Abstract
In this retrospective study, we evaluated changes in corneal higher-order aberrations (HOAs) after PreserFlo MicroShunt (PFM) implantation and examined factors associated with the outcomes. We included 101 PFM implantations performed between February 2023 and June 2024. Visual acuity, intraocular pressure, corneal HOAs, and coma-like and spherical aberrations were analyzed preoperatively, at 1 week, and at 1, 2, and 3 months postoperatively. Generalized linear mixed models were used to analyze factors that might affect HOAs and other aberrations. Preoperatively, HOAs, coma-like aberrations, and spherical-like aberrations were 0.249 ± 0.192, 0.216 ± 0.180, and 0.109 ± 0.093 μm, respectively. Aberrations showed a significant increase up to 1 month postoperatively, peaking at 1 week, with values of 0.396 ± 0.210 μm, 0.346 ± 0.204 μm, and 0.173 ± 0.105 μm, respectively (all P < 0.001). The aberrations were no longer significantly greater than baseline at 2 and 3 months postoperatively. Analysis of risk factors suggested that post-implantation hypotony (≤ 5 mmHg) could influence corneal HOAs and coma-like aberrations. PFM implantation temporarily increased corneal HOAs, coma-like aberrations, and spherical aberrations, but these levels stabilized and had returned to preoperative levels by 2 months postoperatively. Postoperative hypotony was associated with increased corneal HOAs and coma-like aberrations.
Similar content being viewed by others
Introduction
Glaucoma remains one of the leading causes of irreversible blindness worldwide1,2. Lowering intraocular pressure (IOP) is currently the only proven method to slow the progression of the disease and prevent further vision loss2. Although non-surgical treatments, such as medications and laser therapies, are commonly used, these may fail to adequately manage IOP, necessitating surgical intervention. Trabeculectomy, often performed with mitomycin-C, has long been considered the gold standard surgical approach3. Despite its effectiveness in reducing IOP, trabeculectomy is known to cause considerable postoperative changes in the corneal structure, particularly owing to the formation of filtering blebs. These changes may lead to an increase in corneal higher-order aberrations (HOAs), such as coma and spherical aberrations, which could negatively affect retinal image quality, even if good visual acuity is achieved4,5.
The PreserFlo MicroShunt (PFM) (Santen Pharmaceutical Co., Ltd., Osaka, Japan), introduced in Japan in November 2022, is a novel filtration surgery device offering an effective alternative to traditional trabeculectomy. Like trabeculectomy, the PFM is designed to lower IOP, but its minimally invasive nature sets it apart. The device eliminates the need for scleral flap creation, resulting in fewer postoperative interventions6,7.
Recent advances in wavefront analysis have allowed for more detailed evaluation of ophthalmic procedures such as refractive and cataract surgeries. HOAs are residual optical distortions that persist after correcting defocus and astigmatism using spherical-cylindrical lenses. These aberrations can considerably degrade retinal image quality, even when the corrected visual acuity remains good8. Although trabeculectomy has been shown to increase corneal HOAs postoperatively4,5, there are currently no detailed reports on the effect of the PFM on corneal HOAs. The purpose of the present study was to investigate changes in corneal HOAs before and after PFM implantation.
Results
IOP after PFM implantation
A total of 87 patients (101 eyes) were included in the analysis. The clinical characteristics of enrolled participants are summarized in Table 1. Patients’ mean age was 69.9 ± 15.2 years, and 48.5% were women. The preoperative IOP was 20.2 ± 7.1 mmHg. Figure 1 illustrates the trend in IOP over the study period. The mean IOP at baseline was 20.2 ± 7.1 mmHg, which dropped significantly to 7.7 ± 4.2 mmHg (P < 0.0001) at 1 week, 10.4 ± 4.9 mmHg (P < 0.0001) at 1 month, 11.5 ± 5.6 mmHg (P < 0.0001) at 2 months, and had stabilized at 11.5 ± 4.4 mmHg (P < 0.0001) by 3 months postoperatively. A statistically significant reduction in IOP was observed at each postoperative time point.
Visual acuity after PFM implantation
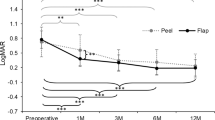
Figure 2 presents the visual acuity data, showing both preoperative and postoperative measurements. Visual acuity initially declined within the first 2 months after surgery but had improved by the 3-month follow-up.
LogMAR visual acuity following PreserFlo MicroShunt implantation. Although the visual acuity was significantly reduced within the first 2 months after surgery, at 3 months after surgery, there was no significant difference found for vision as compared with the baseline. *P < 0.01 compared with baseline.
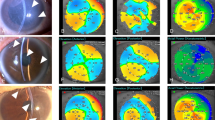
Corneal HOAs, coma-like aberrations, and spherical-like aberrations after PFM implantation
Table 2 provides data on corneal HOAs, coma-like aberrations, and spherical-like aberrations measured before and after surgery. The mean pupil diameter at the time of measurement was 3.3 mm. Corneal HOAs as well as coma-like and spherical-like aberrations increased significantly within the first month postoperatively. However, by 2 months, these values had declined, reaching preoperative levels by the 3-month follow-up. The sole risk factor identified as being associated with an increase in corneal HOAs, coma-like aberrations, and spherical-like aberrations 1 month after PFM implantation was postoperative hypotony (≤ 5 mmHg) (Table 3).
Discussion
In this study, we evaluated and reported the temporal changes in corneal HOAs following PFM implantation. PFM implantation is a novel, minimally invasive surgical technique that has recently gained attention9,10. Compared with conventional filtration surgeries, such as trabeculectomy and Ex-PRESS implantation, PFM implantation is considered to have a less direct impact on corneal shape11. Indeed, Ibarz Barberá et al.12 recently reported that PFM implantation caused only mild and transient increases in corneal astigmatism in the early postoperative period, with these changes returning to baseline within 3 months. Furthermore, visual acuity had recovered to preoperative levels by 3 months, suggesting that the procedure has a minimal long-term impact on corneal optical quality and visual function, which is consistent with the results of our study. Because of these characteristics, PFM implantation is anticipated to enable quicker postoperative recovery of visual function13. However, research regarding its effects on corneal optical properties remains limited11,12. In particular, there are very few prior studies investigating the temporal changes in corneal HOAs after filtering surgery4,5 and, to our knowledge, no previous reports evaluating corneal HOAs after PFM implantation. We found that corneal HOAs, coma-like aberrations, and spherical-like aberrations were significantly increased up to 1 month postoperatively but decreased and returned to preoperative levels by 2 months. This suggests that whereas PFM implantation initially causes a temporary change in corneal optical properties, these changes stabilize over time and eventually recover to baseline levels. The average IOP at 1 week postoperatively was as low as 7.7 ± 4.2 mmHg, suggesting that early postoperative low IOP may have contributed to the initial increase in aberrations. Low IOP is known to affect corneal stability and shape, potentially increasing corneal HOAs by inducing structural changes5,14. A decline in visual quality following IOP reduction is commonly observed after glaucoma surgery15,16,17,18. Studies investigating filtration surgery have suggested the involvement of alterations in refractive factors, including corneal shape, anterior chamber depth, and axial length15,16,17,18. Several studies have reported changes in corneal HOAs following trabeculectomy and Ex-PRESS implantation4,5. Although trabeculectomy is associated with an increase in corneal HOAs that may persist for up to 3 months postoperatively4,5, Ex-PRESS implantation tends to exhibit recovery of corneal HOAs to preoperative levels within 2 months5. Hypotony is reported to be a factor that increases cornel HOAs after glaucoma surgery5. Our findings support those of that previous study. Because many of our patients with hypotony had a shallow or flat anterior chamber, having a shallow or flat anterior chamber was not a factor affecting corneal HOAs. In this study, we found that 12 eyes (12.1%) developed a shallow anterior chamber after surgery, and the anterior chamber depth recovered to normal in a remarkably short period, 14.3 ± 12.7 days.
Because it requires neither the creation of a scleral flap nor the use of iridectomy, the PFM is less invasive than trabeculectomy6,9,10 and may therefore result in fewer direct effects on the corneal shape. However, compared with trabeculectomy, the PFM can lead to lower IOP in the early postoperative period19, potentially contributing to early corneal shape changes and a temporary increase in aberrations. In our study, these changes were most prominent within the first week postoperatively, suggesting that prolonged low IOP requires careful management.
By 2 months postoperatively, we observed that corneal HOAs, coma-like aberrations, and spherical-like aberrations had returned to preoperative levels. This indicated that the corneal shape gradually stabilizes and recovers over time after PFM implantation. These early changes did not persist long term, and the cornea’s optical properties recovered to preoperative levels. In terms of visual acuity, recovery to preoperative levels was observed by 3 months. Thus, the effects of the PFM on visual function appear to be temporary, and visual function recovers relatively quickly. Patients who undergo trabeculectomy sometimes complain of decreased visual quality. In cases with advanced visual field loss, this decline may affect patients’ quality of life, even in the early postoperative period. In this context, PFM implantation, which allows for relatively early recovery of visual function, may serve as a valuable treatment option, especially for patients in whom maintaining postoperative quality of life is a priority.
To examine the reliability of measurements at different time points after surgery, we measured cornel HOAs in normal volunteers on different days. The intra-class correlation coefficient for corneal HOAs, coma-like aberrations, and spherical-like aberrations was 0.82, 0.81, and 0.75, respectively. Therefore, we consider that the reproducibility of wavefront analyzer measurements is high.
This study has several limitations. First, we evaluated visual function using only visual acuity. We did not assess other factors like contrast sensitivity or the impact on patients’ quality of life, which should be considered in future studies. Second, the follow-up period was relatively short. Although we confirmed that HOAs returned to preoperative levels by 2 months, additional data beyond 3 months are necessary to fully understand long-term aberration changes. Third, we did not evaluate the ocular surface condition before and after surgery. Before PFM implantation, most patients were using multiple IOP-lowering medications, but these medications were discontinued postoperatively. This change may have influenced the condition of the tear film and ocular surface. Although we did not evaluate the condition of the tear film after PFM implantation, there was no difference in central corneal thickness between the pre- (504.9 ± 36.5 μm) and postoperative periods (500.7 ± 37.9 μm) (P = 0.16).
In conclusion, whereas PFM implantation surgery led to a temporary increase in corneal HOAs and coma-like and spherical-like aberrations within the first postoperative month, these had stabilized and returned to preoperative levels by the second month postoperatively. Our findings suggest that low IOP contributes to the early increase in aberrations, but long-term recovery of visual function can be expected. Thus, PFM implantation is a promising option in glaucoma surgery, offering early visual recovery and stability.
Methods
Patients
In this retrospective study, we investigated participants’ eyes after they had undergone PFM implantation at Hiroshima University Hospital between February 2023 and June 2024. All patients were followed for at least 3 months after surgery. The study protocol was approved by the Ethics Committee of Hiroshima University (E-2436). In accordance with the principles outlined in the Declaration of Helsinki, all participants provided their written informed consent before enrollment and participation in the study, in addition to standard consent for surgery. Patients who had previously undergone cataract surgery or ab-interno trabeculotomy more than 1 year earlier were included. Exclusion criteria were patients under age 20 years and those with a history of surgeries that could cause conjunctival scarring (e.g., pterygium excision, filtration surgery, long-tube shunt surgery, vitrectomy, or intrascleral fixation of an intraocular lens).
Surgical techniques
First, a fornix-based conjunctival incision was made. A gelatin sponge soaked in 0.4 mg/mL of mitomycin C was then placed under Tenon’s capsule for 3 to 5 min. This area was thoroughly irrigated with 100 to 200 mL of oxyglutathione. The sclera was marked 3 mm posterior to the limbus, and a scleral pocket was created using a double-step knife to guide placement of the PFM. The PFM was inserted, with its fin securely positioned within the scleral pocket. After confirming a continuous flow of aqueous humor from the distal end of the PFM using a cannula, Tenon’s capsule and the conjunctiva were closed with 10-0 nylon sutures. Finally, 1.65 mg of dexamethasone was injected subconjunctivally, and ofloxacin eye ointment was applied before placing a protective eye patch.
Wavefront analysis
A wavefront analyzer (KR-1 W; Topcon Co., Tokyo, Japan) was used to assess anterior, posterior, and total corneal wavefront aberrations both preoperatively as well as at 1 week and 1, 2, and 3 months postoperatively. Aberrometry measurements were conducted automatically, with each measurement repeated three times. All measurements were performed in a dark room without pharmacologic pupil dilation. The analyzed wavefront aberrations included corneal HOAs, trefoil, coma, spherical aberrations, and third- and fourth-order aberrations from Zernike polynomials, all of which were calculated as root mean square values.
Main outcome measures
The main outcomes measured included changes in IOP and corneal HOAs (including coma-like and spherical aberrations). Baseline IOP was defined as the last IOP measurement obtained prior to PFM implantation. IOP was measured using a Goldmann applanation tonometer. Follow-up examinations were conducted for all patients at 1 week, 1 month, 2 months, and 3 months postoperatively. Logistic regression analysis (univariate and multivariate analyses) was used to identify factors associated with corneal HOAs.
Statistical analysis
All analyses were performed using JMP software version 17 (SAS Inc., Cary, NC, USA). To identify factors associated with an increase in corneal HOAs 1 month after PFM implantation, we used univariate and multivariate regression models, incorporating independent variables such as age, sex, central corneal thickness, axial length, preoperative IOP, baseline corneal HOAs, postoperative hypotony (≤ 5 mmHg), shallow anterior chamber, and lens status. For the analysis of data from patients who underwent bilateral surgery, a generalized linear mixed model was used to account for intra-patient correlations, enhancing the accuracy of outcome assessment. All data are reported as mean ± standard deviation, with statistical significance defined as a P-value < 0.05.
Data availability
The datasets generated and/or analyzed during the current study are available from K. Hirooka, the corresponding author, on reasonable request.
References
Quigley, H. A. & Broman, A. T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90, 262–267 (2006).
Tham, Y. C. et al. Global estimates on the number of people blind or visually impaired by glaucoma: A meta-analysis from 2000 to 2020. Ophthalmology 121, 2081–2090 (2014).
Sawchyn, A. K. & Slabaugh, M. A. Innovations and adaptations in trabeculectomy. Curr. Opin. Ophthalmol. 27, 158–163 (2016).
Fukuoka, S. et al. Effect of trabeculectomy on ocular and corneal higher order aberrations. Jpn. J. Ophthalmol. 55, 460–466 (2011).
Kobayashi, N., Nitta, H. K., Ukegawa, E., Tsujikawa, A. & K. & Visual acuity and corneal higher-order aberrations after EX-PRESS or trabeculectomy, and the determination of associated factors that influence visual function. Int. Ophthalmol. 38, 1969–1976 (2018).
Batlle, J. F. et al. Three-year follow-up of a novel aqueous humor MicroShunt. J. Glaucoma 25, e58–e65 (2016).
Saeed, E., Gołaszewska, K., Dmuchowska, D. A., Zalewska, R. & Konopińska, J. The PreserFlo MicroShunt in the context of minimally invasive glaucoma surgery: A narrative review. Int. J. Environ. Res. Public. Health 20, 2904. https://doi.org/10.3390/ijerph20042904 (2023).
Liang, J. & Williams, D. R. Aberrations and retinal image quality of the normal human eye. J. Opt. Soc. Am. Opt. Image Sci. Vis. 14, 2873–2883 (1997).
Pillunat, K. R. et al. PreserFlo MicroShunt versus trabeculectomy: First results on efficacy and safety. Acta Ophthalmol. 100, e779–e790 (2022).
Pawiroredjo, S. S. M. et al. Efficacy of the PreserFlo MicroShunt and a meta-analysis of the literature. J. Clin. Med. 11, 7149. https://doi.org/10.3390/jcm11237149 (2022).
Gambini, G. et al. Early post-operative anterior segment parameters modifications induced by PreserFlo MicroShunt in primary open-angle glaucoma. Int. Ophthalmol. 43, 3055–3044 (2023).
Ibarz Barberá, M., Morales-Fernandez, L., de Gómez, R., Tañá Rivero, P. & Teus, M. A. Changes to corneal topography and biometrics after PreserFlo MicroShunt surgery for glaucoma. J. Glaucoma 30, 921–931 (2021).
Qidwai, U., Jones, L. & Ratnarajan, G. A comparison of iStent combined with phacoemulsificationand endocyclophotocoagulation (ICE2) with the PreserFlo MicroShunt and XEN-45implants. Ther. Adv. Ophthalmol. 14, 25158414221125696 (2022).
Fard, A. M., Sorkhabi, R. D., Nasiri, K. & Tajlil, A. Effect of trabeculectomy on ocular higher-order aberrations in patients with open angle glaucoma. North. Clin. Istanb. 5, 54–57 (2018).
Rosen, W. J., Mannis, M. J. & Brandt, J. D. The effect of trabeculectomy on corneal topography. Ophthalmic Surg. 23, 395–398 (1992).
Dietze, P. J. et al. Visual function following trabeculectomy: Effect on corneal topography and contrast sensitivity. J. Glaucoma 6, 99–103 (1997).
Stewart, W. C. & Shields, M. B. Management of anterior chamber depth after trabeculectomy. Am. J. Ophthalmol. 106, 41–44 (1988).
Francis, B. A. et al. Changes in axial length following trabeculectomy and glaucoma drainage device surgery. Br. J. Ophthalmol. 89, 17–20 (2005).
Bøhler, A. D. et al. Hypotony in the early postoperative period after MicroShunt implantation versus trabeculectomy: A registry study. Acta Ophthalmol. 102, 186–191 (2024).
Acknowledgements
We thank Analisa Avila, MPH, ELS, of Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Data acquisition, T.B.; data analysis and interpretation, T.B., K.H.; figures and tables preparation, T.B., K.H.; writing of main manuscript text, T.B.; critical review and revision of the manuscript, K.H., N.O., H.Onoe., K.T., H.Okumichi., Y.K., H.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Baba, T., Hirooka, K., Okada, N. et al. Changes in corneal higher-order aberrations following PreserFlo MicroShunt implantation. Sci Rep 15, 16748 (2025). https://doi.org/10.1038/s41598-025-01550-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01550-w