainy season, and peak in February or March. Moraga40 and several other authors10,12,13,15,16,41,42,43,44,45,46,47,48,49 agree that climatic conditions substantially influence insect embryonic and post-embryonic development, life cycle duration, population dynamics and density, geographical distribution, and survival. Consistent with these findings, Pulido Fonseca4 reported that environmental factors, especially temperature and relative humidity, affect the developmental duration of each life stage, in Hawaii, the life cycle of Perkinsiella lasted 48 days at 22 °C and 56 days at 25 °C, with the egg stage alone lasting 35–40 days at these temperatures.
In this study, the summer (cooler) season recorded an average temperature of 24.88 °C, while the winter (warmer) season averaged 26.54 °C. Minimum and maximum temperatures were 23.4 °C and 27.07 °C in summer, and 24.5 °C and 27.87 °C in winter, respectively—values consistent with those reported by Pulido Fonseca. Temperature increases, depending on the species, may influence developmental rates, the number of generations per year, population density, genetic composition, host plant use, and both local and geographic distribution. Moreover, elevated temperatures can favor pest outbreaks, weaken natural enemy populations, and reduce host plant resistance14. These alterations can be attributed to both natural global climate change and anthropogenic climate change, which have caused modifications in ecosystems and the extinction of species6.
In this regard, Mendoza et al.39 reported the appearance of the insect during the dry season and note temporary population increases during extended drought periods. Similarly, Contreras et al.50, in studies on the Central American locust (Schistocerca piceifrons Walker), observed population surges and concluded that the insect’s biological development cycle is triggered by rising temperatures and drought conditions. Wilson et al.51 documented that S. saccharivora (Delphacidae), a sugarcane pest in Louisiana, emerged as a significant threat during outbreak years—2012, 2016, 2017, and 2019—with high winter temperatures being associated with these events.
Climate change and extreme weather events exert profound impacts on crop production and pest dynamics. Insect pests, being highly adaptable, respond differently to various climate change drivers and increasingly pose a major threat to food security. Effective pest management strategies are essential to mitigate this issue. Therefore, understanding pest biology and behavior in relation to environmental changes is crucial, as climate change will impact their distribution and activity52,53.
Climate fluctuations, such as increasing temperatures and changes in precipitation, have an impact on agricultural production and insect pests. Global warming is associated with the geographic expansion of insects, increased winter survival, more reproductive cycles, and a higher risk of invasive species and insect-borne diseases. In tropical regions, insect populations may decline as temperatures approach or exceed their physiological optima, whereas increases in pest abundance are expected in temperate zones. Moreover, rising temperatures can indirectly impact crop yields by increasing atmospheric water demand, leading to water stress and reduced soil moisture availability. These conditions can also intensify the frequency and severity of heatwaves, which may influence pest populations (e.g., increased mortality of temperature-sensitive species), as well as the prevalence of weeds and plant diseases6,52,54,55,56,57,58,59.
Conclusions
The climatic conditions during the 2006–2023 period have shown a notable increase in anomalies, particularly in 2023, with significant deviations in temperature, precipitation, and solar radiation parameters. The weather in the study area exhibits two distinct seasonal patterns. The rainy season recorded the highest average minimum and maximum temperatures over the twelve months of each year. Temperatures showed notable variations; currently (2006–2023), there have been increases in means and medians primarily during the dry season (e.g., + 0.25 °C average and + 0.20 °C median in Mean_T).
Perkinsiella showed changes in its abundance from March to October since 2011, in both dry and rainy seasons. The medians of the temperatures during the dry season of recent years (2011–2022) are similar to those of the rainy season from a decade ago (2006–2010), indicating that temperatures are favorable for the insect, since it had only exhibited abundance during the rainy season in former years.
There is a clear relationship between Perkinsiella and the climate during the rainy season, where the highest absolute values (> 0.19) were recorded in Min_T, Helio, and Med_T. However, due to climate change, the values recorded in recent years during the dry season are similar to those of previous rainy seasons, creating a favorable scenario for insect reproduction throughout the years. These findings demonstrate the presence of climate change and its impact on biological elements such as Perkinsiella, which has increased its population in sugarcane shoots, leading to economic losses in production systems.
Data availability
Correspondence and data requests should be addressed to Edwin Jiménez-Ruiz: ejimenez@espol.edu.ec.
References
INEC. Principales Resultados de La Encuesta de Superficie y Producción Agropecuaria Continua, ESPAC. (2024).
Puente Tenezaca, V. & Jimenez Ruiz, E. Influencia de los cambios climáticos en la población del Salta Hoja Hawaiano (Perkinsiella Saccharicida) y sus efectos en la producción de la caña de azúcar, en el Cantón Marcelino Maridueña. Preprint at (2017).
Wilson, B. E. Hemipteran pests of sugarcane in North America. Insects 10, 1–15 (2019).
Pulido Fonseca, J. I. Ciclo Bilógico Y Hábitos Del Perkinsiella saccharicida Kirkaldy (Homoptera:Delphacidae) Plaga De La Caña De Azúcar (Universidad Nacional de Colombia, 1980).
Hoping for better. Nat. Clim. Change 14 1 (2024).
Field, C. B. et al. Climático Impactos, Adaptación y Vulnerabilidad Parte A: Resumen Para Responsables de Políticas: Contribución Del Grupo de Trabajo II al Quinto Informe de Evaluación Del Grupo Intergubernamental de Expertos Sobre El Cambio Climático.Pdf. (2014). (2014).
Houghton, J. T. et al. Climate Change 2001: The Scientific Basis: Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. (2001). https://doi.org/10.1016/S1058-2746(02)86826-4
Houghton, J. T. & Climate Change : The Science of Climate Change: Contribution of Working Group I to the Second Assessment Report of the Intergovernmental Panel on Climate Change. vol. 2 (Cambridge University Press, 1996). (1995).
Stocker, T. F. et al. Cambio Climático 2013: Bases Físicas: Resumen Para Responsables de Políticas, Resumen Técnico y Preguntas Frecuentes: Contribución Del Grupo de Trabajo I al Quinto Informe de Evaluación Del Grupo Intergubernamental de Expertos Sobre El Cambio Climático. (2013).
Hamilton, J. G. et al. Anthropogenic changes in tropospheric composition increase susceptibility of soybean to insect herbivory. Environ. Entomol. 34, 479–485 (2005).
Gregory, P. J., Johnson, S. N., Newton, A. C. & Ingram, J. S. I. Integrating pests and pathogens into the climate change/food security debate. J. Exp. Bot. 60, 2827–2838 (2009).
Vega, J. M. et al. Prevalence of cutaneous reactions to the pine processionary moth (Thaumetopoea pityocampa) in an adult population. Contact Dermat. 64, 220–228 (2011).
Garibaldi, L. A. & Paritsis, J. Cambio climático e insectos herbívoros. (2012).
Kondo, T. & Medellín Insectos plaga del árbol urbano con énfasis en los insectos escama (hemiptera: coccoidea) en Colombia. in MemoriasResúmenes Congreso Colombiano de Entomología. 42. Congreso SOCOLEN. Antioquia vol. 29 364–382 (2015).
Karuppaiah, V. & Sujayanad, G. K. Impact of climate change on population dynamics of insect pests. (2012).
Bale, J. S. et al. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Chang. Biol. 8, 1–16 (2002).
Challinor, A. J. et al. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 4, 287–291 (2014).
Savary, S. et al. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, 430–439 (2019).
Gómez, H. et al. Atlas Marino Costero del Ecuador_Capítulo 1 Oceanografía y Clima. (2015).
World Bank Group. Climate Change Knowledge Portal. Ecuador (2021). https://climateknowledgeportal.worldbank.org/country/ecuador/climate-data-historical
NOAA. Cold (La Niña) Episodes in the Tropical Pacific. (2005). https://www.cpc.ncep.noaa.gov/products/analysis_monitoring/lanina/cold_impacts.shtml
NOAA. Warm (El Niño/ Southern Oscillation - ENSO) Episodes in the Tropical Pacific. (2012). https://www.cpc.ncep.noaa.gov/products/analysis_monitoring/impacts/warm_impacts.shtml
Ingenio San Carlos. Dpto. de Campo, Sección Agronomía. (2015).
Volúmen, W. M. O. I - Normas meteorológicas de carácter general y prácticas recomendadas. 75 Preprint at (2021).
Trewin, B. C. Función de las normales climatológicas en un clima cambiante. vol. 61 1 Preprint at (2007).
Fritsch, F. N. & Carlson, R. E. Monotone piecewise cubic interpolation. Soc. Industrial Appl. Math. 17, 238–246 (1980).
Ramdas, G., Bharti, G. & Deepak, P. Weather parameter analysis using interpolation methods. Artif. Intell. Appl. 1, 260–472 (2023).
Lilliefors, H. W. On the Kolmogorov-Smirnov test for normality with mean and variance unknown. J. Am. Stat. Assoc. 62, 399–402 (1967).
Shapiro, S. S. & Wilk, M. B. An analysis of variance test for normality (complete samples). Biometrika 52, 591–611 (1965).
Kruskal, W. H. & Wallis, W. A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 47, 583–621 (1952).
Wilcoxon, F. Individual comparisons by ranking methods. Biometrics Bull. 1, 80–83 (1945).
Spearman, C. The proof and measurement of association between two things. Am. J. Psychol. 15, 72–101 (1904).
Granger, C. W. Investigating causal relations by econometric models and Cross-spectral methods. Econometrica 37, 424–438 (1969).
Wiener, N. The theory of prediction. Modern mathematics for engineers. New. York. 165, 6 (1956).
Said, S. E. & Dickey, D. A. Testing for unit roots in autoregressive-moving average models of unknown order. Biometrika 71, 599–607 (1984).
NOAA. Cold & Warm Episodes by Season. El Niño / Southern Oscillation (ENSO (2023). https://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php
Barros, V. R. et al. Climate Change 2014 Impacts, Adaptation, and Vulnerability Part B: Regional Aspects: Working Group Ii Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Climate Change 2014: Impacts, Adaptation and Vulnerability: Part B: Regional Aspects: Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (2014). https://doi.org/10.1017/CBO9781107415386
Flores, O. R., Mendoza, M. J. & Gualle, A. D. Biología y dinámica poblacional de Perkinsiella saccharicida, en caña de azúcar. 1–8 (2003).
Mendoza, J., Martínez, I., Alvarez, A. M., Ayora, A. & Luzuriaga, V. Manejo Del Saltahojas de La Caña de Azúcar, Perkinsiella saccharicida (Homoptera: Delphacidae), en el Ecuador. Memorias. Primer Taller Latinoamericano Sobre Plagas de La Caña de Azúcar. Guayaquil-Ecuador 17–22 (2001).
Moraga, E. Q. Plagas de insectos y cambio climático. Phytoma España: La revista profesional de sanidad vegetal 21–31 (2011).
Ayres, M. P. Global change, plant defense, and herbivory. Biotic Interactions and Global Change. Sunderland (MA): Sinauer Associates 75–94 (1993).
Björkman, C. & Niemelä, P. Climate Change and Insect Pests (Cabi, 2015).
Schaefer, H., Jetz, W. & Böhning-Gaese, K. Impact of climate change on migratory birds: community reassembly versus adaptation. Glob. Ecol. Biogeogr. 17, 38–49 (2008).
Cilas, C., Goebel, F. R., Babin, R. & Avelino, J. Tropical crop pests and diseases in a climate change setting—A few examples. Clim. Change Agric. Worldw. 73–82 (2016).
Gustafson, D. I. Climate change: a crop protection challenge for the twenty-first century. Pest Manag Sci. 67, 691–696 (2011).
Hellmann, J. J., Byers, J. E., Bierwagen, B. G. & Dukes, J. S. Five potential consequences of climate change for invasive species. Conserv. Biol. 22, 534–543 (2008).
Juroszek, P. & Von Tiedemann, A. Plant pathogens, insect pests and weeds in a changing global climate: a review of approaches, challenges, research gaps, key studies and concepts. J. Agric. Sci. 151, 163–188 (2013).
Juroszek, P., Racca, P., Link, S., Farhumand, J. & Kleinhenz, B. Overview on the review articles published during the past 30 years relating to the potential climate change effects on plant pathogens and crop disease risks. Plant. Pathol. 69, 179–193 (2020).
Saxony, L. et al. Impact of climate change on the Temporal and regional occurrence of Cercospora leaf spot in lower Saxony. J. Plant Dis. Prot. 118, 168–177 (2011).
Contreras, S. C., Galindo, M. M. G. & Ibarra, Z. E. Metodología para correlacionar los fenómenos de la sequía y El Niño con la presencia de la langosta en México. La plaga de langosta Schistocerca piceifrons piceifrons (Walker) Una vision multidisciplinaria desde la perspectiva del riesgo fitosanitario en México 137–147 (2013).
Wilson, B. E. et al. West Indian canefly (Hemiptera: Delphacidae): An emerging pest of Louisiana sugarcane. J. Econ. Entomol. 113, 263–272 (2020).
Skendžić, S., Zovko, M., Živković, I. P., Lešić, V. & Lemić, D. The impact of climate change on agricultural insect pests. Insects 12, 1–31 (2021).
Subedi, B., Poudel, A. & Aryal, S. The impact of climate change on insect pest biology and ecology: implications for pest management strategies, crop production, and food security. J. Agric. Food Res. 14, 100733 (2023).
Sauer, C. Possible impacts of climate change on Carrot Fly’s population dynamics in Switzerland. IOBC/WPRS Bull. 142, 31–41 (2019).
Mech, A. M., Tobin, P. C., Teskey, R. O., Rhea, J. R. & Gandhi, K. J. K. Increases in summer temperatures decrease the survival of an invasive forest insect. Biol. Invasions. 20, 365–374 (2018).
Deutsch, C. A. et al. Increase in crop losses to insect pests in a warming climate. Sci. (1979). 361, 916–919 (2018).
Schneider, L., Rebetez, M. & Rasmann, S. The effect of climate change on invasive crop pests across biomes. Curr. Opin. Insect Sci. 50, 1–5 (2022).
DeLucia, E. H., Casteel, C. L., Nabity, P. D. & F. O’Neill, B. Insects take a bigger bite out of plants in a warmer, higher carbon dioxide world. Proc. Natl. Acad. Sci. 105, 1781–1782 (2008).
Zhao, C. et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. 114, 9326–9331 (2017).
Acknowledgements
The authors would like to thank the team at Ingenio San Carlos for providing the data needed for the analyses of this research.
Author information
Authors and Affiliations
Contributions
E.J. and W.F designed the research; M.G., and C.O. wrote the paper with; V.P. and O.N. collected measurement data and M.G. performed the statistical analysis of the data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiménez-Ruiz, E., González-Narváez, M., Puente-Tenezaca, V. et al. Impact of climatic variations on the abundance pattern of Perkinsiella saccharicida in the ingenio sugar San Carlos, Ecuador. Sci Rep 15, 16919 (2025). https://doi.org/10.1038/s41598-025-01446-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01446-9