Abstract
This study aimed to evaluate the stability and quality enhancement of Acorus tatarinowii and Atractylodes lancea volatile oils (ATaAL-VO) and their β-cyclodextrin encapsulated and Pickering emulsion forms under ozone exposure. Under an ozone environment, ATaAL-VO were subjected to three treatments: raw oil, β-cyclodextrin encapsulated oil, and pickering emulsion. Peroxide values were quantified. GC-MS was employed to identify compositional variances, while t-tests were used to identify compounds with significant quantitative differences. PCA charts were generated using OmicShare, and line diagrams were created with Rmisc and reshape2. OmicShare was also utilized to construct Upset diagrams for filtering qualitative differential compounds, and charts illustrating newly formed and disappeared qualitative differential compounds were composed. Principal compounds’ box diagrams were crafted through reshape2 and ggplot2. The β-cyclodextrin and pickering emulsion groups exhibited lower oxidation levels compared to the original oil group after ozone exposure. The pickering emulsion and β-cyclodextrin encapsulation groups both demonstrated a marked improvement in the stability of the majority of volatile components. The stability and quality of ATaAL-VO can be markedly enhanced through β-cyclodextrin encapsulation or Pickering emulsion preparation, with the latter offering distinct advantages.
Similar content being viewed by others
Introduction
The main components of Acorus calamus include α-asarone, γ-asarone, methyl eugenol, linalool, trans-methyl isoeugenol, δ-cadinene, caryophyllene, and α-humulene1,2. It is traditionally believed to possess analgesic, antibacterial, anti-inflammatory, and digestive regulatory effects according to traditional herbal medicine theory3,4. Atractylodes lancea primarily contains volatile components, such as Atractylodin, β-eudesmol, and Atractylone5,6. It is considered to possess biological activities including antibacterial, anti-inflammatory, antioxidant, anticancer, and antiallergic properties7,8. Acorus tatarinowii and Atractylodes lancea volatile oils (ATaAL-VO) can be extracted and utilized as herbal ingredients in the preparation of herbal products, traditional Chinese medicine prescriptions, flavorings, food additives, and skincare products9,10. However, the ATaAL-VO are prone to oxidation, and long-term exposure to an ozone environment may lead to degradation and evaporation of their components11.
Pickering emulsion is a new type of emulsion prepared by using ultrafine solid particles or solid colloidal particles instead of traditional surfactants as emulsifiers12,13. As a cutting-edge emulsification technology that employs solid particles as stabilizers, Pickering emulsion has emerged as a focal point across multiple research domains, including chemical engineering, materials science, and food science14. Encapsulating volatile oils in Pickering emulsion is an effective way to improve the stability of volatile oils15. It is simple to prepare, highly biocompatible, and stable. Furthermore, it can be further dehydrated, dried, and solidified, achieving solid powderization16,17,18. This has important value and significance for the subsequent processing and application of traditional Chinese medicine volatile oils19,20. β-cyclodextrin is a natural starch derivative with a cyclic structure and excellent inclusion ability21. β-cyclodextrin can encapsulate volatile oils within its cavity, forming stable inclusion complexes, thereby reducing the evaporation and oxidation of volatile oils, extending their stability and shelf life22. When complexed with β-cyclodextrin, the release of volatile oils can be controlled to achieve a slow-release effect and prolong their activity. β-cyclodextrin can also enhance the solubility of volatile oils in water or other solvents, thereby improving their uniformity and dissolution in formulations23,24. In summary, utilizing β-cyclodextrin to encapsulate volatile oils can enhance their stability, controlled release properties, solubility, and odor-masking advantages. This makes β-cyclodextrin an important carrier and excipient widely used in the fields of pharmaceuticals, food, and cosmetics23,24,25.
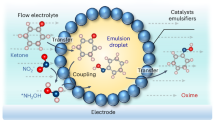
Our research team has previously completed the preparation and characterization of β-cyclodextrin encapsulation and Pickering emulsions, with a focus on optimizing their preparation processes26. Building on this foundation, as shown in Fig. 1 this study further compares the stability and compositional changes of these systems in an ozone environment, aiming to enhance the stability and quality of ATaAL-VO.
Materials and methods
Materials and equipment
Acorus tatarinowii, (Acori tatarinowii Rhizoma) is acorus grassleaf sweetflag rhizome; Atractylodes lancea (Atractylodis lanceae Rhizoma) is the rhizome of the Atractylodes plant in the Asteraceae family. The volatile oils used in this study was purchased from Shaanxi Momentum Qixuehe Pharmaceutical Co., Ltd. and authenticated by Professor Yonggang Yan from Shaanxi University of Chinese Medicine as complying with the required standards.
The LS-F8 multifunctional negative ion oxygen detox machine (Xiamen Laisen Electronics Co., Ltd.), JY-3002 one ten-thousandth analysis balance (Shanghai Puchun Instrument Co., Ltd.), IKA T18 digital high - speed disperser (Shanghai Tusen Vision Technology Co., Ltd.), DHG − 9140 A electrically heated forced - air drying oven (Shanghai Hengkai Scientific Instrument Co., Ltd.), and DZTW electronically temperature - adjustable heating mantle (Beijing Yongguangming Medical Equipment Co., Ltd.) were used in the experiments. The Agilent 7890B/5977B gas chromatography-mass spectrometer (Agilent, USA) was applied.
Sodium thiosulfate (batch no. 20180806), sodium carbonate anhydrous (batch no. 20210506), soluble starch (batch no. 20180808), sodium chloride (batch no. 20210302), and chloroform (batch no. 20210203), were all purchased from Tianjin Tengli Chemical Reagent Co., Ltd. Potassium iodide (Tianjin Kemio chemical reagent Co., Ltd., batch no.20220422), n-Hexane (Grace Chemical Technology Co. LTD, Lot: 2112091), and n-Docosane (Grace Chemical Technology Co. LTD, Lot: G171809, purity: 99.6%) were used in the study. The ATaAL-VO was obtained from Shaanxi Momentum Qixuehe Pharmaceutical Co., Ltd. (batch no. S-230204).
The preparation of β-cyclodextrin encapsulation of ATaAL-VO, and pickering emulsion
The encapsulation of ATaAL-VO with β-cyclodextrin, as well as the Pickering emulsion preparation, were previously conducted by our previous study. The optimal encapsulation process of ATaAL-VO was determined as follows: a grinding duration of 33 min, a ratio of β-cyclodextrin to volatile oil of 6.3:1, and a water to β-cyclodextrin ratio of 2.6:1. The most optimal method for the preparation of the Pickering emulsion included the usage of PEG4000, a PEG4000 to natural indigo ratio of 5:1, a melting temperature of 228 °C, a melting duration of 5 min, an oil to water ratio of 13:7, an addition of 0.5 g of modified natural indigo, a stirring speed of 10,000 rpm, and a stirring duration of 2 min26.
Preparation of modified particles: PEG4000 and finely milled Indigo Naturalis were weighed separately, PEG4000 was placed in an electric heating jacket to melt it, and then indigo was immediately added to dry and crush for later use27.
Preparation of Pickering emulsion: the modified indigo was weighed and it was combined with ATaAL-VO and water in the centrifuge tube. Then the mixture was put into a high-speed shear machine for operation, culminating in the formation of a modified Indigo Naturalis Pickering emulsion.
Preparation of β-cyclodextrin encapsulation of ATaAL-VO: β-cyclodextrin was weighed meticulously and introduced into a ball mil. Next volume of purified water, equating to 1.5 times its original volume (108 mL) was added in β-cyclodextrin, resulting in a β-cyclodextrin slush.
After slush formation, a diluted solution made by mixing equal volumes of volatile oil and ethanol (12 mL in total) was gradually added and carefully mixed into the ball mill. Then it filtered under vacuum, followed by three washing schemes. The resulting product was dried and finally roughly absorbed into a 40 mesh product for collection. Based on the above conditions, the process of β-cyclodextrin inclusion of ATaAL-VO and Pickering emulsion was optimized.
Collection of volatile oils under ozone exposure and determination of peroxide contents
Crude oil (a mixture of ATaAL-VO extractions) and Pickering emulsion were each placed in 25 mL open colorimetric tubes, while β-cyclodextrin encapsulated ATaAL-VO was placed in a 250 mL conical flask. Ozone at a concentration of 4.28 ppm was continuously introduced into the colorimetric tubes and the conical flask for 10, 15, and 20 min.The mixed extraction of ATaAL-VO from the crude oil group was then retrieved and set aside. The β-cyclodextrin encapsulation group and the Pickering emulsion group were subjected to steam distillation to extract the ATaAL-VO within, distilled for 5 h, the oil phase was separated and set aside for future use. These procedures were performed in triplicate.
Accurately pipette 500 µL of the volatile oil sample into a 10 mL conical flask containing 10 mL of a chloroform/acetic acid mixture (4:6, v/v). Then, 1mL of saturated potassium iodide solution was added, the flask was tightly sealed, shaken for 0.5 min, and left in a dark place for 3 min. The flask was then taken out and 3 mL of water was added, followed by the addition of 1.00 mL of 1% starch indicator. The solution was titrated with 0.00001 mol/L sodium thiosulfate standard solution until the blue color disappeared, and the volume of sodium thiosulfate standard solution consumed was recorded. The peroxide value was calculated based on these measurements28.
Determination of volatile oil components in ozone environment by GC-MS
Preparation of internal standard solution
Precise weighing of 50.50 mg of the n-docosane standard substance was performed, and it was then placed into a 5 mL vial. n-Hexane was added to the calibration line, followed by thorough shaking to obtain a concentrated n-docosane internal standard solution with a concentration of 10 mg/mL.
Preparation of the test sample solution
Accurately transfer 100 µL of volatile oil samples from each group into 10 mL vials. Add 100 µL of n-docosane internal standard solution, then bring the volume to the calibration line with n-hexane. Add an appropriate amount of anhydrous sodium sulfate to remove moisture. The mixture was shaken thoroughly and filtered through a 0.22 μm organic filter membrane to obtain the test solution of volatile oils from different groups26.
Analytical conditions for GC-MS
The test samples were prepared using liquid injection method, and the influence of different GC and MS conditions on the chromatographic information of the samples was investigated. Using chromatographic information abundance as the evaluation index, the optimal conditions for maximizing component information acquisition were determined as follows: Employ an HP-5 MS quartz capillary column (30 m × 0.25 mm × 0.25 μm), with helium gas (purity 99.999%) serving as the carrier gas at a flow rate of 1 mL/min. A 1 µL sample was injected with a split ratio of 10:1. The injector temperature was 230 ℃. The column temperature started at 50 ℃, rose at 15 ℃/min to 140 ℃, then at 0.4 ℃/min to 144 ℃ with a 5 min hold. It then increased at 10 ℃/min to 250 ℃ with a 2 - min hold, and finally at 4 ℃/min to 280 ℃ with a 2 - min hold. Electron ionization (EI) mode with 70 eV electron energy was used. The ion source temperature was maintained at 230 °C, and the quadrupole temperature was kept at 150 °C. The mass scan range was set from 35 to 500 m/z, with a solvent delay of 3 min. After the acquisition of GC-MS data, the Agilent database analysis software Data Analysis was used to access the NIST14.0 database and summarize the components using n-alkanes as references27.
Volatile oil quantitative change difference compounds in ozone environment
Selection of volatile oil quantitative change difference compounds in ozone environment
A t-test was conducted to screen for differential components under this environment. We compared the crude oil group with the groups exposed to ozone for 10, 15, and 20 min. We performed a t-test on the concentration data of each component to check for significant differences between groups. The pheatmap package was used to generate heatmap plots of the 37 differential components28. The PCA plots of the 37 differential components in the crude oil group, β-cyclodextrin encapsulation group, and Pickering emulsion group were generated using the OmicShare online platform (https://www.omicshare.com/tools).
Analysis of volatile oil quantitative change difference compounds in ozone environment
Line plots of the 37 quantitative variable differential components were generated using the Rmisc and reshape2 packages29,30.
Volatile oil qualitative change difference compounds in ozone environment
Selection of volatile oil qualitative change differential compounds in ozone environment
The Upset diagram of the crude oil group, β-cyclodextrin encapsulation group, and Pickering emulsion group under ozone exposure was generated using the OmicShare online platform. The horizontal bars on the left denote the compound count for each set. The individual points in the matrix below the graph indicate compounds unique to a specific set, while the connecting lines between points represent intersections among statistically associated sets. The vertical bars above represent the number of compounds that are unique or intersectional in the statistical analysis15,31.
Analysis of newly generated qualitative change differential compounds in volatile oil under ozone environment
The heatmap stack plot of newly generated compounds under ozone exposure in the crude oil group, β-cyclodextrin encapsulation group, and Pickering emulsion group was generated using the OmicShare online platform. The horizontal axis of the heatmap represents the samples and groups, while the vertical axis represents the compounds. Each square in the heatmap represents the abundance of a compound in a sample, indicated by the color. In the stack plot, the horizontal axis denotes the abundance of compound groups, while the vertical axis indicates the compounds. Each bar’s color distinguishes different groups, and its height reflects the compound’s abundance within each group.
Analysis of disappear qualitative change differential compounds in volatile oil under ozone environment
The stack plot of disappearance compounds under ozone exposure in the crude oil group, β-cyclodextrin encapsulation group, and Pickering emulsion group was created using the OmicShare online platform.
Analysis of the main components of volatile oil in ozone environment
The main components of Acorus calamus include α-asarone (CAS number: 002883-98-9), γ-asarone (CAS number: 005353-15-1), methyl eugenol (CAS number: 000093-15-2), linalool (CAS number: 000078-70-6), trans-methyl isoeugenol (CAS number: 006380-24-1), δ-cadinene (CAS number: 000483-76-1), caryophyllene (CAS number: 000087-44-5), α-humulene (CAS number: 006753-98-6), and others. The main component of Curcuma zedoaria is β-eudesmol (CAS number: 000473-15-4). The Rmisc, reshape2, and ggplot2 packages were used to plot the boxplots of the main component compounds in the crude oil group, β-cyclodextrin encapsulation group, and Pickering emulsion group in ozone environment29,30,32.
Results
Collection of volatile oils under ozone exposure and determination of peroxide contents
As shown in Fig. 2, following 10, 15, and 20 min of ozone exposure, both β-cyclodextrin encapsulated and Pickering emulsion groups exhibited significantly lower peroxide content compared to the crude oil group (p < 0.001). No statistically significant difference was observed in peroxide levels between the β-cyclodextrin encapsulated group and the Pickering emulsion group (p > 0.05). These findings indicate that both β-cyclodextrin and Pickering emulsion were capable of reducing the peroxide content in the ATaAL-VO to a certain extent under ozone exposure.
Determination of volatile oil components in ozone environment by GC-MS
The results of GC-MS volatile components of ATaAL-VO in each group under ozone environment are shown in Table 1, and the GC-MS chromatogram stack plot is shown in Fig. 3A. The chromatograms show that at 5 min, crude oil and Pickering emulsion groups exhibit compound loss, while the β-cyclodextrin encapsulation group has new compounds. At 15 min, the crude oil group’s compound abundance is slightly lower than the other groups. By 20 min, the Pickering emulsion group’s compound abundance is higher than the others. At 25 min, the crude oil group’s compound abundance is lower. Overall, the overlaid chromatograms more intuitively highlight the protective effects of Pickering emulsion and β-cyclodextrin encapsulation group on crude oil, enhancing its stability.
Volatile oil quantitative change difference compounds in ozone environment
Selection of volatile oil quantitative change differential compounds in ozone environment
As shown in Fig. 3B, compared to the crude oil, there were 3 differential components (p < 0.05) after 10 min of ozone exposure. Similarly, there were 3 differential components (p < 0.05) after 15 min of ozone exposure. In comparison, there were 33 differential components (p < 0.05) after 20 min of ozone exposure, resulting in a total of 37 differential components after removing duplicates. As shown in Fig. 3C. in the heatmap (generated via the pheatmap package) of 37 differential components under various ozone conditions, the nine groups were divided into six clusters:①: Crude oil groups after 15 and 20 min ozone exposure.②: Crude oil group after 10 min ozone exposure.③: β-cyclodextrin encapsulation groups after 10 and 15 min ozone exposure.④: β-cyclodextrin encapsulation group after 20 min ozone exposure.⑤: Pickering emulsion groups after 10 and 15 min ozone exposure.⑥: Pickering emulsion group after 20 min ozone exposure. In clusters ① and ②, the 15 and 20 min crude oil groups had similar component concentrations, which differed from the 10 min group. This might be because oxidation wasn’t stable after 10 min ozone exposure, causing significant concentration changes. In clusters ③ and ④, the 10 and 15 min β-cyclodextrin encapsulation groups had similar concentrations but differed from the 20 min group. This could be due to the protective effect of β-cyclodextrin encapsulation in the early stage, reducing oxidation loss. However, as ozone exposure time increased, the stability of the inclusion complexes might have decreased, causing more components to be oxidized or released. In clusters ⑤ and ⑥, the 10 and 15 min pickering emulsion groups had similar concentrations but differed from the 20 min group. This might be because the stability of Pickering emulsion wasn’t significantly affected in the short term, maintaining a relatively stable interface. However, after 20 min ozone exposure, the stability of the emulsion might have decreased due to changes in emulsifier properties and interfacial tension, possibly leading to breaking of the emulsion and different component concentrations compared to the 10 and 15 min groups (Tables 2, 3, 4).
Based on the clustering results of the volatile components, the characteristic components could be divided into 6 categories: (1) alpha-Bisabolol (CAS No. 000515-69-5), (+)-DELTA-CADINENE (CAS No. 000483-76-1), Germacrene B (CAS No. 015423-57-1), etc.; (2) Geraniol (CAS No. 000106-24-1), 1-Ethenyl-1-Methyl-4-Propan-2-Ylidene-2-Prop-1-En-2-Ylcyclohexane (CAS No. 003242-08-8), 4-Allylanisole (CAS No. 000140-67-0), etc.; (3) Methyl eugenol (CAS No. 000093-15-2), (-)-GUAIOL (CAS No. 000489-86-1), ISOEUGENYLMETHYLETHER (CAS No. 006380-24-1), etc.; (4) (+)-LONGICYCLENE (CAS No. 001137-12-8), Terpinen-4-ol (CAS No. 000562-74-3), (+)-Acorenone B (CAS No. 021653-33-8), etc.; (5) delta-elemene (CAS No. 020307-84-0), Elemol (CAS No. 000639-99-6), b-Eudesmol (CAS No. 000473-15-4); (6) Camphor (CAS No. 000464-48-2), Cyclopenta[c]pentalene (CAS No. 138752-24-6), Naphthalene (CAS No. 058893-88-2). As shown in Fig. 3D, in the PCA analysis, after 10 min of ozone exposure, the Pickering emulsion group and crude oil group were in the upper part of the plot, while the β-cyclodextrin encapsulation group was in the lower part. The three groups were clearly separated, indicating significant differences. After 15 min, the β-cyclodextrin encapsulation group moved to the top of the plot, and some overlap occurred between the three groups, suggesting some components had similar properties. After 20 min, the overlap between groups increased, especially between the crude oil and Pickering emulsion groups. As ozone exposure time increased, the differences between groups decreased, and component properties became more similar.
Selection of differential components in ozone environment. (A) GC-MS chromatogram stack plot of Pickering emulsion, β-cyclodextrin encapsulation, and crude oil group under ozone conditions. (B) Bar chart of different time intervals under ozone exposure. (C) Heatmap of the 37 differential components. (D) PCA diagram of differential components in ozone environment. (a) PCA plot of differential components in different groups after 10 min of ozone exposure. (b) PCA plot of differential components in different groups after 15 min of ozone exposure. (c) PCA plot of differential components in different groups after 20 min of ozone exposure. (d) Overall PCA plot of differential components in different groups under ozone exposure.
Analysis of volatile oil quantitative change difference compounds in ozone environment
As shown in Fig. 4, in the Pickering emulsion group, the declining trends of differential components 000084-74-2, 000464-48-2, 003242-08-8, and 020085-19-2 were alleviated. Meanwhile, the increasing trends of differential components 000483-76-1, 000515-69-5, 005353-15-1, 006380-24-1, 010208-80-7, 015423-57-1, and 068269-87-4 were mitigated. The decreasing trends of differential components 000489-86-1, 002387-78-2, 138752-24-6, and 150320-52-8 in the β-cyclodextrin encapsulation group were alleviated. The increasing trend of differential component 729602-94-2 in the β-cyclodextrin encapsulation group was mitigated. The decreasing trend of differential component 000106-22-9 in both the Pickering emulsion group and β-cyclodextrin encapsulation group was alleviated.
Volatile oil qualitative change difference compounds in ozone environment
Selection of volatile oil qualitative change differential compounds in ozone environment
As shown in Fig. 5A, compared to the crude oil, after 10 min of ozone exposure, the crude oil group generated 12 new compounds and lost 14; the Pickering emulsion group generated 18 new compounds and lost 17; and the β-cyclodextrin encapsulation group generated 15 new compounds and lost 17. The unique compounds in each group were 7 for crude oil, 4 for the crude oil group, 5 for the Pickering emulsion group, and 6 for the β-cyclodextrin encapsulation group. As shown in Fig. 5B, after 15 min of ozone exposure, the crude oil group generated 12 new compounds and lost 13; the Pickering emulsion group generated 13 new compounds and lost 21; and the β-cyclodextrin encapsulation group generated 15 new compounds and lost 19. A total of 43 compounds were common to all four groups, with 3 shared among the crude oil group, β-cyclodextrin encapsulation group, and Pickering emulsion group. The unique compounds were 10 for crude oil, 5 for the crude oil group, 5 for the Pickering emulsion group, and 7 for the β-cyclodextrin encapsulation group. As shown in Fig. 5C, after 20 min of ozone exposure, the crude oil group generated 20 new compounds and lost 14; the Pickering emulsion group generated 18 new compounds and lost 21; and the β-cyclodextrin encapsulation group generated 24 new compounds and lost 21. A total of 39 compounds were common to all four groups, with 6 shared among the crude oil group, β-cyclodextrin encapsulation group, and Pickering emulsion group. The unique compounds were 10 for crude oil, 7 for the crude oil group, 6 for the Pickering emulsion group, and 17 for the β-cyclodextrin encapsulation group. As shown in Fig. 5D, after 10, 15, and 20 min of ozone exposure, 34 compounds were common between the crude oil and all groups, while only 3 compounds were common among all groups. Crude oil had 4 unique compounds. After 10 min, the Pickering emulsion and β-cyclodextrin encapsulation group had 1 and 3 unique compounds, respectively. After 15 min, the crude oil and β-cyclodextrin encapsulation group had 1 and 3 unique compounds, respectively. After 20 min, the crude oil, Pickering emulsion, and β-cyclodextrin encapsulation group had 1, 2, and 13 unique compounds, respectively.
Analysis of newly generated qualitative change differential compounds in volatile oil under ozone environment
As shown in Fig. 5E-a, after 10 min of ozone exposure, compound 000106-24-1 exhibited higher relative abundance in the crude oil and β-cyclodextrin encapsulation group than in the Pickering emulsion group. Additionally, compounds 000076-49-3, 058893-88-2, 000084-74-2, and 997340-11-2 showed greater relative abundance in the crude oil group and lower in the Pickering emulsion group, with the latter two being undetected in the β-cyclodextrin encapsulation group. Figure 5E-b highlights that after 15 min of ozone exposure, compound 003779-61-1 had the highest relative abundance in the crude oil group, lower in the β-cyclodextrin encapsulation group, and was undetected in the Pickering emulsion group. Compound 000076-22-2 showed higher relative abundance in the crude oil group and lower in the Pickering emulsion group, while being undetected in the β-cyclodextrin encapsulation group. Compounds 019912-62-0 were highly abundant in the crude oil and Pickering emulsion groups but absent in the β-cyclodextrin encapsulation group. Compounds 029803-81-4 and 000515-69-5 demonstrated greater relative abundance in the crude oil and Pickering emulsion groups compared to the β-cyclodextrin encapsulation group. As depicted in Fig. 5E-c, after 20 min of ozone exposure, compounds 000084-74-2, 005655-61-8, 018252-44-3, and 000105-90-8 had higher relative abundance in the crude oil group and lower in the Pickering emulsion group, with the latter two being undetected in the β-cyclodextrin encapsulation group. Compounds 000523-47-7 and 019912-62-0 showed higher relative abundance in the crude oil and Pickering emulsion groups but were absent in the β-cyclodextrin encapsulation group. Compounds 029803-81-4 and 000106-24-1 exhibited greater relative abundance in the crude oil group and lower in the other groups. Compound 000515-69-5 had higher relative abundance in the crude oil and Pickering emulsion groups than in the β-cyclodextrin encapsulation group.
Analysis of disappear qualitative change differential compounds in volatile oil under ozone environment
As shown in Fig. 5F-a, after 10 min of ozone exposure, the compounds 094482-89-0 and 029873-99-2, which were disappearing, were effectively preserved in both the Pickering emulsion and β-cyclodextrin encapsulation groups. Additionally, the Pickering emulsion group effectively preserved the disappearing compounds 001117-61-9, 092618-89-8, and 000106-25-2. The disappearing compounds 013744-15-5 and 017334-55-3 could be effectively preserved in the β-cyclodextrin encapsulation group. According to Fig. 5F-b, after 15 min of ozone exposure, the disappearing compound 112421-19-9 could be effectively preserved in the Pickering emulsion group and β-cyclodextrin encapsulation group. The disappearing compounds 000106-25-2 and 017334-55-3 could be effectively preserved in the β-cyclodextrin encapsulation group. According to Fig. 5F-c, after 20 min of ozone exposure, the disappearing compounds 000104-20-1, 000624-15-7, and 013744-15-5 could be effectively preserved in the Pickering emulsion group and β-cyclodextrin encapsulation group. The disappearing compound 092618-89-8 could be effectively preserved in the β-cyclodextrin encapsulation group.
Selection and analysis of Volatile oil qualitative change difference compounds in ozone environment. (A) Upset diagram of different groups after 10 min of ozone exposure. (B) Upset diagram of different groups after 15 min of ozone exposure. (C) Upset diagram of different groups after 20 min of ozone exposure. (D) Overall Upset diagram of different groups under ozone exposure. (E) The heat map stacking diagram of newly generated compounds in each group under ozone environment (a) Heatmap stack plots of newly generated compounds in ATaAL-VO after 10 min of ozone exposure in different groups. (b) Heatmap stack plots of newly generated compounds in ATaAL-VO after 15 min of ozone exposure in different groups. (c) Heatmap stack plots of newly generated compounds in ATaAL-VO after 20 min of ozone exposure in different groups. (F) The stacking connection diagram of disappeared compounds in each group under ozone environment. (a) Stacked line plots of disappearing compounds in ATaAL-VO after 10 min of ozone exposure. (b) Stacked line plots of disappearing compounds in ATaAL-VO after 15 min of ozone exposure. (c) Stacked line plots of disappearing compounds in ATaAL-VO after 20 min of ozone exposure.
Analysis of the main components of volatile oil in ozone environment
According to Fig. 6, compared to the crude oil group, the overall trends of the main components 000078-70-6, 000093-15-2, 000087-44-5, 006380-24-1, 006753-98-6, 005353-15-1, 002883-98-9, and 000473-15-4 in the Pickering emulsion group are more stable. The relative content of the main components 000087-44-5, 006753-98-6, and 000473-15-4 in the β-cyclodextrin encapsulation group is higher than that in the crude oil group.
Discussion
The pharmacological activity of volatile oils in traditional Chinese medicine is significant; however, their volatility and susceptibility to oxidation lead to poor formulation stability33. Ozone, as a strong oxidizing agent, has a high oxidation potential (2.07 V) and can attack unsaturated bonds (such as double and triple bonds) in volatile oils, triggering radical reactions34. Ozone’s strong oxidizing ability enables it to react quickly with unsaturated components in volatile oils, thereby inducing oxidation reactions or altering the structure and composition of compounds in the oils35,36.
This could result in structural alterations of certain compounds in the volatile oils, leading to their decomposition or degradation into other compounds and thus altering the composition of the volatile oil. The oxidative action of ozone may further reduce the stability of volatile oils and shorten their shelf life37.
The application of Pickering emulsions and β-cyclodextrin encapsulation has shown significant effectiveness in reducing the peroxide values of ATaAL-VO. Compared to the crude oil group, these technologies led to a marked reduction in peroxide values (p < 0.01). GC-MS analysis revealed that these technologies could partially mitigate the increasing or decreasing trends of most differential components in the crude oil group when exposed to ozone. Additionally, they help reduce the loss of certain components in the crude oil group under ozone exposure, stabilize the variations of major components, and decrease the oxidative loss of key constituents.
These findings indicate that Pickering emulsions and β-cyclodextrin encapsulation, through physical barrier and chemical inclusion effects, reduce the direct contact between volatile oils and ozone, thereby inhibiting peroxide formation. They can also partially prevent the formation of new differential components and delay the compositional changes of volatile oils caused by ozone exposure. By reducing the oxidative loss of major components, these technologies help maintain the bioactivity and chemical stability of volatile oils, demonstrating consistent antioxidant capabilities14,38,39,40,41.
This study compares and analyzes the variations of peroxide value, differential components, quantitative components, qualitative components, and major components of ATaAL-VO in the presence of ozone, as well as the stabilizing effects of Pickering emulsion and β-cyclodextrin. The advantages of Pickering emulsion and β-cyclodextrin in stabilizing the different groups of ATaAL-VO are elucidated. This research provides valuable insights for enhancing the quality of ATaAL-VO.
Conclusion
In this study, based on the concept of drug-assisted combination, Pickering emulsion was successfully prepared using PEG 4000 and finely ground indigo Naturalis via the melting method. It was applied to the stability of ATaAL-VO and rhizome in ozone environment. At the same time, we compared the change trend of volatile components of β-cyclodextrin and Pickering emulsion in ozone environment. In the ozone environment, the variation of volatile components of essential oils with time was analyzed from three aspects: quantity change, quality change and principal component change. In the above research process, we found that compared with the original oil group, the β-cyclodextrin encapsulation group and the Pickering emulsion group exposed to ozone for 10, 15 and 20 min showed a lower degree of oxidation. In addition, GC-MS analysis showed that compared with the crude oil group, the stability of volatile components in the Pickering emulsion group and the β-cyclodextrin encapsulation group was significantly improved. In summary, both the complexation of β-cyclodextrin with ATaAL-VO and the formation of Pickering emulsions can significantly enhance the stability and quality of ATaAL-VO. Numerous studies have demonstrated that Pickering emulsions offer substantial advantages for the stabilization of volatile oils across different environments. Given these benefits, Pickering emulsions represent a promising approach for improving the stability and quality of volatile oils and are expected to have good development prospects in the future.
Data availability
Data is provided within the manuscript information files.
References
Khwairakpam, A. D. et al. Acorus Calamus: a bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 29, 107–122. https://doi.org/10.1515/jbcpp-2016-0132 (2018).
Rajput, S. B., Tonge, M. B. & Karuppayil, S. M. An overview on traditional uses and Pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine 21, 268–276. https://doi.org/10.1016/j.phymed.2013.09.020 (2013).
Olas, B. & Bryś, M. Is it safe to use Acorus calamus as a source of promising bioactive compounds in prevention and treatment of cardiovascular diseases? Chemico-Biol. Interact. 281, 32–36. https://doi.org/10.1016/j.cbi.2017.12.026 (2017).
He, X., Chen, X., Yang, Y., Liu, Y. & Xie, Y. Acorus calamus Var. Angustatus Besser: insight into current research on ethnopharmacological use, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Phytochemistry 210, 113626. https://doi.org/10.1016/j.phytochem.2023.113626 (2023).
Deng, A. P. et al. Advances in studies on chemical compositions of atractylodes lancea and their biological activities. China J. Chin. Materia Med. 41, 3904–3913. https://doi.org/10.4268/cjcmm20162104 (2017).
Bailly, C. Atractylenolides, essential components of Atractylodes-based traditional herbal medicines: antioxidant, anti-inflammatory and anticancer properties. Eur. J. Pharmacol. 891. https://doi.org/10.1016/j.ejphar.2020.173735 (2020).
Koonrungsesomboon, N., Na-Bangchang, K. & Karbwang, J. Therapeutic potential and pharmacological activities of atractylodes lancea (Thunb.) DC. Asian Pac. J. Trop. Med. 7, 421–428. https://doi.org/10.1016/S1995-7645(14)60069-9 (2014).
Jun, X., Fu, P., Lei, Y. & Cheng, P. Pharmacological effects of medicinal components of atractylodes lancea (Thunb.) DC. Chin. Med. 13, 59. https://doi.org/10.1186/s13020-018-0216-7 (2018).
Yang, L. et al. A review of the ethnopharmacology, phytochemistry, pharmacology, application, quality control, processing, toxicology, and pharmacokinetics of the dried rhizome of Atractylodes macrocephala. Front. Pharmacol. 12, 727154. https://doi.org/10.3389/fphar.2021.727154 (2021).
Peng, L. et al. Pickering emulsion technology based on the concept of the combination of medicine and adjuvant to enhance the oxidation stability of volatile oils in solid preparations—taking Lingzhu pulvis as an example. RSC Adv. 12, 27453–27462. https://doi.org/10.1039/d2ra04433a (2022).
Albuquerque, P. M. et al. Biotechnological applications of nanoencapsulated essential oils: A review. Polymers 14, 5495. https://doi.org/10.3390/polym14245495 (2022).
Cahyana, Y. et al. Pickering emulsions as vehicles for bioactive compounds from essential oils. Molecules 27, 7872. https://doi.org/10.3390/molecules27227872 (2022).
Keramat, M. et al. Oxidative stability of pickering emulsions. Food Chem. X. 14, 100279. https://doi.org/10.1016/j.fochx.2022.100279 (2022).
Hong, X., Zhao, Q., Liu, Y. & Li, J. Recent advances on food-grade water-in-oil emulsions: instability mechanism, fabrication, characterization, application, and research trends. Crit. Rev. Food Sci. Nutr. 63, 1406–1436. https://doi.org/10.1080/10408398.2021.1964063 (2021).
Ru, H. et al. Cinnabaris modified with SiO2 nanoparticles stabilized Pickering emulsion to improve the photostability of volatile oil: A Lingzhu San case study. Arab. J. Chem. 14, 31367–31384. https://doi.org/10.1016/j.arabjc.2023.105442 (2023).
He, J. et al. Natural food preservation with ginger essential oil: biological properties and delivery systems. Food Res. Int. 173. https://doi.org/10.1016/j.foodres.2023.113221 (2023).
Feng, L. et al. Cyclodextrin drugs in liposomes: Preparation and application of anticancer drug carriers. AAPS PharmSciTech. 26. https://doi.org/10.1208/s12249-024-02999-0 (2024).
Abdellatif, A. A. H. et al. Recent advances in the pharmaceutical and biomedical applications of Cyclodextrin-Capped gold nanoparticles. Int. J. Nanomed. 18, 3247–3281. https://doi.org/10.2147/ijn.s405964 (2023).
Apostolidis, E., Stoforos, G. N. & Mandala, I. Starch physical treatment, emulsion formation, stability, and their applications. Carbohydr. Polym. 305, 120554. https://doi.org/10.1016/j.carbpol.2023.120554 (2023).
Araj, S. K. et al. A review on cyclodextrins/estrogens inclusion complexes. Int. J. Mol. Sci. 24, 8780. https://doi.org/10.3390/ijms24108780 (2023).
Ma, J. et al. Preparation of aromatic β-cyclodextrin nano/microcapsules and corresponding aromatic textiles: A review. Carbohydr. Polym. 308. https://doi.org/10.1016/j.carbpol.2023.120661 (2023).
Xiao, Z., Zhang, Y., Niu, Y., Ke, Q. & Kou, X. Cyclodextrins as carriers for volatile aroma compounds: A review. Carbohydr. Polym. 169, 118292. https://doi.org/10.1016/j.carbpol.2021.118292 (2021).
Nutho, B., Nunthaboot, N., Wolschann, P., Kungwan, N. & Rungrotmongkol, T. Metadynamics supports molecular dynamics simulation-based binding affinities of eucalyptol and beta-cyclodextrin inclusion complexes†. RSC Adv. 7, 50899–50911 (2017).
Tian, Y. et al. Pickering emulsions stabilized by β-cyclodextrin and cinnamaldehyde essential oil/β-cyclodextrin composite: A comparison study. Food Chem. 377, 131995. https://doi.org/10.1016/j.foodchem.2021.131995 (2021).
Wang, Y. et al. Enhanced preservation effects of clove (Syzygium aromaticum) essential oil on the processing of Chinese bacon (preserved meat products) by beta cyclodextrin metal organic frameworks (β-CD-MOFs). Meat Sci. 195, 108998. https://doi.org/10.1016/j.meatsci.2022.108998 (2022).
Chen, Z. Y. et al. The investigation of thermal stability and GC-MS analysis of Acorus Tatarinowii and atractylodes lancea volatile oils treated by Β cyclodextrin inclusion and pickering emulsion technologies. Heliyon 10, e25909. https://doi.org/10.1016/j.heliyon.2024.e25909 (2024).
Lin, X. et al. The light stability and GC-MS analysis of volatile oil of Acorus Tatarinowii Schott and atractylodes lancea under the treatment of β-cyclodextrin inclusion and pickering emulsion technology. Chin. Herb. Med. 56, 441–457 (2025).
Kolde, R. Pretty Heatmaps. R Package Version 1.0.12. https://CRAN.R-project.org/package=pheatmap (2019).
Hope, R. M. Rmisc: Ryan Miscellaneous. R Package Version 1.5.1. https://CRAN.R-project.org/package=Rmisc (2022).
Wickham, H. Reshaping data with thereshapepackage. J. Stat. Softw. 21. https://doi.org/10.18637/jss.v021.i12 (2007).
Zhu, M. et al. Development, stability, and in vitro/in vivo studies of volatile oil pickering emulsion. Stabilized Modified Amber Pharmaceuticals. 17, 1117. https://doi.org/10.3390/ph17091117 (2024).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Elsebai, M. F. & Albalawi, M. A. Essential oils and COVID-19. Molecules (Basel Switzerland) 27. https://doi.org/10.3390/molecules27227893 (2022).
Chen, F. et al. Ozone meets peroxides: A symphony of hybrid techniques in wastewater treatment. Chem. Eng. J. 483, 149129. https://doi.org/10.1016/j.cej.2024.149129 (2024).
Angulo Milhem, S., Verriele, M., Nicolas, M. & Thevenet, F. Does the ubiquitous use of essential oil-based products promote indoor air quality? A critical literature review. Environ. Sci. Pollut. Res. 27, 14365–14411. https://doi.org/10.1007/s11356-020-08150-3 (2020).
Fomenkov, D. I., Budekhin, R. A. & Vil, V. A. The Ozone and hydroperoxide teamwork: synthesis of unsymmetrical geminal bisperoxides from alkenes. Org. Lett. 25, 4672–4676. https://doi.org/10.1021/acs.orglet3t.3c01477 (2023).
Floare, A. D. et al. Enhancing the antimicrobial effect of ozone with Mentha piperita essential oil. Molecules 28, 2032. https://doi.org/10.3390/molecules28052032 (2023).
Cahyana, Y. et al. Pickering emulsions as vehicles for bioactive compounds from essential oils. Molecules 27, 7872. https://doi.org/10.3390/molecules27227872 (2022).
He, J. et al. Natural food preservation with ginger essential oil: biological properties and delivery systems. Food Res. Int. 173, 113221. https://doi.org/10.1016/j.foodres.2023.113221 (2023).
Xu, D., Liu, J. & Liu, Y. Research progress on β-cyclodextrin and its derivatives in increasing the solubility of guest molecules. Food Ind. Sci. Technol. 42, 404–411. https://doi.org/10.13386/j.issn1002-0306.2020080221 (2021).
Shen, Y., Wang, Q., Guo, Y., Niu, J. & Sun, M. Research progress on cyclodextrin and its derivatives as delivery systems in the food field. Food Ind. Sci. Technol. 43, 496–505. https://doi.org/10.13386/j.issn1002-0306.2022060180 (2022).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82274105), the Science and Technology Innovative Talent Program of Shaanxi University of Chinese Medicine (No. 2024-CXTD-03), the Shaanxi Province Traditional Chinese Medicine Pharmaceutical Key Discipline Funding Project (No. 303061107), the Discipline Innovation Team Project of Shaanxi University of Chinese Medicine (No. 2019-YL11), and the Shaanxi Provincial Administration of Traditional Chinese Medicine Key Discipline of Traditional Chinese Medicine Pharmaceutical Engineering (No. 2017001).
Author information
Authors and Affiliations
Contributions
Fei Luan and Junbo Zou designed this study and amended the final manuscript. Junping Li and Zhongying Chen performed the experiment and wrote the manuscript. Yajun Shi, Jing Sun, and Xiaofei Zhang provided technical help and did data analysis for this experiment. Dongyan Guo, Bingtao Zhai and Fei Luan polished and revised the manuscript. All of the authors have read and approved the final manuscript. All authors contributed substantially to this study and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Chen, Z., Zou, J. et al. Application of β-cyclodextrin and pickering emulsion to enhance the ozone stability of Acorus tatarinowii and Atractylodes lancea volatile oils. Sci Rep 15, 17683 (2025). https://doi.org/10.1038/s41598-025-01607-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01607-w