Abstract
Although the addition of olanzapine to conventional antiemetic therapy has been reported to be useful for systemic chemotherapy with highly emetogenic agents such as cisplatin, no studies have evaluated its efficacy in transcatheter arterial chemoembolization (TACE) for patients with hepatocellular carcinoma (HCC). We evaluated the antiemetic efficacy of olanzapine in patients with HCC undergoing cisplatin-based TACE. This prospective study included 68 patients with HCC scheduled for cisplatin-based TACE between 2021 and 2022. Patients were divided into two groups: an olanzapine group receiving olanzapine 5 mg plus the conventional triple antiemetic combination and a control group receiving only the conventional triple combination therapy. The incidence of digestive symptoms and adverse events (AEs) in both groups were compared. For TACE-induced nausea and vomiting, the olanzapine group had similar antiemetic complete response (aCR) and complete control (CC) rates at 12 h post-TACE as the control group but significantly higher aCR and CC rates during the delayed-phase after 24 h and better patient satisfaction scores. No significant differences were noted in the occurrence of severe AEs in the two groups. The use of olanzapine, in addition to conventional antiemetics, may be a new standard for patients undergoing cisplatin-based TACE.
Similar content being viewed by others
Introduction
Liver cancer is among the most prevalent malignancies, ranking as the sixth most common cancer globally1, with its incidence projected to reach 1 million cases annually by 2025 2. Hepatocellular carcinoma (HCC) is the predominant form of primary liver cancer, accounting for approximately 90% of cases2. Transcatheter arterial chemoembolization (TACE) is a widely used treatment for unresectable intermediate-stage HCC3,4,5, as it selectively delivers chemotherapy agents and embolic materials to the tumor while preserving surrounding liver function. TACE is also performed in selected early-stage cases where resection or radiofrequency ablation is not feasible. While effective, TACE frequently induces post-embolization syndrome, which includes gastrointestinal symptoms, fever, abdominal pain, and leukocytosis. Among these adverse effects, nausea and vomiting are particularly common due to both post-embolization syndrome6,7 and the highly emetogenic properties of cisplatin, a standard chemotherapeutic agent used in TACE. These symptoms not only diminish patients’ quality of life but also contribute to poor treatment adherence, potentially compromising therapeutic outcomes.
Cisplatin is classified as a highly emetogenic chemotherapy (HEC), with an incidence of vomiting exceeding 90% in the absence of prophylactic antiemetics8. The standard antiemetic regimen for HEC consists of a triple combination therapy that includes a neurokinin-1 (NK1) receptor antagonist, a 5-hydroxytryptamine (5-HT) receptor antagonist, and dexamethasone. However, despite this standard approach, nausea and vomiting—especially during the delayed-phase occurring more than 24 h after chemotherapy—remain inadequately controlled9,10. Olanzapine, an atypical antipsychotic with antiemetic properties, has been shown to suppress multiple neurotransmitter receptors involved in nausea and vomiting, including dopamine, serotonin, histamine, and adrenergic receptors11. Clinical trials have demonstrated that the addition of olanzapine to conventional antiemetic therapy significantly improves the prevention of chemotherapy-induced nausea and vomiting, leading to its inclusion in the American Society of Clinical Oncology (ASCO) guidelines for HEC, including cisplatin-based regimens12.
Despite its established role in systemic chemotherapy, the use of olanzapine for managing TACE-induced nausea and vomiting remains unexamined. Currently, there is limited evidence regarding optimal antiemetic strategies for TACE, and no studies have specifically evaluated the efficacy of olanzapine in this setting. Given the high prevalence of gastrointestinal symptoms following TACE and the potential benefits of olanzapine in preventing delayed nausea and vomiting, further investigation is warranted.
This prospective study aims to assess the antiemetic efficacy of olanzapine when added to the conventional triple antiemetic combination therapy in HCC patients undergoing cisplatin-based TACE. Additionally, we evaluate the safety profile of olanzapine in this population, considering that many HCC patients are older adults or have underlying cirrhosis, which may influence drug metabolism and tolerability.
Methods
Ethics approval
Written informed consent was obtained from all the patients. The present study was approved by the research ethics committee of the Kagawa University Faculty of Medicine (Ethics approval 2020 − 221), conducted in accordance with relevant guidelines and regulations, and registered in the UMIN Clinical Trial Registry (UMIN000043188: Registration date January 30, 2021. https://center6.umin.ac.jp/cgi-bin/icdr/ctr_view_reg.cgi?recptno=R000049292). Furthermore, this study was conducted in compliance with the Ethical Guidelines for Medical and Biological Research Involving Human Subjects, revised in 2022 by the Japanese Ministry of Health, and the Helsinki Declaration of 1975, as revised in 2008.
Study design and protocol
This non-randomized prospective intervention study enrolled patients aged 20 years or older with HCC scheduled to undergo TACE at Kagawa University Hospital between January 2021 and December 2022. HCC was diagnosed based on tumor markers—including des-γ-carboxyprothrombin and alpha-fetoprotein—and imaging modalities such as contrast-enhanced computed tomography (CE-CT) and magnetic resonance imaging. When typical imaging findings were not evident, a needle biopsy was performed to confirm the diagnosis. In addition, only patients with TNM stage I, II, or III (according to the LCSGJ 6th edition) were eligible; patients with advanced disease or extrahepatic metastases were excluded. Furthermore, patients with significant liver dysfunction (Child-Pugh class C), severe renal impairment (creatinine clearance < 30 mL/min), or notable heart dysfunction (New York Heart Association class III–IV) were excluded.
All enrolled patients received the conventional triple antiemetic regimen, comprising NK1 receptor antagonists, 5-HT receptor antagonists, and dexamethasone. To maintain patient safety and ensure consistency in treatment assignment, patients with contraindications to olanzapine were not assigned to the olanzapine group. Specifically, patients receiving medication for diabetes mellitus or those using antipsychotic or antiepileptic drugs were excluded from receiving olanzapine. Patients allocated to the olanzapine group received olanzapine 5 mg in addition to the conventional triple antiemetic regimen, while patients allocated to the control group received only conventional triple antiemetic regimen. Furthermore, some patients in the olanzapine group underwent a second TACE with cisplatin but without olanzapine within 6 months. For these patients, outcomes of the first TACE (with olanzapine) were compared with those of second procedure (without olanzapine).
Treatment protocol
As conventional antiemetic therapy, an NK1 receptor antagonist (fosaprepitant 150 mg) was administered intravenously to patients one hour before TACE, followed by a 5-HT receptor antagonist (palonosetron 0.75 mg). Dexamethasone was administered intravenously at doses of 8.25 mg immediately before TACE and 3.3 mg for 3 consecutive days, beginning from the day after TACE. In addition to this triple antiemetic combination, patients in the olanzapine group began taking 5 mg of olanzapine orally at bedtime on the day before TACE, continuing until day 5.
TACE involved inserting a catheter into the hepatic artery during treatment in conjunction with CT angiography to identify the exact location of the tumor and its feeding artery, thereby avoiding unnecessary arterial injection of cisplatin or embolic substances. A microcatheter was inserted as selectively as possible in the hepatic artery feeding the tumor. The required dose of cisplatin emulsified with lipiodol was injected with an upper limit of 100 mg, followed by injection of a 1-mm square gelatin sponge as the embolizing material until the tumor-feeding vessel was completely embolized.
Evaluation of outcomes and parameters for treatment
Patients recorded items related to nausea and vomiting and completed the questionnaire regarding symptoms at 12 h after TACE and every 24 h thereafter. The frequency and time of first vomiting, degree of nausea on a Likert scale (0 = no nausea, 1 = mild nausea, 2 = moderate nausea, 3 = severe nausea), frequency and time of first rescue medication administration, degree of appetite loss (0 = not at all, 1 = a little bit, 2 = quite a bit, 3 = very much), and severity of sleepiness (0 = not at all, 1 = a little bit, 2 = quite a bit, 3 = very much) were assessed. Attending physicians assessed the patients every 24 h for the predicted AEs after olanzapine administration, including constipation, hiccups, somnolence, insomnia, dry mouth, and dizziness, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 5.0. At the end of the observation period, the patients responded to a questionnaire regarding their satisfaction with the antiemetic therapy on a 7-point scale (0 = very satisfied, 1 = satisfied, 2 = somewhat satisfied, 3 = rather satisfied, 4 = rather dissatisfied, 5 = dissatisfied, 6 = very dissatisfied).
The main outcomes of antiemetic therapy were antiemetic complete response (aCR), complete control (CC), and total control (TC), which were evaluated during the early phase (0–24 h post-TACE) and the delayed phase (24–120 h post-TACE). aCR was defined as the absence of vomiting or retching and no use of rescue antiemetic medication. CC was defined as a condition in which the patient achieved aCR and experienced no more than mild nausea, corresponding to a nausea scale of 0 or 1 on a 4-point scale. TC, the most stringent endpoint, was defined as a condition in which the patient achieved aCR and experienced no nausea at all (nausea scale = 0). These definitions form a hierarchical structure, with TC representing the highest level of antiemetic effectiveness, followed by CC and then aCR. Patient-reported outcomes were monitored daily by the attending physician.
The general condition of the patient was evaluated using Eastern Cooperative Oncology Group performance status (PS)13. The liver condition was evaluated based on imaging findings, especially related to liver cirrhosis or chronic hepatitis, and liver function was assessed by the Child-Pugh classification. The clinical stage of HCC was classified according to the tumor-lymph node-metastasis classification based on the criteria of the Liver Cancer Study Group of Japan14. CE-CT findings performed 1–3 months after TACE were evaluated with the modified Response Evaluation Criteria in Solid Tumors (mRECIST)15, and the therapeutic effects were classified as tumor complete response (tCR), partial response (PR), stable disease (SD), or progressive disease (PD).
Statistical analysis
The continuous variables are expressed as medians, and these differences between the olanzapine and control groups were examined using the Mann–Whitney U test. Differences in the frequencies of various parameters in the two groups were examined using the chi-squared or Fisher’s exact test. For analysis of the antiemetic effects, the aCR rates for up to 120 h after TACE were calculated using the Kaplan–Meier method, and the difference between the two groups was determined using the log-rank test. Logistic regression analysis was performed on factors associated with aCR, and the significance of each factor was compared using the Wald test. Among the explanatory variables, the cutoff values for tumor size, number of tumors, and cisplatin dose were calculated using receiver operating characteristic analysis, and the cutoff values for other factors were defined as the median. For statistical analyses and figure creation, JMP Pro 17.0.0 (JMP Statistical Discovery, Cary, NC, USA) and GraphPad Prism 8.4.3 (GraphPad Software, San Diego, CA, USA) were used, and statistical significance was set at P < 0.05.
Results
Patient characteristics
The study enrolled 108 patients undergoing TACE, and a flowchart of the patient selection is presented in Fig. 1. Due to the exclusion criteria for regimens other than cisplatin, 29 patients who underwent TACE with miriplatin and 11 patients who underwent TACE with epirubicin were excluded. A total of 68 HCC patients who had undergone TACE with cisplatin were included in the analysis, with 31 patients in the olanzapine group and 37 patients in the control group. The reasons for not administering olanzapine were diabetes mellitus (n = 26), use of antipsychotic drugs (n = 2), use of antiepileptic drugs (n = 1), patient refusal (n = 3), and physician judgment (n = 5).
Flowchart of patient selection. Initially, 108 patients with HCC undergoing TACE were eligible. Twenty-nine and eleven patients undergoing TACE with miriplatin and epirubicin, respectively, were excluded. Finally, 68 patients undergoing TACE with cisplatin were included in this study. If the records on olanzapine administration were accurate, the olanzapine and control groups included 31 and 37 patients, respectively. The patients in the olanzapine and control groups were compared. HCC; hepatocellular carcinoma, TACE; transcatheter arterial chemoembolization.
The baseline patient characteristics are presented in Table 1. The median ages were 75 (57–86) and 71 (42–82) years for the olanzapine and control groups, respectively, and the difference was not statistically significant. There were also no significant differences in the body mass index (BMI) and PS between the two groups. As diabetes mellitus is a contraindication for olanzapine administration and the main reason for not administering it, there were no patients with diabetes mellitus in the olanzapine group. However, the control group included 26 patients (70.3%) with diabetes mellitus. Otherwise, there were no significant differences between the two groups in the baseline characteristics such as etiology, liver function, kidney function, tumor markers, clinical stage, cisplatin dose, and embolization of the hepatic segment.
Therapeutic effects of TACE
The therapeutic effects based on the mRECIST evaluation of CE-CT findings at 1–3 months after TACE are presented in Table 2. The overall response rates (tCR + PR) were 74.2% and 70.3%, while the disease control rates (tCR + PR + SD) were 93.5% and 86.5% for the olanzapine and control groups, respectively. The therapeutic effects of TACE in the two groups were not significantly different.
Therapeutic effects of olanzapine
The effects of the antiemetic agents after TACE with cisplatin are presented in Table 3. The two groups showed no significant difference in the aCR rates within 12 h after TACE. However, the aCR rate was significantly higher for the olanzapine group than for the control group during the delayed-phase from 24 h to 120 h. The CC and total TC rates were also significantly better for the olanzapine group than for the control group during the delayed-phase. According to the responses about their satisfaction with the antiemetics at the end of the observation period, 93.5% of the patients in the olanzapine group and 32.4% in the control group were very satisfied or satisfied; the difference was marked. Of the 31 patients in the olanzapine group, seven patients underwent a second cisplatin-based TACE and were treated with the conventional triple antiemetic regimen without olanzapine. A comparison of the antiemetic efficacy with and without olanzapine after the first and second TACE procedures in the same patients is shown in Table 4. All patients achieved aCR, CC, and TC within 120 h after the first TACE with the conventional triple antiemetic regimen plus olanzapine, whereas several patients did not demonstrate good antiemetic effects after the second TACE with conventional triple antiemetic regimen without olanzapine. In addition, the satisfaction scores for the first TACE were better than for the second TACE, indicating that patients were more satisfied with the antiemetic effect.
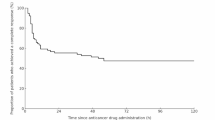
The Kaplan–Meier method was used to examine the aCR rate for up to 120 h after TACE, and the results are shown in Fig. 2. The aCR rates for up to 24 h were similar for the olanzapine and control groups. However, the difference gradually became apparent after 24 h and remained significant until 120 h. Furthermore, the results of the logistic regression analysis for the factors related to aCR are shown in Supplementary Table 1. In the univariate analysis, only assignment to the olanzapine group was significantly associated with aCR, with an odds ratio of 4.48 (95% CI 1.13–17.72; p < 0.05). As no additional factors reached statistical significance in univariate analysis, we did not perform a multivariate analysis. These results suggested that the addition of olanzapine to conventional triple antiemetic regimen provided better efficacy in the delayed-phase, and that the use of olanzapine was an important predictor of achieving aCR.
aCR rates for up to 120 h of TACE derived using the Kaplan–Meier method. The aCR rates for up to 24 h were 90.3% and 89.2% for the olanzapine and control groups, respectively. After 24 h, there was a gradual difference between the two groups, with the aCR rate of 90.3% for olanzapine and 70.3% for the control group at 120 h after TACE. The Mantel-Haenszel HR for the olanzapine group compared to the control group was 0.26 (95% CI 0.09–0.77, p < 0.05), indicating a significantly lower hazard of nausea and vomiting in the olanzapine group. aCR; antiemetic complete response, TACE; transcatheter arterial chemoembolization, HR: hazard ratio, CI; confidence interval.
Occurrence of AEs
The AEs caused by TACE are shown in Supplementary Table 2. The common AEs were post-embolization syndrome (fever, abdominal pain, malaise, etc.) and renal dysfunction, while Grade 3 or higher adverse events included elevated liver enzymes, biliary infection, and hepatic failure.
Furthermore, the AEs associated with the antiemetic therapy are presented in Table 5. Constipation, hiccups, somnolence, insomnia, dry mouth, and dizziness, among others, were observed in the olanzapine group. However, they were all Grade 1 and not severe. Several of these AEs were also common in the control group.
Discussion
This study demonstrated the efficacy of olanzapine in preventing nausea and vomiting after cisplatin-based TACE. While cisplatin has a strong antitumor effect, its highly emetogenic properties pose a significant challenge in clinical practice. Nausea and vomiting remain among the most frequent adverse events, impairing patients’ quality of life and treatment adherence. Although the standard antiemetic regimen, consisting of an NK1 receptor antagonist, a 5-HT receptor antagonist, and dexamethasone, is effective in controlling acute-phase nausea and vomiting, its efficacy in preventing delayed-phase symptoms remains insufficient9,10. The addition of olanzapine, a multi-receptor antagonist targeting dopamine, serotonin, histamine, and adrenergic receptors, has been proven to be effective in suppressing delayed emesis in patients undergoing systemic chemotherapy16,17, but its role in TACE had not been previously studied. This study is the first to provide clinical evidence supporting the use of olanzapine in this setting.
Olanzapine is a monoaminergic antagonist that binds with high affinity to serotonin 5-HT2A and 5-HT2C receptors; dopamine D1, D2, D3, and D4 receptors; and alpha1-adrenergic receptors11. The mechanism underlying the reduction of chemotherapy-induced emesis by olanzapine remains unclear, but its effects on serotonin and dopaminergic receptors have been reported11,18. Furthermore, olanzapine targets and antagonizes several key receptors with a single agent, and it may improve patient compliance in managing chemotherapy-induced gastrointestinal symptoms relative to the use of multiple antiemetic drugs. A large RCT demonstrating the antiemetic effects of olanzapine showed that olanzapine 10 mg in combination with the conventional triple antiemetic drugs significantly improved nausea and vomiting relative to the conventional triple antiemetic combination regimen alone in patients treated with anthracycline plus cyclophosphamide and cisplatin-based systemic chemotherapy16. Another RCT showed that olanzapine 5 mg in combination with the conventional triple antiemetic combination therapy was beneficial in patients receiving cisplatin-based systemic chemotherapy17. In the updated ASCO antiemetic guidelines, olanzapine 5–10 mg plus triple therapy is recommended as the standard antiemetic therapy for HEC, and the addition of olanzapine should be reduced to 5 mg for cases of oversedation or in older patients because of the risk of oversedation12. However, uncertainties remain regarding the optimal administration antiemetic therapy during cisplatin-based TACE for HCC. Nevertheless, the 5-mg dose of olanzapine used in this study was appropriate in terms of safety, considering that several patients with HCC have cirrhosis or are older adults.
There is limited evidence supporting the use of antiemetic therapy during TACE in patients with HCC. A meta-analysis of four RCTs on the antiemetic efficacy of dexamethasone in patients with HCC undergoing TACE showed a significant reduction of the cumulative incidence of nausea and vomiting, as well as AEs, including fever and abdominal pain19. In addition, several studies have shown that combination therapy of dexamethasone and a 5-HT receptor antagonist has superior antiemetic efficacy during TACE. An RCT randomized patients to receive a regimen of dexamethasone and granisetron before TACE followed by dexamethasone on days 2 and 3 or placebo and granisetron before TACE followed by placebo on days 2 and 3. The cumulative incidence of nausea and vomiting was lower for the dexamethasone regimen than for the control regimen20. A retrospective study compared an antiemetic group administered dexamethasone and palonosetron before TACE, followed by dexamethasone on days 2 and 3, with a control group that did not receive antiemetic premedication. The results showed that premedication with dexamethasone significantly reduced the occurrence and severity of nausea and vomiting during the acute-phase but had an insufficient effect during the delayed-phase21. Another retrospective observational study compared dexamethasone and palonosetron administered 30 mininites before TACE with no pretreatment antiemetic and showed that dexamethasone and palonosetron combination therapy resulted in a significantly lower occurrence of nausea and vomiting but did not increase the risk of AEs, including infection22. Thus, similar to systemic anticancer therapy, antiemetic therapy with an NK1 receptor antagonist, a 5-HT receptor antagonist, and dexamethasone is effective in preventing nausea and vomiting after TACE in patients with HCC and has become the standard antiemetic therapy.
Our findings showed that adding olanzapine 5 mg to the conventional triple antiemetic regimen significantly improved the control of delayed-phase nausea and vomiting in patients undergoing cisplatin-based TACE. Compared to the control group receiving standard therapy alone, the olanzapine group exhibited higher complete response (aCR) rates for nausea and vomiting in the delayed-phase (24–120 h post-TACE) and reported greater satisfaction with symptom management. Furthermore, among patients who underwent two TACE sessions—one with olanzapine and one without—those who received olanzapine during their first session experienced better symptom relief, reinforcing its potential efficacy in this population. Importantly, no significant differences in TACE therapeutic outcomes were observed between the olanzapine and control groups, suggesting that symptom suppression did not interfere with treatment effectiveness. Additionally, the incidence of severe adverse events was comparable between groups, supporting the safety of olanzapine even in HCC patients, many of whom are elderly or have underlying cirrhosis.
Despite the promising results, this study has some limitations. First, it was a single-center, prospective interventional study with a limited sample size. Additionally, the open-label design of this study may have introduced bias, particularly in the assessment of subjective outcomes. Future studies employing a randomized, double-blind design with a larger sample size are needed to confirm these findings. Second, given olanzapine’s known effects on glucose metabolism, patients receiving medications for diabetes mellitus were excluded from the olanzapine group, while diabetic patients were included in the control group. This approach may have introduced selection bias and baseline differences between the groups. Future research should explore the feasibility of safely including diabetic patients in the olanzapine group, with careful monitoring of metabolic side effects, or apply statistical adjustments to mitigate these imbalances. Third, the study focused exclusively on cisplatin-based TACE; thus, the efficacy of olanzapine in patients undergoing TACE with other chemotherapeutic agents—such as epirubicin or miriplatin, which have a lower emetogenic potential—remains to be determined. Investigating whether olanzapine provides similar benefits in these alternative regimens could further refine antiemetic strategies for TACE.
Despite these limitations, our findings provide novel evidence supporting the use of olanzapine to control TACE-induced nausea and vomiting. By improving the management of delayed-phase symptoms, olanzapine has the potential to enhance patient comfort and treatment compliance, ultimately contributing to better overall management of HCC. Future research should aim to expand the patient population, investigate olanzapine’s role in various TACE regimens, and optimize antiemetic strategies to further improve treatment outcomes.
Conclusion
Olanzapine can be safely used to suppress symptoms such as nausea and vomiting during the delayed-phase after TACE in patients with HCC. The use of olanzapine plus the conventional triple combination antiemetic therapy, including NK1 receptor antagonists, 5-HT receptor antagonists, and dexamethasone, may be a new standard antiemetic therapy for patients with HCC undergoing cisplatin-based TACE.
Data availability
The datasets generated during the study are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 7, 6. https://doi.org/10.1038/s41572-020-00240-3 (2021).
Marrero, J. A. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 68, 723–750. https://doi.org/10.1002/hep.29913 (2018).
Omata, M. et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 11, 317–370. https://doi.org/10.1007/s12072-017-9799-9 (2017).
European Association for the Study of the Liver. Electronic address, E. E. E. & European association for the study of the, L. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 69, 182–236. https://doi.org/10.1016/j.jhep.2018.03.019 (2018).
Rmilah, A. A. et al. Association of cirrhosis and other patient and procedural characteristics with postembolization syndrome after bland hepatic artery embolization for hepatic malignancy. AJR Am. J. Roentgenol. 218, 1030–1039. https://doi.org/10.2214/AJR.21.26806 (2022).
Lee, H. N. & Hyun, D. Complications related to transarterial treatment of hepatocellular carcinoma: A comprehensive review. Korean J. Radiol. https://doi.org/10.3348/kjr.2022.0395 (2023).
Hesketh, P. J. et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy.J. Clin. Oncol. 15, 103–109. https://doi.org/10.1200/JCO.1997.15.1.103 (1997).
Aapro, M. et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann. Oncol. 25, 1328–1333. https://doi.org/10.1093/annonc/mdu101 (2014).
Barton, D. L. et al. Phase III double-blind, placebo-controlled study of Gabapentin for the prevention of delayed chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy, NCCTG N08C3 (Alliance). Cancer 120, 3575–3583. https://doi.org/10.1002/cncr.28892 (2014).
Bymaster, F. P. et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14, 87–96. https://doi.org/10.1016/0893-133X(94)00129-N (1996).
Hesketh, P. J. et al. Antiemetics: ASCO guideline update. J. Clin. Oncol. 38, 2782–2797. https://doi.org/10.1200/JCO.20.01296 (2020).
Oken, M. M. et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am. J. Clin. Oncol. 5, 649–655 (1982).
Kudo, M., Kitano, M., Sakurai, T. & Nishida, N. General rules for the clinical and pathological study of primary liver cancer, nationwide Follow-Up survey and clinical practice guidelines: The outstanding achievements of the liver cancer study group of Japan. Dig. Dis. 33, 765–770. https://doi.org/10.1159/000439101 (2015).
Lencioni, R., Llovet, J. M. & Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 30, 52–60. https://doi.org/10.1055/s-0030-1247132 (2010).
Navari, R. M. et al. Olanzapine for the prevention of Chemotherapy-Induced nausea and vomiting. N Engl. J. Med. 375, 134–142. https://doi.org/10.1056/NEJMoa1515725 (2016).
Hashimoto, H. et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 21, 242–249. https://doi.org/10.1016/S1470-2045(19)30678-3 (2020).
Bymaster, F. P. et al. Potent antagonism of 5-HT(3) and 5-HT(6) receptors by olanzapine. Eur. J. Pharmacol. 430, 341–349. https://doi.org/10.1016/s0014-2999(01)01399-1 (2001).
Chang, L. et al. Dexamethasone prevents TACE-induced adverse events: A meta-analysis. Med. (Baltim). 99, e23191. https://doi.org/10.1097/MD.0000000000023191 (2020).
Ogasawara, S. et al. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology 67, 575–585. https://doi.org/10.1002/hep.29403 (2018).
Sakamoto, T. et al. Effect of palonosetron and dexamethasone administration on the prevention of gastrointestinal symptoms in hepatic arterial chemoembolization with epirubicin. Support Care Cancer. 28, 3251–3257. https://doi.org/10.1007/s00520-019-05178-1 (2020).
Lu, H., Zheng, C., Liang, B. & Xia, X. Efficacy and safety analysis of dexamethasone + palonosetron in prevention of post-embolization syndrome after D-TACE: A retrospective study. Med. (Baltim). 102, e35433. https://doi.org/10.1097/MD.0000000000035433 (2023).
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the study. K.O. and A.M. provided study materials or patients. All authors collected data. K.O. and H.K. analyzed and interpreted the data. K.O . and A.M. wrote the manuscript. All authors gave final approval of the manuscript. K.O. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oura, K., Morishita, A., Manabe, T. et al. Efficacy of olanzapine as an antiemetic drug for transcatheter arterial chemoembolization for hepatocellular carcinoma. Sci Rep 15, 18095 (2025). https://doi.org/10.1038/s41598-025-01632-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01632-9