Abstract
Heavy metal contamination in agricultural soils is a growing environmental concern, particularly due to the increasing accumulation of cadmium (Cd) and chromium (Cr) from industrial discharge, wastewater irrigation, and excessive fertilizer use. These toxic metals severely impact crop productivity by disrupting nutrient uptake, damaging root structures, and inducing oxidative stress, which collectively inhibit plant growth and development. Maize (Zea mays L.), a globally important cereal crop, is highly susceptible to heavy metal toxicity, making it essential to develop cost-effective and sustainable mitigation strategies. Spent mushroom substrate (SMS) biochar has emerged as an effective and sustainable method due to its ability to absorb heavy metals. Spent mushroom substrate biochar improves compost quality, soil fertility, and health. Its high porosity and surface area immobilize toxic metals, reducing nutrient losses and oxidative stress in plants. Pyrolysis temperature affects its surface area, nutrient composition, and adsorption abilities. This study aims to address this gap by evaluating the effectiveness of SMS biochar at varying application rates in mitigating Cd and Cr toxicity in maize. By assessing key physiological and agronomic parameters, this research provides novel insights into the potential of SMS biochar as a sustainable soil amendment for heavy metal-contaminated soils. Five treatments, i.e., 0, 50, 100, 150 and 200B were applied under Cd and Cr toxicity in 3 replications following the completely randomized design (CRD). Results exhibited that 200B caused an increase in maize plant height (26.1%), root dry weight (99.7%), grain yield (98.2%), and chlorophyll contents (50%) over control under Cd and Cr stress. In conclusion, 200B can mitigate Cd and Cr stress in maize plants. More investigations are suggested to declare 200B as a promising amendment for mitigation of Cd and Cr stress in other crops.
Similar content being viewed by others
Introduction
Heavy metals toxicity imposed significant threats to crops growth and productivity1. These metals become the part of food chain via industrial and anthropogenic activities. In these heavy metals, Cd and Cr are acute toxins that have contaminated approximaly 137,000 sites in europe while 11% of agricultural soils in china2,3,4. In case of maize increase in Cd toxicity (2.14–8.29-folds) in various plant parts has been documented where sewage water was applied as irrigation compared to freshwater5,6. Researcher found that in India, maize crops irrigated with wastewater in the industrial belts of Punjab and Uttar Pradesh were found to contain Cd levels between 0.25 and 0.78 mg/kg and Cr levels between 0.56 and 2.45 mg/kg, reflecting considerable health risks to local populations through dietary intake7. Khan et al.8 also found that in Pakistan the districts of Sargodha, Mianwali, and Bhakkar, Cr concentrations in forage samples used for livestock feeding were found to range between 0.672–1.631 mg/kg, 1.493–2.612 mg/kg, and 0.7804–1.047 mg/kg, respectively. Correspondingly, the levels of Cr detected in goat blood plasma from these regions were observed within the ranges of 0.666–1.269 mg/L in Sargodha, 0.657–0.752 mg/L in Mianwali, and 1.39–2.37 mg/L in Bhakkar8. Ghazzal et al.9 validated the above fact and noted that among different fodder crops, maize was prime suspect where different concentrations and an elevated level of Cr were observed. Both metals i.e., Cr and Cd have potential to disrupt key biochemical and physiological processes when taken up even at concentrations10,11. Toxicity of Cd disturb the uptake of nutrients i.e., zinc and calcium12,13,14. It also caused higher synthesis of reactive oxygen species (ROS) which induced oxidative stress in plants10,15. On the other hand, hexavalent form chromium (Cr⁶⁺) adversely affects the chloroplast structure which decreased the photosynthetic efficiency16,17. Impairment of water relationship in plants, alteration in enzymatic activities and handiness in cellular metabolism are allied negative effects of Cr in plants18.
Maize is major cereal crop that have high economic and nutritional values worldwide. It is staple food for millions of people, because of high value of essential calories, micronutrients and proteins19. Presence of phytochemicals that have potential health benefits i.e., carotenoids, phytosterols and phenolic compounds also make maize a valuable crop for consumers. However, both Cr and Cd negatively affects its physiological and morphological attributes of maize. These heavy metals minimize the maize growth via reduction in photosynthetic rate and alteration in water balance20. Leaf chlorosis, decreased stem diameter and reduced stomatal conductance have also been documented in maize when it is cultivated in Cd and Cr contaminated soil20,21. Among different maize plant organs, accumulation of these metals is higher in roots which also induced oxidative stress22,23. That’s why in-depth study and exploration of effective strategy is need of time for the resolution of this problem in agriculture especially for improvement of maize growth and productivity.
Spent mushroom substrate (SMS) biochar has recently gained attention as a sustainable and effective material for heavy metal adsorption24. Literature showed that it is effective in improving the quality of compost by decreasing electrical conductivity and loss of organic matter25,26. It can also enhance soil fertility and health due to high porosity and surface area26. These attributes allow biochar to immobilize toxic metals and decrease the nutrient losses, thereby decreasing oxidative stress in plants25,27,28. Furthermore, SMS biochar shows high adsorption abilities for heavy metals, i.e. lead (Pb), zinc (Zn), copper (Cu), and Cd25,29,30. However, pyrolysis temperature is a key factory which influences its surface area, nutrient composition, and adsorption abilities26,31. High-temperature pyrolysis generally improves its heavy metal removal performance30.
Therefore, the current study was planned to explore the potential of SMS biochar on growth of maize cultivated under Cd and Cr toxicity. The aim of study was to find out most representable attribute of maize which get modified in maize and it play key role in enhancement of its growth under Cd and Cr toxicity. The novelty of study lies in exploration of SMS biochar effectives in decreasing Cd and Cr uptake in maize for mitigation of its adverse effects in maize. The study is also covering the knowledge gap regarding use of SMS biochar effect level for alleviation of Cd and Cr stress in maize. It is hypothesized that SMS biochar might have potential to decrease Cd and Cr uptake in maize for improvement in growth.
Material and methods
Experimental design and setup
A pot experiment was conducted in glass house of Department of Soil and Environmental Sciences, The University of Agriculture Peshawar (34°01’N, 71°71’E). The experiment was carried out in completely randomized design (CRD) and was replicated three times.
Soil collections and characterization
Bulk soil was brought from the university research farm field. The soil was dried and sieved through 2 mm. The characteristics of soil is provided in (Table 1).
Soil spiking with Cd and Cr
The soil was contaminated artificially with Cd and Cr at 5 and 150 mg kg−1 concentration each. After that incubation was carried out for 21 days under control conditions i.e., 25 ± 5˚C temperature, 60% humidity and 70% field capacity. The mixing was done manually after each 5 days for achievement of homogenization. Cadmium nitrate and chromium nitrate were used as Cd and Cr sources for spiking of soil. Total 10 kg soil was then placed in each pot.
Biochar preparation and application
Spent mushroom substrate biochar was prepared in muffle furnace at 500 °C for 2 h pyrolysis. The spent mushroom substrate was washed with distilled water to remove the dust and other extra materials and were air dried. After pyrolysis the biochar was cooled at room temperature and analyzed of basic properties i.e. [surface area (m2/g) = 5.10, cation exchange capacity (cmol/kg) = 31.69, ash content (%) = 30, volatile matter (%) = 26, fixed carbon (%) = 44, total nitrogen (%) = 0.71, pH = 8.15, EC (dS/m) = 2.09] were determined. The different levels of biochar such as, 0.5, 1, 1.5, and 2% were applied to 10 kg soil in pots.
Nutrients application
NPK was then applied at the rate of 90, 75, and 60 kg ha−1 as basal dose from urea, single super phosphate, and murate of potash uniformly to all treatment pots. Ten plants per pot were sown. After germination 2 healthy seedlings were maintained in each pot.
Treatment plan
In this study, five distinct treatments of SMS biochar were applied to assess its impact on maize growth under Cd and Cr stress. Each treatment involved incorporating 0, 50, 100, 150, or 200 g of SMS biochar into pots containing 10 kg of soil. The pots measured 20 cm in width and 45 cm in depth, providing a consistent environment for plant growth. To ensure the reliability and validity of the results, each treatment was replicated three times, allowing for statistical analysis and verification of data consistency.
Harvesting and data collection
The plants were harvested at the time of maturity (after 125 days of sowing). To determine the average plant height in each treatment plot, 10 plants was randomly selected and their height was measured using a tape measure from the bottom to the top of the plant.
Root dry weight (pot−1)
Maize plants were harvested with roots. Harvested plants were tied together with rope and transported to the laboratory in a plastic bag. They were brushed first and then washed with distilled water. Roots were separated for the purpose to weight it, and scattered on a table inside the laboratory to air dry. After air drying, roots for each pot were individually weighed, placed in paper bags, and baked for 48 h at 70 °C.
Number of Grain cob−1
Cobs were threshed for each pot, and the number of grains obtained from each cob were counted. Finally, the average number of grains per cob was calculated.
Grain yield (g pot−1)
The amount of maize harvested from the entire pot was measured by weighing the threshed grain after it had been dried for a week. The results were expressed as g pot−1.
Biological yield (g pot−1)
The entire pot was harvested and left to dry for seven days to measure the biological yield. The dried plants were weighed and the results were expressed as g pot−1.
Determination of cadmium and chromium
Samples were taken from roots, shoots, and grains of 0.5 g each and treated with 10 mL concentrated HNO3 separately. Then the samples were kept overnight and 4 mL of concentrated HClO4 was added and the digestion was done up to the time when the samples produced an off-white color in a conical flask. Let to be cooled down the samples and filtered using filter paper Whattman. No. 42. Then samples were taken in 100 mL volumetric flask and added distilled water to make the volume up to 100 mL. Then readings were taken on an atomic absorption spectrophotometer for the elemental analysis of Cd and Cr37.
Chlorophyll content
SPAD reading on the mid-point and on ear of the youngest fully expanded leaf at tasseling stage. Three leaves were measured randomly in pot and a main SPAD value was calculated for each pot38.
Antioxidant enzymes
The activities of peroxidase39 and superoxide dismutase40 in leaves were measured by using a spectrophotometer. Briefly, leaf samples were collected at tasseling stage in fresh form to analyze the antioxidant enzymes. The ground samples of leaves were homogenized with a phosphate buffer of molarity 0.05 M and pH 7.8, in cold via the use of liquid nitrogen. This mixture was centrifuged for a time duration of 10 min at 4ºC, afterwards the required absorbance for superoxide dismutase and peroxidase was set to calculate the intensity of supernatants with the help of a spectrophotometer i.e. 560 nm and 470 nm, respectively. Ascorbate peroxidase (APX) activity was assessed spectrophotometrically by monitoring the decrease in absorbance at 290 nm as ascorbate is oxidized. GSH levels was measured using high-performance liquid chromatography (HPLC) with pre-column derivatization using o-phthalaldehyde (OPA). GR activity was determined by monitoring the oxidation of NADPH to NADP⁺, observed as a decrease in absorbance at 340 nm. Proline concentration was determined using the acid-ninhydrin method which produce a colored complex and absorbance of which was measured at 520 nm. Total phenolic content was assessed using the Folin-Ciocalteu reagent leading to a blue color whose absorbance was measured at 765 nm. Ascorbic acid (AsA) was assessed following the reaction between ascorbic acid and 4-chloro-7-nitrobenzofurazane (NBD-Cl) in alkaline conditions, that produce a bluish-green compound with maximum absorption at 582 nm.
Translocation factor for heavy metals
The translocation factor (TF) was calculated according to the procedure of Ahmed and Slima (2018).
FTIR and XRD
FTIR spectra were recorded over a wavenumber range of 400 to 4000 cm⁻1 with a resolution of 4.0 cm⁻1. For XRD analysis, the biochar samples were scanned using an Ultima IV X-ray diffractometer (operating at 40 kV and 40 mA. The scanning range was from 5 to 85° 2θ, with a scanning speed of 6° min⁻1.
Statistical analysis
Standard statistical procedure was followed for the analysis of data. One way ANOVA was applied for examining the significance of treatments. Graphs were made using OriginPro 2021 software41.
Results
Plant height, yield, and yield components
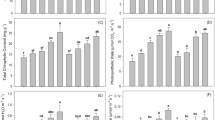
Results showed that control plants showed an average plant height of 71.3 cm. At 50 g biochar /kg soil (50B), plant height was increased by 5.8% (75.43 cm) compared to the control. Applying 100 g biochar /kg soil (100B) resulted in an 11.6% increase in plant height (79.57 cm) over control. Compared to control at 150 g biochar /kg soil (150B), plant height was improved by 17.4% (83.7 cm). Treatment 200 g biochar /kg soil (200B), showed the maximum plant height of 89.9 cm, corresponding to a 26.1% increase over the control (Fig. 1A).
Effect of variable doses (control having Cd-Cr but no B = 0 g), 50, 100, 150 and 200 g) of spent mushroom substrate derived biochar (B) on maize plant height (A), root dry weight (B), grains/cob (C) and grain yield (D) cultivated under chromium and cadmium toxicity. Bars are means of 3 replicates ± SE. Variable bar letters showed significant changes at p ≤ 0.05 compared by Fisher’s LSD.
The control plants showed a root dry weight of 3.07 g. Root dry weight was enhanced by 18.5% (3.63 g) compared to control at 50B. Treatment 100B further improved root dry weight to 4.57 g, showing a 48.9% increase over the control. An enhancement was observed at 150B, where root dry weight reached 5.47 g, representing a 78.3% increase compared to the control. The highest biochar level (200 g/kg, 200B) led to the maximum root dry weight of 6.43 g, marking a 99.7% increase over the control treatment (Fig. 1B).
Under control treatment, the number of grains per cob was observed 111.67. Biochar application 50B led to a 29.6% increase, reaching 144.67 grains per cob over control. Treatment 100B resulted in a 75.8% improvement (196.33 grains per cob) compared to control. An enhancement (122.1%) was observed at 150B, where grains per cob was 248, over control. Applying 200B led to the maximum number of grains per cob (310 grains), represented a 177.6% increase over the control (Fig. 1C).
In the control treatment, grain yield was 103.33 g per pot. The application of 50B resulted in a 29% increase, raising grain yield to 133.33 g per pot. At 100B, grain yield further improved to 163.33 g per pot, marking a 58% increase over the control. A significant enhancement was observed in the 150B treatment, where grain yield reached 193.33 g per pot, representing an 87.1% increase compared to the control. The highest biochar level, 200B, resulted in the maximum grain yield of 213 g per pot, which accounted for a 98.2% increase over the control (Fig. 1D).
In the control treatment, biological yield was 248 g per pot. The introduction of 50B led to a 20.9% increase, raising the biological yield to 299.67 g per pot compared to control. At 100B, biological yield further improved to 351.33 g per pot, representing a 41.7% increase over the control. A significant increase was observed at 150B, where biological yield reached 408.17 g per pot, marking a 64.6% improvement than control. The highest biochar application, 200B, resulted in the maximum biological yield of 450.53 g per pot, reflecting an 81.7% increase from the control (Fig. 2A).
Effect of variable doses (control having Cd–Cr but no B = 0 g), 50, 100, 150 and 200 g) of spent mushroom substrate derived biochar (B) on maize biological yield (A), chlorophyll (SPAD) (B), peroxidase (POD) (C) and superoxide dismutase (SOD) (D) cultivated under chromium and cadmium toxicity. Bars are means of 3 replicates ± SE. Variable bar letters showed significant changes at p ≤ 0.05 compared by Fisher’s LSD.
Physiological parameters
In the control treatment, the chlorophyll content was 33.07 SPAD units. The application of 50B resulted in a 6.2% increase, raising chlorophyll content to 35.13 SPAD units than control. At 100B, chlorophyll content improved further to 40.3 SPAD units, showing a 21.9% increase from control. A significant enhancement was recorded in the 150B treatment, where chlorophyll content increased to 44.43 SPAD units, reflecting a 34.4% improvement over control. The highest biochar level, 200B, resulted in the maximum chlorophyll content of 49.6 SPAD units, marking a 50% increase compared to the control (Fig. 2B).
Antioxidant enzyme activities
In the control treatment, POD activity was recorded at 45.57 µg/g FW. Applying 50B resulted in a 37.4% increase, raising POD activity to 62.62 µg/g FW. At 100B, POD activity further increased to 70.06 µg/g FW, representing a 53.8% enhancement over the control. A significant increase was observed in the 150B treatment, where POD activity reached 82.15 µg/g FW, showing an 80.3% increase compared to the control. Treatment 200B resulted in the maximum POD activity of 94.34 µg/g FW, marking a 107.1% increase from the control (Fig. 2C).
In the control treatment, SOD activity was 33.38 µg/g FW. Treatment 50B resulted in a 18.3% increase, reaching 39.47 µg/g FW. Furthermore, at 100B, where SOD activity increased by 39.6% over the control, reaching 46.60 µg/g FW. At 150B, SOD activity reached 54.77 µg/g FW, reflecting a 64.2% increase from the control. At 200B, showed a maximum SOD activity of 68.10 µg/g FW, corresponding to a 104.1% increase compared to the control (Fig. 2D).
At control treatment, APX activity was the highest (53.22 U/mg protein). Applying 50B, APX activity decreased by 17.9%, reaching 43.67 U/mg protein than control. Under 100B, APX activity declined by 31.6% over the control, reaching 36.39 U/mg protein. Treatment 150B, APX activity was decreased (29.86 U/mg protein), marking a 43.9% decline compared to the control. The lowest APX activity was found at 200B, with a value of 23.23 U/mg protein, indicating a 56.4% decrease than control (Fig. 3A).
Effect of variable doses (control having Cd–Cr but no B = 0 g), 50, 100, 150 and 200 g) of spent mushroom substrate derived biochar (B) on maize ascorbate peroxidase (APX) (A), glutathione reductase (GR) (B) and glutathione (GSH) (C) cultivated under chromium and cadmium toxicity. Bars are means of 3 replicates ± SE. Variable bar letters showed significant changes at p ≤ 0.05 compared by Fisher’s LSD.
At control treatment, GR activity was showed as 31.65 U/mg protein. Resulted showed that 50B resulted in a 22.7% decrease, reducing GR activity to 24.46 U/mg protein from control. At 100B, GR activity was decreased by 40.3% over the control, reaching 18.90 U/mg protein. A significant decline was noted at 150B, where GR activity decreased to 14.00 U/mg protein, showing a 55.7% decline compared to the control. The lowest GR activity was recorded in 200B treatment (10.44 U/mg protein) showing 67% decrease over the control (Fig. 3B).
In the control treatment, GSH content was 24.85 μmol/g FW. The addition of 50B led to a 27.3% reduction, bringing GSH levels down to 18.08 μmol/g FW over control. At 100B, GSH content further declined by 41.4% compared to the control, reaching 14.57 μmol/g FW. A significant decline was observed at 150B, where GSH was 10.90 μmol/g FW, marking a 56.1% decrease over the control. Applying 200B treatment showed 8.20 μmol/g FW GSH, indicating a 67% decline compared to the control (Fig. 3C).
Results showed that ascorbic acid (AsA) content was 13.75 mg/g FW in control treatment. With 50B, a 26.8% reduction was observed in AsA (10.07 mg/g FW) than control. Treatment 100B caused 48.4% decline (AsA = 7.1 mg/g FW) compard to control. A significant decrease (4.65 mg/g FW) was noted at 150B, over the control. The lowest AsA level was found at 200B, i.e., 3.39 mg/g FW, representing a 75.4% decline over the control (Fig. 4A).
Effect of variable doses (control having Cd-Cr but no B = 0 g), 50, 100, 150 and 200 g) of spent mushroom substrate derived biochar (B) on maize ascorbic acid (AsA) (A), proline (B) and phenolics (C) cultivated under chromium and cadmium toxicity. Bars are means of 3 replicates ± SE. Variable bar letters showed significant changes at p ≤ 0.05 compared by Fisher’s LSD.
With control treatment, proline accumulation was 17.06 μmol/g FW. Applying 50B resulted in 20.5% decrease (13.56 μmol/g FW) than control. At 100B, a 35.7% decline in proline (10.97 μmol/g FW) was noted compared to control. However, at 150B, proline content was decreased by 49.9%, reaching 8.54 μmol/g FW over control. The 200B treatment resulted in 66.3% decline in proline (5.75 μmol/g FW) compared to control (Fig. 4B).
In the control treatment, phenolic content was 14.81 mg/g FW. The application of 50B resulted in a 28.7% reduction, bringing phenolic levels down to 10.56 mg/g FW compared to control. With 100B, a further 46.4% decline was observed, reducing phenolic content to 7.95 mg/g FW over control. At 150B, phenolics decreased by 60.2%, reaching 5.9 mg/g FW than control. The highest biochar application 200B caused a 67.9% reduction, with phenolic levels dropping to 4.75 mg/g FW from control (Fig. 4C).
Cadmium and chromium concentrations in plant root, shoot and grains
Under control treatment, root Cd concentration was 25.63 µg/g. Treatment 50B reduced root Cd by 20.8%, lowering it to 20.28 µg/g compared to control. Further at 100B resulted in a 37.7% decrease, reducing Cd accumulation to 15.98 µg/g over control. Applying 150B, caused root Cd levels declined by 52.9%, reaching 12.06 µg/g, whereas the highest biochar application 200B led to a significant 73.7% reduction, bringing root Cd down to 6.75 µg/g from control (Fig. 5A).
Effect of variable doses (control having Cd-Cr but no B = 0 g), 50, 100, 150 and 200 g) of spent mushroom substrate derived biochar (B) on maize root Cd (A), shoot Cd (B) and grains Cd (C) cultivated under chromium and cadmium toxicity. Bars are means of 3 replicates ± SE. Variable bar letters showed significant changes at p ≤ 0.05 compared by Fisher’s LSD.
In the control treatment, shoot Cd concentration was 21.25 µg/g. The application of 50B decreased shoot Cd by 29.8%, lowering it to 14.91 µg/g over control. With 100B, shoot Cd was further reduced by 50.1%, reaching 10.60 µg/g compared to control. At 150B, shoot Cd levels dropped by 69.8%, declining to 6.41 µg/g from control. The 200B dose, led to an 88.6% reduction, minimizing shoot Cd accumulation to just 2.42 µg/g than control (Fig. 5B).
In the control treatment, Cd concentration in grains was 18.35 µg/g. Applying 50B reduced grain Cd by 44.7%, lowering it to 10.15 µg/g than control. With 100B, grain Cd was declined by 65.6%, reaching 6.32 µg/g than control. At 150B, grain Cd content decreased by 82.3%, declining to 3.26 µg/g over control. The highest biochar dose, 200B, resulted in a remarkable 94.2% reduction, bringing grain Cd levels down to just 1.06 µg/g from control (Fig. 5C).
In the control treatment, root Cr concentration was 21.35 µg/g. The addition of 50B led to a 25.0% reduction, bringing root Cr down to 16.02 µg/g. With 100B, Cr levels further declined by 40.0%, reaching 12.81 µg/g. At 150B, Cr concentration was reduced by 60.0%, with a value of 8.54 µg/g. The highest biochar dose, 200B, resulted in a significant 75.0% decrease, lowering root Cr to 5.33 µg/g (Fig. 6A).
Effect of variable doses (control having Cd-Cr but no B = 0 g), 50, 100, 150 and 200 g) of spent mushroom substrate derived biochar (B) on maize root Cr (A), shoot Cr (B) and grains Cr (C) cultivated under chromium and cadmium toxicity. Bars are means of 3 replicates ± SE. Variable bar letters showed significant changes at p ≤ 0.05 compared by Fisher’s LSD.
In the control treatment, shoot Cr concentration was 16.84 µg/g. The application of 50B led to a 31.3% reduction, lowering Cr levels to 11.57 µg/g. Further, at 100B, shoot Cr decreased by 53.7%, reaching 7.79 µg/g. A significant decline was observed at 150B, with a 73.5% reduction, bringing shoot Cr down to 4.45 µg/g. The highest biochar dose, 200B, resulted in an 85.8% decrease, limiting shoot Cr accumulation to 2.40 µg/g (Fig. 6B).
In the control treatment, Cr concentration in grains was 12.54 µg/g. With the addition of 50B, grain Cr levels decreased by 31.5%, reaching 8.60 µg/g. A further reduction was observed at 100B, where grain Cr dropped by 60.5%, reaching 4.96 µg/g. At 150B, grain Cr concentration declined by 82.7%, reducing it to 2.17 µg/g. The highest dose of 200B resulted in a remarkable 91.4% reduction, lowering grain Cr to 1.07 µg/g (Fig. 6C).
Translocation of Cd and Cr from root to shoot and from shoot to grains
In the control treatment, the TF was 0.8167. The application of 50B led to an 8.6% reduction, lowering the TF to 0.7467. Further, at 100B, the TF declined by 23.3%, reaching 0.6267. A significant decrease was observed at 150B, with a 34.3% reduction, bringing the TF down to 0.5367. The highest biochar dose, 200B, resulted in a 43.3% reduction, limiting the TF to 0.4633 (Fig. 7A).
Effect of variable doses (control having Cd–Cr but no B = 0 g), 50, 100, 150 and 200 g) of spent mushroom substrate derived biochar (B) on maize translocation factor of Cr from root to shoot (A), translocation factor of Cr from shoot to grains (B), translocation factor of Cd from root to shoot (C) and translocation factor of Cd from shoot to grains (D) cultivated under chromium and cadmium toxicity. Bars are means of 3 replicates ± SE. Variable bar letters showed significant changes at p ≤ 0.05 compared by Fisher’s LSD.
In the control treatment, the translocation factor (TF) was 0.7693. The application of 50B had a negligible effect, maintaining the TF at 0.7673, showing only a 0.26% reduction. However, at 100B, a more noticeable decline of 14.5% was observed, lowering the TF to 0.6577. A significant reduction occurred at 150B, where the TF decreased by 34.6%, reaching 0.5033. The highest biochar application, 200B, resulted in a 39.8% decrease, limiting the TF to 0.4630 (Fig. 7B).
In the control treatment, the translocation factor (TF) was 0.8567. The application of 50B reduced the TF by 11.3%, lowering it to 0.7600. At 100B, the TF further declined by 20.2%, reaching 0.6833. A more significant reduction occurred with 150B, where the TF decreased by 36.2%, limiting it to 0.5467. The highest biochar application, 200B, resulted in a drastic 56.8% reduction, bringing the TF down to 0.3700 (Fig. 7C).
In the control treatment, the shoot-to-grain Cd translocation factor was 0.8933. The application of 50B led to a 21.3% reduction, decreasing the TF to 0.7033. With 100B, the TF further dropped by 31.0%, reaching 0.6167. A more significant decrease was observed at 150B, where the TF declined by 41.0%, lowering it to 0.5267. The highest biochar application, 200B, resulted in a 49.2% reduction, bringing the TF down to 0.4533 (Fig. 7D).
The FTIR spectra of the biochar revealed several functional groups indicative of its chemical composition. A broad band around 3417–3423 cm⁻1 corresponded to O–H stretching vibrations, suggesting the presence of hydroxyl groups. Peaks near 1618 cm⁻1 were attributed to C=C and C=O stretching in aromatic rings, while bands around 1045 cm⁻1 indicated C–O stretching vibrations (Fig. 8A).
(A) FTIR spectrum of spent mushroom substrate-derived biochar (SMS-B), showing characteristic absorption peaks at 3417–3423 cm⁻1 (O–H stretching), 1618 cm⁻1 (C = C and C = O stretching in aromatic rings), and 1045 cm⁻1 (C–O stretching). (B) XRD pattern of SMS-B, indicating the presence of crystalline minerals such as SiO₂, CaCO₃, KCl, and CaSO₄, with distinct peaks around 25.5, 30°, and other relevant 2θ positions.
XRD analysis identified the presence of crystalline minerals within the biochar. Characteristic peaks corresponding to SiO₂ and CaCO₃ were observed across all samples, with varying intensities depending on the pyrolysis conditions. For instance, a significant peak for KCl was detected around 30° 2θ in certain samples, aligning with higher potassium content. Additionally, peaks for CaSO₄ were noted at approximately 25.5° 2θ, particularly in biochar produced at lower pyrolysis temperatures (Fig. 8B).
Discussion
Biochar, as an enriched carbon amendment, is produced through the pyrolysis of organic waste material42. It has gained significant attention as a soil amendment due to its ability to mitigate heavy metal toxicity in plants43. In the case of maize, heavy metal such as Cd and Cr stress significantly reduces physiological growth and yield due to oxidative damage, nutrient imbalance, and metabolic disruptions44,45,46. The oxidative stress disrupts cellular homeostasis, damages biomolecules, and hampers plant growth and development. The application of biochar in contaminated soils plays a crucial role in reducing the bioavailability of heavy metals by several mechanisms47. One of the primary modes of action includes the immobilization of heavy metals through precipitation, electrostatic attraction, and complex formation. For instance, biochar rich in oxygen-containing functional groups (e.g., hydroxyl, carboxyl, and phenolic groups) can strongly bind heavy metal ions, effectively reducing their phytoavailability48,49,50. The high surface area, porous structure, and functional groups of biochar enhance its ability to adsorb heavy metal ions, thereby limiting their uptake by plant roots51. This reduction in heavy metal bioavailability directly contributes to improved maize root growth, which is otherwise inhibited under metal stress due to metal-induced oxidative damage46,52.
Additionally, biochar contributes to an increase in soil pH, which further reduces the solubility and mobility of heavy metals, making them less available for plant absorption. Alongside heavy metal immobilization, biochar enhances soil properties such as, cation exchange capacity, organic matter content, and microbial diversity53. These factors collectively enhance soil fertility, leading to better plant growth even under heavy metal stress. In maize, improved soil fertility due to biochar has been linked to increased root biomass, leaf chlorophyll content, and grain yield54. Moreover, biochar-mediated changes in soil structure facilitate improved water retention and aeration, which further supports plant health55.
One of the most significant benefits of biochar application is its ability to regulate antioxidant enzyme activities in plants, which counteracts oxidative stress56. Under heavy metal toxicity, ROS accumulation leads to lipid peroxidation, membrane damage, and impaired photosynthetic efficiency in maize57,58. Superoxide dismutase (SOD) serves as the first line of defense by catalyzing the dismutation of superoxide radicals (O₂⁻) into hydrogen peroxide (H₂O₂) and molecular oxygen (O₂). This reaction is crucial in preventing the toxic accumulation of superoxide radicals, which can cause severe cellular damage. However, the presence of H₂O₂ still poses a threat to cellular integrity. Peroxidase (POD) plays an essential role in detoxifying H₂O₂ by converting it into H₂O and O₂ using electron donors, thus preventing oxidative damage to lipids, proteins, and nucleic acids. Furthermore, catalase (CAT) also contributes to H₂O₂ decomposition, reinforcing the plant’s antioxidant defense system. In addition to improving antioxidant defense, biochar enhances nutrient uptake, which is crucial for sustaining plant metabolism under heavy metal stress59. Application of SMS biochar under Cd and Cr stress has been observed to enhance SOD and POD activities, indicating a strengthened antioxidant defense in plants. Conversely, activities of ascorbate peroxidase (APX) and levels of reduced glutathione (GSH) tend to decline, possibly due to nutrient immobilization or altered metal bioavailability affecting these specific antioxidant pathways. Similar trends have been reported in studies where biochar amendments influenced antioxidant enzyme activities under heavy metal stress60. The increased availability of essential nutrients such as N, P, and K supports better physiological functioning, including enhanced chlorophyll biosynthesis. Improved chlorophyll content results in more efficient photosynthesis, leading to higher energy production and better overall plant growth. Additionally, biochar-induced improvement in nutrient absorption supports enzymatic activities and gene expression related to stress tolerance mechanisms61. Moreover, biochar positively influences microbial communities in the rhizosphere by providing a favorable habitat for beneficial microorganisms. These microbes play a crucial role in organic matter decomposition, nutrient cycling, and the degradation of toxic compounds, further alleviating heavy metal stress in plants62. Specifically, maize roots in biochar-amended soils exhibit increased colonization by plant growth-promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF), which enhance phosphorus solubilization and root nutrient63,64. Certain microbes associated with biochar application, such as PGPR and mycorrhizal fungi, enhance root development and increase the plant’s ability to tolerate environmental stressors65. Biochar amendments have been found to enhance the expression of ZmHSP70 and ZmHSP90, which encode heat shock proteins essential for plant protection under temperature stress. Notably, studies indicate that biochar application significantly upregulates ZmHSP70 and ZmHSP90, with their expression levels increasing by approximately 4.6-fold and 8.2-fold, respectively, under high-temperature conditions66.
Conclusion
In conclusion, using 200 g SMS biochar has the potential to improve maize growth under heavy metal stress. Applying 200 g SMS biochar exhibits the ability to regulate Cd and Cr antioxidants, thereby mitigating Cd and Cr adverse effects on maize. Further extensive field investigations are suggested to validate the effectiveness of 200 g SMS biochar as a primary solution for alleviating Cd and Cr stress in maize, ensuring its practical application in agriculture.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Ren, M. et al. Enhanced fertilizer utilization and heavy metals immobilization by ball-milling bentonite with NH4Cl: Experiments and DFT calculations. J. Hazard. Mater. 466, 133616 (2024).
Sana, S. et al. Differential responses of chili varieties grown under cadmium stress. BMC Plant Biol. 24, 7 (2024).
Ramzan, M. et al. Modulation of sunflower growth via regulation of antioxidants, oil content and gas exchange by arbuscular mycorrhizal fungi and quantum dot biochar under chromium stress. BMC Plant Biol. 23, 629 (2023).
FAO and UNEP. Global Assessment of Soil Pollution. https://www.unep.org/resources/report/global-assessment-soil-pollutionhttps://doi.org/10.4060/cb4894en (2021)
Awan, N., Fatima, A. & Farhan, M. Comparative analysis of chromium and cadmium in various parts of wheat and maize. Pol. J. Environ. Stud. 28, 1561–1566 (2019).
Anjum, S. A. et al. Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 22, 17022–17030 (2015).
Sharma, R. K., Agrawal, M. & Marshall, F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 66, 258–266 (2007).
Khan, Z. I. et al. A study on the transfer of chromium from meadows to grazing livestock: an assessment of health risk. Environ. Sci. Pollut. Res. 27, 26694–26701 (2020).
Ghazzal, M. et al. Chromium poisoning in buffaloes in the vicinity of contaminated pastureland, Punjab, Pakistan. Sustainability 14, 15095 (2022).
Nazar, R. et al. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant Sci. 03, 1476–1489 (2012).
Rashid, A. et al. Heavy metal contamination in agricultural soil: environmental pollutants affecting crop health. Agronomy 13, 1521 (2023).
Hussan, M. U. et al. Calcium oxide nanoparticles ameliorate cadmium toxicity in alfalfa seedlings by depriving its bioaccumulation, enhancing photosystem II functionality and antioxidant gene expression. Sci. Total Environ. 955, 176797 (2024).
Hussan, M. U. et al. Comparative role of calcium oxide nanoparticles and calcium bulk fertilizer to alleviate cadmium toxicity by modulating oxidative stress, photosynthetic performance and antioxidant-defense genes expression in alfalfa. Plant Physiol. Biochem. 215, 109002 (2024).
Nizar, M. et al. Exogenous application of salicylic acid and hydrogen peroxide ameliorate cadmium stress in milk thistle by enhancing morpho-physiological attributes grown at two different altitudes. Front. Plant Sci. 12, 809183 (2022).
Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A. & Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 17, 3782 (2020).
Irfan, M. et al. Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci. Rep. 11, 18416 (2021).
Danish, S. et al. Effect of foliar application of Fe and banana peel waste biochar on growth, chlorophyll content and accessory pigments synthesis in spinach under chromium (IV) toxicity. Open Agric. 4, 381–390 (2019).
Younis, U., Danish, S., Datta, R., Alahmadi, T. A. & Ansari, M. J. Sustainable remediation of chromium-contaminated soils: boosting radish growth with deashed biochar and strigolactone. BMC Plant Biol. 24, 115 (2024).
Tanumihardjo, S. A. et al. Maize agro-food systems to ensure food and nutrition security in reference to the sustainable development goals. Glob. Food Sec. 25, 100327 (2020).
Masood, S. A. et al. Effects of heavy metals (Cd & Cr) on physiological and morphological parameters in Zea mays L.: An overview. Life Sci. J. 15, 38–43 (2018).
Pál, M., Horváth, E., Janda, T., Páldi, E. & Szalai, G. Physiological changes and defense mechanisms induced by cadmium stress in maize. J. Plant Nutr. Soil Sci. 169, 239–246 (2006).
Anjum, S. A. et al. Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. CLEAN—Soil Air Water 44, 29–36 (2016).
Hussain, I. et al. Cadmium dose and exposure-time dependent alterations in growth and physiology of maize (Zea mays). Int. J. Agric. Biol. 14, 959–964 (2012).
Phan, C.-W. & Sabaratnam, V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 96, 863–873 (2012).
Wu, Q. et al. Adsorption characteristics of Pb(II) using biochar derived from spent mushroom substrate. Sci. Rep. 9, 15999 (2019).
Lou, Z. et al. Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar. Chemosphere 169, 23–31 (2017).
Chang, K.-L. et al. Spent mushroom substrate biochar as a potential amendment in pig manure and rice straw composting processes. Environ. Technol. 38, 1765–1769 (2017).
Zhang, X., Chang, K., Wang, X., Li, M. & Wang, Y. Effects of biochar from spent mushroom substrate on physic-chemical properties during pig manure composting. Front. Environ. Sci. 3, 67–74 (2014).
Zhang, G. et al. Efficient removal of Cu(II), Zn(II), and Cd(II) from aqueous solutions by a mineral-rich biochar derived from a spent mushroom (Agaricus bisporus) Substrate. Materials (Basel) 14, 35 (2020).
Jin, Y. et al. Characterization of biochars derived from various spent mushroom substrates and evaluation of their adsorption performance of Cu(II) ions from aqueous solution. Environ. Res. 196, 110323 (2021).
Zhao, Z. et al. Properties of biochar derived from spent mushroom substrates. BioResources 14, 5254–5277 (2019).
Gee, G. W. & Bauder, J. W. Particle-size analysis. In Methods of Soil Analysis. Part 1 Physical and Mineralogical Methods (ed. Klute, A.) (John Wiley Sons Inc., 2018).
Rhoades, J. D. Salinity: electrical conductivity and total dissolved solids. In Methods of Soil Analysis, Part 3, Chemical Methods (eds Sparks, D. L. et al.) (Soil Science Society of America, 1996).
Thomas, G. W. Soil pH and soil acidity. In Methods of Soil Analysis, Part 3: Chemical Methods (eds Sparks, D. L. et al.) (John Wiley & Sons, 2018).
Nelson, D. W. & Sommers, L. E. Total carbon organic carbon and organic matter. Methods Soil Anal. Part 3 Chem. Methods 5, 961–1010 (1996).
Hanlon, E. A. Elemental determination by atomic absorption spectrophotometery. In Handbook of Reference Methods for Plant Analysis (ed. Kalra, Y.) (CRC Press, 1998).
Russell, R. D. Soil Testing and Plant Analysis. In: LM Walsh, JD Beaton (eds) Soil Science Society of America Exp. Agric. 11, 75 (1975).
Uddling, J., Gelang-Alfredsson, J., Piikki, K. & Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 91, 37–46 (2007).
Pütter, J. Peroxidases. In Methods of Enzymatic Analysis (ed. Pütter, J.) (Elsevier, 1974).
Bergmeyer, H. U. Methods of Enzymatic Analysis (Elsevier, 2012).
OriginLab Corporation. OriginPro (OriginLab, 2021).
Sri, S. S., Palanivelu, K., Ramachandran, A. & Raghavan, V. Biochar from biomass waste as a renewable carbon material for climate change mitigation in reducing greenhouse gas emissions—A review. Biomass Convers. Biorefinery 11, 2247–2267 (2021).
Khan, Z. et al. The toxicity of heavy metals and plant signaling facilitated by biochar application: Implications for stress mitigation and crop production. Chemosphere 308, 136466 (2022).
Zhou, C. et al. Enterobacter asburiae reduces cadmium toxicity in maize plants by repressing iron uptake-associated pathways. J. Agric. Food Chem. 67, 10126–10136 (2019).
Razzaq, M., Akram, N. A., Ali, S. & Ashraf, M. Induction of chromium (Cr) stress tolerance in maize by foliar applied vanillic acid: growth, gas exchange characteristics and antioxidants. Pak. J. Bot. 55, 419–428 (2023).
Al-Wabel, M. I. et al. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 22, 503–511 (2015).
Yuan, C. et al. A meta-analysis of heavy metal bioavailability response to biochar aging: Importance of soil and biochar properties. Sci. Total Environ. 756, 144058 (2021).
Lu, Y. et al. Application of biochar-based photocatalysts for adsorption-(photo)degradation/reduction of environmental contaminants: mechanism, challenges and perspective. Biochar 4, 45 (2022).
Qiu, M. et al. Biochar for the removal of contaminants from soil and water: a review. Biochar 4, 19 (2022).
Uchimiya, M., Chang, S. C. & Klasson, K. T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 190, 432–441 (2011).
Mansoor, S. et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 271, 129458 (2021).
Irfan, M. et al. Effect of wheat straw derived biochar on the bioavailability of Pb, Cd and Cr using maize as test crop. J. Saudi Chem. Soc. 25, 101232 (2021).
Singh, H., Northup, B. K., Rice, C. W. & Prasad, P. V. V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: a meta-analysis. Biochar 4, 8 (2022).
Parvage, M. M., Ulén, B., Eriksson, J., Strock, J. & Kirchmann, H. Phosphorus availability in soils amended with wheat residue char. Biol. Fertil. Soils 49, 245–250 (2013).
Jatav, H. S. et al. Sustainable approach and safe use of biochar and its possible consequences. Sustainability https://doi.org/10.3390/su131810362 (2021).
Zheng, X. et al. The effects of biochar and its applications in the microbial remediation of contaminated soil: A review. J. Hazard. Mater. 438, 129557 (2022).
Kumar, P., Tewari, R. K. & Sharma, P. N. Cadmium enhances generation of hydrogen peroxide and amplifies activities of catalase, peroxidases and superoxide dismutase in maize. J. Agron. Crop Sci. 194, 72–80 (2008).
Anjum, S. A. et al. Phyto-toxicity of chromium in maize: Oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27, 262–273 (2017).
Faizan, M. et al. Unraveling the nano-biochar mediated regulation of heavy metal stress tolerance for sustaining plant health. Plant Stress 14, 100615 (2024).
Baptista, F. et al. Nutraceutical potential of lentinula edodes’ spent mushroom substrate: A comprehensive study on phenolic composition, antioxidant activity, and antibacterial effects. J. Fungi 9, 1200 (2023).
Feng, L., Xu, W., Tang, G., Gu, M. & Geng, Z. Biochar induced improvement in root system architecture enhances nutrient assimilation by cotton plant seedlings. BMC Plant Biol. 21, 1–14 (2021).
Nigam, N. et al. Biochar amendment reduced the risk associated with metal uptake and improved metabolite content in medicinal herbs. Physiol. Plant. 173, 321–339 (2021).
Alotaibi, M. O. et al. Examining the role of AMF-Biochar in the regulation of spinach growth attributes, nutrients concentrations, and antioxidant enzymes in mitigating drought stress. Plant Stress 10, 100205 (2023).
Anbuganesan, V. et al. Combined application of biochar and plant growth-promoting rhizobacteria improves heavy metal and drought stress tolerance in Zea mays. Plants 13, 1143 (2024).
Li, Z. et al. Silicon-rich biochar detoxify multiple heavy metals in wheat by regulating oxidative stress and subcellular distribution of heavy metal. Sustainability 14, 16417 (2022).
Kutlu, I. Biochar-mediated stimulation of antioxidant defense and heat shock protein expression in maize under extreme temperature stress. J. Plant Growth Regul. https://doi.org/10.1007/s00344-024-11600-8 (2024).
Acknowledgement
This research has been supported by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R241), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
This research has been supported by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R241), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
K.D.; A.U.K.; I.A.M.; contributed to the conceptualization and design of the study; K.D.; A.U.K.; I.A.M.; data collection; A.M.; S.S.A.; S.S.; M.A.M.; M.O.A.; A.M.G. analysis, M.A.M.; M.O.A.; A.M.G. interpretation of the data. All authors have reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable. Study protocol must comply with relevant institutional, national, and international guidelines and legislation. Our experiment follows the with relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dawar, K., Khan, A.U., Al-Mutairi, M. et al. Utilizing spent mushroom substrate biochar to improve Zea mays L. growth and biochemical resilience against cadmium and chromium toxicity. Sci Rep 15, 17511 (2025). https://doi.org/10.1038/s41598-025-01617-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01617-8