Abstract
Multicomponent training is suggested as an efficient way to address the side effects of long-term treatment in breast cancer survivors and reduce the age-related relapse risk in these patients. This study aimed to evaluate the impact of a multicomponent training intervention on breast cancer survivors’ physical fitness and body composition. This experimental and controlled study included 19 breast cancer survivors with 64.0 ± 8.6 years, to evaluate long-term effects (32 weeks) of multicomponent training on body composition [body weight (kg), body mass index, body fat (%), lean mass (kg), body water (%), basal metabolism (Kcal) and visceral fat (index)] and physical fitness [Upper limb strength (repetitions), lower limb strength (repetitions), upper limb flexibility (cm), lower limb flexibility (cm), dynamic balance (seconds), and aerobic fitness (repetitions)]. Bayesian statistical tests were employed to analyze the reduced dataset size, considering a Bayes factor ≥ 10 as the cutoff for significant differences. Hierarchical clustering identified participant improvements using Manhattan distance, and clusters were ranked by responsiveness. After 32 weeks, the experimental group showed significant reductions in body weight (Δ = − 1.67 kg; BF = 15.15; Cohen’s d = 0.19) and body fat percentage (Δ = − 3.99%; BF = 34.87; Cohen’s d = 0.73), while no relevant changes were observed in the control group. Improvements were also observed in upper limb strength (Δ = + 14.14 reps; BF = 1022.02; Cohen’s d = 3.45), strength in the surgically affected arm (Δ = + 13.57 reps; BF = 121.39; Cohen’s d = 2.37), lower limb strength (Δ = + 7.86 reps; BF = 206.55; Cohen’s d = 2.24), and aerobic fitness (Δ = + 97.57 reps; BF = 157.28; Cohen’s d = 0.10). Flexibility and dynamic balance also improved, with moderate to large effect sizes. The multicomponent physical exercise program effectively improved all physical fitness variables but was limited in body composition, exposing improvements only in body weight and % body fat. The intervention did not cause any side effects or injury to the participants
Similar content being viewed by others
Introduction
Breast cancer is the most prevalent form of cancer worldwide, characterized by the development of malignant cells in the mammary glands, which can invade surrounding tissues and metastasize to other areas of the body1. This disease is highly heterogeneous, with a complex etiology influenced by genetic predispositions, behavioral (lifestyle) and environmental factors2. In 2020, an estimated 2.3 million new cases of breast cancer were diagnosed globally3. In 2022, the disease led to approximately 670,000 deaths1, solidifying its position as the leading cause of cancer-related mortality among women4. The survival rates for breast cancer vary significantly across the globe. The 5-year survival rate in developed countries is approximately 80%, while in developing nations, it drops to below 40%5. Limited resources and infrastructure in developing countries often impede efforts to improve outcomes, particularly in early detection, diagnosis, and treatment6.
Over the past few decades, the breast cancer rate has increased by 0.5% annually, driven by disease stage at diagnosis, aging populations, and overall population growth7,8,9,10. Projections suggest that by 2040, there will be more than 3 million new cases of breast cancer annually11,12. The current breast cancer treatment and management includes targeted therapies, hormonal treatments, radiotherapy, chemotherapy, and surgery4. However, survivors often face heightened risks of comorbid conditions such as sarcopenia, osteoporosis, and cardiovascular disease, which can adversely affect their quality of life, cardiorespiratory fitness, muscle strength, and bone health13. The treatments can also lead to fatigue, insomnia, nausea, vomiting, neuropathy, cardiotoxicity, and reduced muscle strength, resulting in both physiological and psychological challenges4,14,15,16. Additionally, significant loss of upper limb mobility, strength, and muscle mass in the arms, compromised or not by neoplasms, can further compromises life satisfaction and quality of life for these patients17,18,19,20,21. For these reasons, behavioral strategies based on active living and wellbeing have been promoted in cancer patients and survivors22,23.
Physical exercise complementary therapies have emerged as a powerful non-pharmacological strategy to mitigate treatment side effects and enhance the quality of life, cardiorespiratory fitness, and muscle strength among breast cancer survivors24, this is also supported by physical activity guidelines25, and exercise can improve cancer-related health indicators, support a healthy energy balance, and reduce the risk of cardiovascular diseases, which are a leading cause of death among survivors26. Various studies have explored different exercise modalities, including aquatic exercises27, Pilates interventions28, cognitive-behavioral therapy29, and aerobic and resistance-based exercises30. While substantial evidence supports the benefits of exercise interventions in improving physical abilities and body composition among cancer survivors, research focusing specifically on the long-term effects of multicomponent training programs—particularly those exceeding 24 weeks—remains limited. Survivors often experience persistent psychological and physiological issues, as well as complications from surgery31,32. Thus, understanding the long-term impact of exercise programs is crucial from a clinical perspective for breast cancer survivors33. Long-term analyses are hard to perform because of the difficulties related to drop-in and -out34,35. Strong evidence supporting physical activity and exercise-specific programs like multicomponent training shows potential for improvements but currently lack sufficient robust evidence36. The multicomponent training is an exercise program that integrate of strength, cardiorespiratory endurance, flexibility, and balance into a single session, typically lasting about 60 min37. As medical advancements extend life expectancy, understanding the rehabilitation needs of older adults becomes increasingly important38,39. Establishing new theoretical, methodological, and analytical frameworks is essential for guiding cancer research39. It is important to highlight that exercise programs that last more than 24 weeks are considered long-term30,40,41,42,43,44, and the research misses studies that have longer durations. In aged people there are studies with multicomponent training of 24, 36 and 60 weeks33,45. For this reason, the authors seek to explore the effects of 32 weeks of multicomponent training in the breast cancer survivors.
Recent evidence suggests that physical exercise interventions, including aerobic, resistance, and multicomponent programs, are effective strategies to improve body composition and physical fitness in individuals with overweight and obesity46,47. Studies have demonstrated that structured exercise can lead to significant reductions in body fat, improvements in lean mass, and enhancements in strength, balance, and cardiorespiratory fitness in these populations46. Moreover, multicomponent exercise programs—which combine resistance, aerobic, balance, and flexibility training—have gained popularity for their holistic benefits, offering effective, practical, and sustainable approaches to managing excess body weight and improving functional health outcomes47. Given the high prevalence of overweight among breast cancer survivors, as indicated by the mean BMI in this sample, it becomes critical to explore interventions that can address both oncological recovery and weight management concurrently.
The individual’s response to training programs can vary due to factors such as prior physical condition, intensity and frequency of physical activity, and individual biological characteristics48. Therefore, it is essential to recognize that although everyone can show improvements, the magnitude of these changes tends to differ for each person49,50.
That said, concerning the difficulties of long-term analysis in this type of population and the lack of research regarding multicomponent exercise-based therapy. Thus, this study aims to evaluate the long-term effects of multicomponent training on body composition and functional fitness among breast cancer survivors. We propose the following hypotheses: (1) multicomponent training will improve body composition in breast cancer survivors; and (2) it will enhance functional fitness.
Materials and methods
Study design
This is an experimental and controlled study, enrolling the long-term effects of a multicomponent training program in the breast cancer survivors’ body composition and physical fitness. The training sessions had a duration of 60 min, with evaluations conducted over the course of 48 h. Participants were also encouraged to continue their daily activities throughout the study. Following the exercise guidelines for cancer survivors by Campbell et al.51 which refers to the range of intensity, duration, and frequency of the exercise sessions considering subjects’ responsiveness and adherence levels, we adjusted the exercise sessions to three sessions per week, each lasting approximately 60 min. The total duration of the intervention was 8 months (32 weeks of intervention) for the experimental group (EG), while the control group (CG) did not perform any regular intervention for 5 months (20 weeks) because some participants needed to restart cancer treatment having a shorter follow-up than EG. Furthermore, the intensity and volume of these sessions can vary, often incorporating multiple sets and repetitions to optimize physical fitness outcomes52. Multicomponent training programs for breast cancer survivors typically range from 20 to 90 min per session53. Overall, the flexibility in session duration allows for tailored programs that can accommodate the varying fitness levels and preferences of breast cancer survivors, ultimately enhancing adherence and effectiveness of the training interventions54. Figure 1 illustrates the timeline of the evaluation and exercise phases during the program.
Sample and sampling
A total of 19 breast cancer survivors were included in this study, 7 in the EG, and 12 in the CG. The participants were conveniently included because the EG were breast cancer survivors who voluntarily enrolled in a physical exercise program from the Polytechnic Institute of Bragança. The CG comprised participants without time to participate in the physical exercise intervention. Nevertheless, the statistical power was calculated a priori in R, computing language, and following Cohe’s guidelines where the minimal power of 0.80, a moderate effect size for paired samples (Cohen’s d = 0.5), and alpha of 95% to accept the alternative hypothesis55 Unfortunately, the sample recruited of this study did not reach minimal statistical power. This way, alternative Bayesian statistical procedures were applied to possibly reliable comparisons56,57, (see Statistical analysis section).
The following inclusion criteria were considered for participation in the multicomponent training sessions: (i) previous diagnosis of breast cancer; (ii) having undergone definitive surgery, regardless of the type; (iii) having undergone post-surgical treatment, such as chemotherapy, radiotherapy, immunotherapy, endocrine therapy, or a combination of these; (iv) having finished treatment before starting the multicomponent training program; and v) have not participated in any type of intervention one year before the start of the study The established exclusion criteria were: (i) the presence of serious clinical conditions related to other chronic diseases that would hinder participation in the multicomponent training sessions; (ii) the use of medications that could compromise participation in the sessions; and (iii) the presence of severe cardiovascular, muscular, metabolic, or articular complications that would impair the ability to participate in the program; and (iv) have participated in any regular intervention one year before the study. At the end of the intervention, seven women completed the program, with one dropout due to the need for a new surgical procedure.
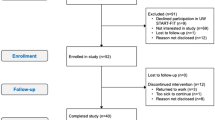
The control group was composed of breast cancer survivors who did not participate in any intervention during the experimental period. Unlike the group that underwent the multicomponent training, these participants were not subjected to any exercise or specific protocol during the study. Unfortunately, due to the reduced personnel of researchers and exercise coaches and the lack of time available by the subjects of CG, there was no possibility of engaging these patients in an alternative physical exercise intervention in parallel to the EG subjects. However, they were regularly (every 4 weeks) instructed to maintain their daily life routines, especially physical activity levels. The absence of intervention allowed the results to be compared directly with the intervention group, highlighting the possible effects of physical training on survivors’ recovery and quality of life. The study design is illustrated in the flowchart (Fig. 2).
Trial registration
This trial was retrospectively registered at OSF (https://osf.io/8apsm/) on April 29, 2025.
Data collection
For the data collection, a team of interns was systematically trained to ensure the standardized application of research instruments and obtain reliable information on body composition, functional fitness, and quality of life in breast cancer survivors. The training process included theoretical instruction on measurement protocols, practical demonstrations, and supervised application to ensure consistency and minimize inter-rater variability. The assessments were conducted in two stages: the baseline and at the end of the intervention after eight months. To standardize the procedures, all measurements were performed within a 48-h window for all participants, always at the same time of the day, between 7:00 and 11:00 PM, in the same laboratory setting, ensuring controlled environmental conditions. The same evaluators performed the assessments at both pre- and post-intervention time points to reduce measurement inconsistencies.
Body composition was assessed through the calculation of Body Mass Index (BMI) and bioelectrical impedance analysis. BMI was determined using the standard formula, weight in kilograms divided by height squared in meters (kg/m2)58. Height measurements were obtained using a stadiometer (SECA 213, Hamburg, Germany) with a precision of 0.1 cm, while body weight was assessed using a digital scale (Tanita DC-430, Illinois, USA) with a precision of 0.1 kg. The classification of BMI followed the standards established by the World Health Organization (WHO), categorizing values between 18.50 and 24.99 kg/m2 as normal weight, between 25 and 29.99 kg/m2 as overweight, between 30 and 34.99 kg/m2 as Class I obesity, and between 35 and 39.99 kg/m2 as Class II obesity59. In addition to BMI, body composition was assessed through bioimpedance analysis, providing data on lean muscle mass in kilograms, body fat percentage, bone mass in kilograms, visceral fat rating, basal metabolic rate in kcal/day, and total body water percentage. The bioimpedance measurements were taken using a Tanita DC-430 digital bioimpedance scale, ensuring a precision of 0.1 g. To maintain consistency in the conditions of measurement, all participants were instructed to avoid caffeine, alcohol, and strenuous exercise for at least 24 h prior to the assessment. Measurements were conducted in a fasted state, with participants wearing lightweight clothing and standing barefoot to ensure optimal electrode contact.
Functional fitness assessments followed the validated Senior Fitness Test (SFT) battery developed by Rikli and Jones60, which evaluates key physical components related to functional mobility. The assessment protocol included six standardized tests measuring different aspects of functional fitness. Upper limb strength was evaluated using the arm curl test, in which participants performed as many full biceps curls as possible within 30 s while holding a dumbbell weighing 2.27 kg for women or 3.63 kg for men. The number of correctly executed repetitions was recorded. Lower limb strength was assessed using the 30-s chair stand test, in which participants sat on a standardized chair with a height of 43 cm and performed as many complete sit-to-stand repetitions as possible within 30 s, without using their arms for support. The total number of completed stands was recorded as the final score. Upper limb flexibility was evaluated through the back scratch test, where participants attempted to reach one hand over the shoulder and the other behind their back, trying to touch their fingertips. The distance between the fingertips was measured in centimeters, with positive values indicating overlap and negative values indicating a gap. Lower limb flexibility was measured using the sit-and-reach test in a seated position, where participants extended one leg forward and reached toward their toes while keeping the other knee flexed at 90 degrees. The distance between the fingertips and the toes was recorded in centimeters, with positive values indicating that the participant reached beyond their toes and negative values indicating an inability to reach the toes.
Dynamic balance and mobility were assessed using the Timed Up and Go (TUG) test, which required participants to stand up from a chair, walk 2.45 m, turn around, and return to a seated position as quickly as possible. The total time taken to complete the task was recorded in seconds. Aerobic endurance was evaluated using the 2-min step test, in which participants were instructed to march in place for two minutes while ensuring that their knees reached a predefined height corresponding to the midpoint between their patella and iliac crest. The total number of times the right knee reached the target height was recorded as the final score. To minimize the effects of fatigue, rest intervals of one to two minutes were provided between each test. Standardized verbal instructions and demonstrations were given before each test to ensure consistency in execution. All measurements were taken under controlled conditions, using the same evaluators at both pre- and post-intervention time points to ensure reliability.
Multicomponent training
The program was held at the Gymnasium of the School of Education at the Polytechnic Institute of Bragança, with three sessions per week, on Mondays, Thursdays, and Fridays, lasting one hour per training session. The multicomponent training sessions were divided into three stages: the first one, a general warm-up, aimed at preparing the participants’ cardiorespiratory, joint, and muscular systems; the second stage, the main core of the session, where exercises focused on resistance, aerobic activities, flexibility, and static and dynamic balance were performed; and finally, the third stage, focused on relaxation, aiming to provide rest and recovery for the body after training. The weight progression was individually adjusted based on baseline strength levels and gradual overload principles. Figure 3 below shows the full multicomponent training protocol for breast cancer survivors. To ensure adequate stimulus for neuromuscular adaptation, the load for strength exercises was individually adjusted based on the gradual overload principle. Initially, the training load allowed participants to perform 10–12 repetitions with moderate difficulty, corresponding approximately to a perceived exertion of 12–14 on the Borg RPE scale. Every two weeks, the load was systematically increased by approximately 5–10%, regardless of individual performance, following a biweekly progression model to maintain continuous training stimulus. Adjustments were made considering participant feedback and exercise tolerance, aiming to preserve the target repetition range without inducing excessive fatigue or risk of injury.
Statistical analysis
The normality of the data was checked with the Shapiro–Wilk test. The statistical power was calculated a priori in R, statistical computing language (34), to define the minimal sample size for this study. Due the reduced statistical power, calculated a priori, the paired and two-sample Bayesian T-tests were employed56,57. The Wilcoxon signed-rank test for paired samples were employed for the non-parametric datasets57. Bayesian paired and unpaired statistical tests are well-described to work robustly in small datasets where assumptions such as statistical power are broken61. The main metric of significance62 was used with the following cut-offs of (≤ 3 = Anecdotal evidence, between 3 and 10 = Moderate evidence, > 10 = Strong evidence) a 95% confidence interval57,62.
A hierarchical clustering method was employed considering the absolute pre-post differences to characterize the participants with higher or smaller improvements based on a between/within-subjects comparison (personalized clustering)63 by the Manhattan distance62 for small datasets where the individual differences can bias the clustering results64. The Silhouette score was calculated to check the best final number of clusters, considering the cut-off < 0.5 to consider a good separation for the determined number of clusters65. All the statistical clustering analysis and respective graphic creation were performed in R, a statistical programming language66.
Results
Baseline comparison results
Table 1 shows the results of body composition and physical fitness at the study’s baseline originated from the comparison between the experimental and control groups. The Bayesian tests showed that, after comparing prior to post probabilities, EG had higher height, higher visceral fat concentration at the baseline, reduced upper limb strength the arm surgically underwent, and smaller aerobic fitness than the control group.
Experimental results
Table 2 shows the results of the moments (pre-post) comparison between CG and EG groups. The Bayesian paired tests revealed significant post-intervention improvements in the EG after 32 weeks of physical exercise training. Notably, there were reductions in body weight (pre = 73.16 ± 2.22 kg, post = 71.49 ± 12.1 kg; BF = 15.15; Cohen’s d = 0.19) and body fat percentage (pre = 36.9 ± 5.03%, post = 32.91 ± 5.86%; BF = 34.87; Cohen’s d = 0.73). Improvements were also observed across all physical fitness variables, including upper limb strength (pre = 13.57 ± 4.65, post = 27.71 ± 3.45 repetitions; BF = 1022.02; Cohen’s d = 3.45), upper limb strength on the surgically intervened arm (pre = 7 ± 4.16, post = 20.57 ± 6.92 repetitions; BF = 121.39; Cohen’s d = 2.37), lower limb strength (pre = 17.57 ± 3.69, post = 25.43 ± 3.31 repetitions; BF = 206.55; Cohen’s d = 2.24), and aerobic fitness (pre = 37 ± 14.65, post = 134.57 ± 28.18 repetitions; BF = 157.28; Cohen’s d = 0.10). Additional positive changes were seen in upper limb flexibility (Cohen’s d = 1.85), lower limb flexibility (Cohen’s d = 1.09), and dynamic balance (Cohen’s d = 1.56), although with varying levels of statistical evidence. In contrast, BMI, lean mass, body water percentage, basal metabolism, and visceral fat index showed small or negligible effect sizes (Cohen’s d < 0.33) and were not statistically significant. No significant changes were observed in the control group after 20 weeks, with all effect sizes being small (Cohen’s d < 0.20), supporting the specificity of the training effects observed in the EG.
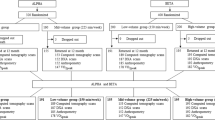
Figure 4 shows the hierarchical clustering results from the variables with significant pre-post 32 weeks of multicomponent training in the EG. In general, regarding the variables with statistically significant differences, all participants have individual improvements. Specifically within the hierarchical cluster results, respective to the body weight reductions (Silhouette score = 0.77) (Fig. 4-A), there were two clusters, cluster 1 [higher responders (28%)]: participant 1 [3.3 kg], and 5 [2.7 kg], and cluster 2 [lower responders (71%)]: participants 2 [0.8 kg], 3 [1.4 kg], 4 [1.2 kg], 6 [0.9 kg], and 7 [1.4 kg]. For body fat percentage reductions (Silhouette score = 0.75) (Fig. 4-B), the HC identified also two clusters, were cluster 1 [higher responders (57%)]: participants 1 [4.9%], 2 [4.8%], 3 [6.9%) and 4 [4.7%], and cluster 2 [lower responders (43%)]: participants 5 [2%], 6 [2%], and 7 [2.6%]. For upper limb strength improvements (Silhouette score = 0.68) (Fig. 4-C) there were three clusters, cluster 1 [high responders (86%)]: participants 1, 3, and 4 [17 repetitions], cluster 2 [mild responders (43%)]: participants 2 [13 repetitions], 5 [14 repetitions], and 7 [12 repetitions], and cluster 3 [lower responder (14%)]: characterized by only the participant 6 [9 repetitions]. For the upper limb strength improvements of the arm underwent to surgical procedure (Silhouette score = 0.56) (Fig. 4-D), there were also two clusters, cluster 1 [higher responders (71%)]: participants 1 and 7 [13 repetitions], 2 and 4 [16 repetitions], and 3 [21 repetitions], and cluster 2 [lower responders (29%)]: participants 5 [9 repetitions], and 6 [9 repetitions]. For the lower limb strength (Silhouette score = 0.80) (Fig. 4-E) there were two principal clusters, were cluster 1 [higher responders (43%)]: participants 1 [11 repetitions], 6 and 7 [10 repetitions], and cluster 2 [lower responders (57%)]: participants 2 [7 repetitions] 3, 4 [6 repetitions], and 5 [5 repetitions]. Regarding aerobic fitness (Silhouette score = 0.62) (Fig. 4-F), there were two clusters, were cluster 1 [higher responders (29%)]: participants 3 [154 repetitions], and 7 [124 repetitions], and cluster 2 [lower responders (71%)]: participants 1 [70 repetitions], 2 [95 repetitions], 4 [72 repetitions], 5 [70 repetitions], and 6 [98 repetitions]. These results prove that all the participants improved similarly, and there were fewer responder subjects, indicating that the intervention was positive, but not in the same meaningful for all patients.
Hierarchical Clustering results of variables with significant pre-post differences after 32 weeks of multicomponent training in the experimental group. Part. (participant), BW (body weight), BF % (body fat percentage), ULS (upper limb strength), ULS – Interv. arm (arm which underwent surgical procedure), LLS (lower limb strength), AF (aerobic fitness).
After outputting the clustering results, we developed a mind map (Fig. 5) to offer a structured approach to guide clinical decisions after identifying each patient’s individual response to multicomponent training. Based on the analysis of performance variables and each patient’s specific responses, the mind map suggests personalized directions for the continuation of treatment, either through intensifying certain components of the program or adjusting the training modality. This process ensures a more effective intervention adapted to the needs and progress of each individual.
Discussions
Overview of the main findings
Regarding the objective of this study, based on the statistical results, weight and fat percentage reduction was observed in the experimental group after the 36-week intervention. Additionally, improvements were noted across all physical fitness variables.
The intervention was made based on the multicomponent training exercise program. Research indicates that such training can lead to enhanced neuromuscular adaptations, including improved motor unit recruitment and coordination, which contribute to increased strength independent of muscle mass changes67. Additionally, multicomponent training has been shown to positively influence cardiovascular fitness, which is vital for recovery and long-term health in cancer survivors. Enhanced cardiovascular fitness can lead to improved oxygen delivery and utilization during physical activities, thereby reducing fatigue and enhancing exercise tolerance68. Furthermore, the psychological benefits of participating in structured exercise programs, such as improved mood and reduced anxiety, can motivate survivors to engage more consistently in physical activity, further amplifying the positive effects on physical fitness69. Finally, the incorporation of balance and flexibility exercises within multicomponent training can help mitigate the risk of falls and improve functional mobility, which is particularly important for older breast cancer survivors who may experience declines in these areas due to treatment side effects68.
Body composition
As previously mentioned, both body weight and body fat percentage were reduced. Regarding body weight, cluster analysis reveals that multicomponent training resulted in predominantly lower responses among the patients in the study. In contrast, when analyzing the reduction in fat percentage, the clusters indicate that the intervention had varying effects on individuals: 4 participants were classified as higher responders, while 3 were lower responders, suggesting that the intervention had a moderate impact on the participants’ weight status. Reductions in body weight are crucial for preventing cancer6, during70, or after treatment6. Excess body fat tissue releases inflammatory cytokines that contribute to low-grade chronic inflammation, commonly known as “inflammaging.” This process involves inflammation that disrupts cell cycle regulation, damages cells, and impairs proteostasis, all of which promote the expression of carcinogenic genes and increase cancer risk71. A recent one-year intervention with multicomponent physical exercise highlighted reductions in body fat and increased the mass of the patients45. This prior evidence aligns with our findings and strengthens the positive aspect of multicomponent exercise for the body composition of breast cancer patients45.
In contrast, BMI, lean mass, body water percentage, basal metabolism, and visceral fat did not present significant changes after the follow-up. BMI is an overall indicator of nutritional status and is conventionally used in healthcare settings due to its simplicity and low-cost application58. Nevertheless, a deeper analysis considering another body compartment (muscle, fat, or even bone tissue) can enrich the quality of inferences about the body composition changes72.
In this study, the non-changes in the participants’ BMI were accompanied by manutention of lean mass, body water percentage, basal metabolism, visceral fat index, and significant reductions in body fat percentage. This finding is a positive factor for breast cancer patients in different phases of the treatment since body fat reduction contributes to attenuating the body inflammation profile, a risk factor for cancer progression and relapse73. On the other hand, the non-change in lean mass is positive in preventing sarcopenia/cachexia in these patients74; nevertheless, the most idealistic scenario is increasing the participants’ muscle mass75, which unfortunately was not reached on our study.
Although weight and body fat percentage significantly decreased following the intervention, no significant changes were observed in BMI, lean mass, body water percentage, basal metabolism, or visceral fat index. A possible explanation is that while multicomponent training effectively reduced adiposity, the overall muscle mass was preserved rather than significantly increased. As lean mass remained stable, there was no substantial impact on body water content or basal metabolic rate, which are closely linked to muscle mass levels76. Furthermore, visceral fat reduction may require longer or higher-intensity interventions, combined with specific dietary control, to produce measurable changes. The preservation of lean mass without muscle wasting is still a positive outcome for cancer survivors, given that sarcopenia is a common complication during and after cancer treatment77.
The findings of the present study align with prior evidence demonstrating the beneficial effects of multicomponent exercise programs on body composition and physical fitness among overweight populations. A previous study reported significant improvements in body composition and strength parameters in obese women following a 40-weeks multicomponent training intervention78. Similarly, recent investigations have confirmed that multicomponent programs combining aerobic, resistance, flexibility, and balance exercises are highly effective for improving cardiorespiratory fitness, muscular strength, and reducing body fat percentage in overweight individuals, regardless of cancer79,80. However, specific studies focusing on breast cancer survivors with overweight remain limited. The present results add to this emerging body of evidence by demonstrating that even in a clinical and aging population, multicomponent training can effectively enhance functional fitness and induce moderate changes in body composition.
Physical fitness
Regarding the change in strength in the upper limbs of the study participants, there was a similar increase in strength in the upper limb that underwent surgical intervention and in the one that was not submitted. The cluster analysis for this variable was subdivided into three groups for increased strength in the upper limbs, in which 3 patients were considered high responders, 3 patients were mild responders and only 1 patient was low responder. This allows us to understand that multicomponent training tends to be efficient in improving the strength of patients’ upper limbs after the intervention since 6 of the 7 participants analyzed were responsive, whether high or light. In contrast, only one patient was slightly responsive, showing increased upper limb strength. When analyzing the improvement in strength in the upper limb submitted to surgical intervention, cluster 1 was observed with five patients as the greatest responders, and cluster 2, with two patients as the lowest responders. This makes it clear that multicomponent training effectively improves the strength of the upper limbs of patients undergoing the surgical process, given the characteristics of patients’ responsiveness to this issue. This shows the relevance of this type of activity for improving the quality of life of patients recovering from breast cancer, as it contributes to the return to routine instrumental activities of daily living which require good control of the upper limbs such as managing finances or medications, food preparation, housekeeping, and laundry, thus contributing to the recovery of their independence81.
Research indicates that resistance training can lead to improvements in muscle function and strength, even in the presence of muscle atrophy54,82. These adaptations can occur through various mechanisms, including increased neuromuscular efficiency and changes in muscle fiber recruitment patterns, which can enhance strength without necessitating an increase in muscle mass83. For instance, a study highlighted that breast cancer survivors who engaged in resistance training exhibited notable improvements in upper limbs strength, despite not experiencing significant changes in lean body mass54. This framework is based on neuromuscular adaptations, where there is a notable increase of muscle even with an absence of increases in muscle mass, but being still essential to presenve the patients’ functionality while muscle mass is preserved74.
Two clusters were observed when analyzing the variability in the participants’ lower limb strength. The first cluster was linked to higher responders, with three patients as group members. The second cluster covered the lowest responders (four patients) submitted to the present study. This distribution allows us to understand that multicomponent training presented a divergent impact on the individuals’ lower limb strength, showing that the training did not evoke the same level of changes in the subjects. However, even at different levels, the intervention proposed by the present study proved to improve the strength of the lower limbs, which is a positive result, considering that this contributes to helping patients in their daily lives, facilitating their ambulation and independence of performance during daily tasks such as climbing stairs, walking at the street and moving carrying objects81. This result is consistent with previous studies reporting strength gains with regular practice of muscle resistance exercises, both for the lower and upper limbs, as evidenced by the meta-analysis of Hasenoehrl et al.84, where isolated aerobic and resistance training were effective in increasing muscle strength of lower extremities of breast cancer patients. The study of Dong et al.33, also finds improvements in the leg strength of breast cancer patients after a one-year remote multicomponent intervention. Moreover, in support of the cluster outputs, the study of Klassen et al.85, warned about the possible variability of muscle strength responses in breast cancer patients undergoing different treatments and at different stages of the pathology.
Regarding changes in aerobic fitness, the clustering results revealed the presence of two clusters. The first cluster is composed of higher responders, consisting of two individuals, while the second cluster comprises lower responders, consisting of five individuals. These results indicate that the intervention primarily led to low-grade improvements among participants. Despite this predominance of lower responders, the intervention still resulted in increases in aerobic fitness for all participants, which is a positive outcome of the training. Our results align with the prior meta-analysis of Ficarra et al.3, where aerobic, resistance or combined exercise interventions were able to produce an overall effect of 65% increases in the patients’ VO2max. Strengthening the direction of this evidence, the study of Shinde et al.45 showed large effect size improvements in the aerobic fitness of breast cancer patients after a one-year multicomponent exercise.
In addition, it is important to highlight the cluster analysis performed in this study as an efficient and personalized way to understand the biological individuality for adapt to the training stimulus. It was crucial to adjust the intervention when necessary. For example, if a patient is not responding well to the initial treatment, cluster analysis can help identify what is happening and suggest changes, either in the approach or type of exercise. This process is similar to medical practice, where doctors adjust a patient’s medication doses according to the subject’s prognostic, always seeking a more effective and personalized treatment86.
In the present study, we had some relevant limitations that should be considered in future studies on this field, given more realistic and concrete results. Among these points, we can mention our small experimental sample, making it challenging to have a broader view of the population reality, which could be considerably different and complex. Furthermore, another limiting factor was the shorter analysis period of the control group (20 weeks) compared to the group undergoing the proposed intervention (32 weeks). Analysis for both groups for at least the same period is necessary to acquire more equivalent comparisons. Additionally, the absence of interim evaluations during the intervention period should be considered a limitation. Although the intervention lasted several months (32 weeks), body composition and physical fitness were assessed only at baseline and after the intervention. It is possible that significant changes in the measured variables could have occurred earlier during the program. Without periodic assessments, it was not possible to track the timing, rate, or trajectory of individual responses throughout the intervention, which could have provided more detailed insights into the effectiveness and dynamics of the multicomponent training over time. The nutritional status, including frequency of tobacco and/or alcohol use, was also not collected, and it can be a significant factor influencing variability in the patients’ responsiveness levels87, time and better understand.
For subsequent studies with larger samples from different ages, ethnicities, and income levels, followed by the collection of data about lifestyle (fitness and nutritional habits), biochemical and molecular tests are relevant to understand even more in-depth, the results of multicomponent training in the target groups of breast cancer patients in different stages of the pathology. Moreover, the outputs provided by the clustering method also highlighted that the multicomponent training did not provide equalized stimulus across the different physical fitness components. Improving technical aspects of this intervention in future studies considering outputs from clustering or other related methods will be essential to evaluate the effect of the maximized stimulus on breast cancer patients’ physical fitness and body composition adaptations.
Future research should explore longer intervention durations, greater sample sizes, and more diverse exercise protocols, including dose–response relationships, to optimize exercise prescription for breast cancer survivors. Investigating potential mediators such as hormonal status, body composition trajectories, and quality of life measures would also contribute to a more comprehensive understanding. From a practical perspective, multicomponent exercise programs should be considered as a first-line non-pharmacological strategy to promote functional recovery, improve physical fitness, and manage body weight in breast cancer survivors, particularly those with overweight. Implementing these programs in community and clinical settings could significantly improve survivorship outcomes and public health.
However, it is important to highlight some strengths of the present study: (i) This is a novelty approach with 32 weeks of duration using multicomponent training exercise-based therapy; (ii) this is a controlled trial with a high level of evidence; (iii) the study incorporated a cluster of reactions to the physical exercise treatment, helping identify individual variability in response to training, allowing for personalized exercise prescriptions; (iv) it is possible to define strategies for treatment in agreement with the responders. The study suggests that each participant reacts differently to the exercise-based therapy and should be reevaluated periodically to adjust the treatment. The combination of these strengths makes this study unique when compared to the existing literature about other types of exercise3, and specifically multicomponent training for breast cancer patients54,82,83,88,89,90,91.
Conclusions
In conclusion, this study demonstrated that a 32-week multicomponent training program significantly improved physical fitness parameters, including strength, flexibility, dynamic balance, and aerobic capacity, among breast cancer survivors. Moderate improvements in body composition were also observed, particularly in reducing body weight and body fat percentage. These findings reinforce the vital role of long-term multicomponent exercise interventions as effective, safe, and sustainable strategies to support physical rehabilitation and body composition management in aging and overweight breast cancer survivors. Finally, the intervention did not cause any side effects or injury for the participants.
Data availability
Data is provided within supplementary information files.
References
DeSantis, C. E. et al. Breast cancer statistics, 2019. CA Cancer J. Clin. 69, 438–451. https://doi.org/10.3322/caac.21583 (2019).
Sun, Y.-S. et al. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 13, 1387–1397. https://doi.org/10.7150/ijbs.21635 (2017).
Ficarra, S. et al. Impact of exercise interventions on physical fitness in breast cancer patients and survivors: A systematic review. Breast Cancer 29, 402–418. https://doi.org/10.1007/s12282-022-01347-z (2022).
Akram, M. et al. Awareness and current knowledge of breast cancer. Biol. Res. 50, 33. https://doi.org/10.1186/s40659-017-0140-9 (2017).
Coleman, M. P. et al. Cancer survival in five continents: A worldwide population-based study (CONCORD). Lancet Oncol. 9, 730–756. https://doi.org/10.1016/S1470-2045(08)70179-7 (2008).
Anderson, B. O. et al. Guideline implementation for breast healthcare in low-income and middle-income countries: Overview of the Breast Health Global Initiative Global Summit 2007. Cancer 113, 2221–2243. https://doi.org/10.1002/cncr.23844 (2008).
Collaboration GB of DC. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 3, 524. https://doi.org/10.1001/jamaoncol.2016.5688 (2017).
Iacoviello, L. et al. Epidemiology of breast cancer, a paradigm of the ‘common soil’ hypothesis. Semin. Cancer Biol. 72, 4–10. https://doi.org/10.1016/j.semcancer.2020.02.010 (2021).
Kelsey, J. L. & Gammon, M. D. The epidemiology of breast cancer. CA Cancer J. Clin. 41, 146–165. https://doi.org/10.3322/canjclin.41.3.146 (1991).
Giaquinto, A. N. et al. Breast cancer statistics, 2022. CA Cancer J. Clin. 72, 524–541. https://doi.org/10.3322/caac.21754 (2022).
Xu, Y. et al. Global trends and forecasts of breast cancer incidence and deaths. Sci. Data. 10, 334. https://doi.org/10.1038/s41597-023-02253-5 (2023).
Arnold, M. et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 66, 15–23. https://doi.org/10.1016/j.breast.2022.08.010 (2022).
Ording, A. G. et al. Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis—A Danish nationwide matched cohort study. PLoS ONE 8, e76013. https://doi.org/10.1371/journal.pone.0076013 (2013).
Shapiro, C. L. & Recht, A. Side effects of adjuvant treatment of breast cancer. N. Engl. J. Med. 344, 1997–2008. https://doi.org/10.1056/NEJM200106283442607 (2001).
Neil-Sztramko, S. E. et al. Aerobic capacity and upper limb strength are reduced in women diagnosed with breast cancer: A systematic review. J. Physiother. 60, 189–200. https://doi.org/10.1016/j.jphys.2014.09.005 (2014).
Parker, P. A. et al. Short-term and long-term psychosocial adjustment and quality of life in women undergoing different surgical procedures for breast cancer. Ann. Surg. Oncol. 14, 3078–3089. https://doi.org/10.1245/s10434-007-9413-9 (2007).
Hayes, S. C. et al. Upper-body morbidity after breast cancer: Incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer 118, 2237–2249. https://doi.org/10.1002/cncr.27467 (2012).
Hidding, J. T. et al. Treatment related impairments in arm and shoulder in patients with breast cancer: A systematic review. PLoS ONE 9, e96748. https://doi.org/10.1371/journal.pone.0096748 (2014).
Mols, F. et al. Quality of life among long-term breast cancer survivors: A systematic review. Eur. J. Cancer 41, 2613–2619. https://doi.org/10.1016/j.ejca.2005.05.017 (2005).
Kilbreath, S. L. et al. Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: A randomized controlled trial. Breast Cancer Res. Treat. 133, 667–676. https://doi.org/10.1007/s10549-012-1964-1 (2012).
Todd, J. et al. A randomised controlled trial of two programmes of shoulder exercise following axillary node dissection for invasive breast cancer. Physiotherapy 94, 265–273. https://doi.org/10.1016/j.physio.2008.09.005 (2008).
Grant, A. R. et al. What do cancer survivors and their health care providers want from a healthy living program? Results from the first round of a co-design project. Support Care Cancer 29, 4847–4858. https://doi.org/10.1007/s00520-021-06019-w (2021).
Santin, O. et al. A comparative analysis of the health and well-being of cancer survivors to the general population. Support Care Cancer 20, 2545–2552. https://doi.org/10.1007/s00520-011-1372-9 (2012).
Speck, R. M. et al. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 4, 87–100. https://doi.org/10.1007/s11764-009-0110-5 (2010).
Runowicz, C. D. et al. American Cancer Society/American Society of clinical oncology breast cancer survivorship care guideline. CA Cancer J. Clin. 66, 43–73. https://doi.org/10.3322/caac.21319 (2016).
Zagar, T. M., Cardinale, D. M. & Marks, L. B. Breast cancer therapy-associated cardiovascular disease. Nat. Rev. Clin. Oncol. 13, 172–184. https://doi.org/10.1038/nrclinonc.2015.171 (2016).
Lindquist, H. et al. Water exercise compared to land exercise or standard care in female cancer survivors with secondary lymphedema. Lymphology 48, 64–79 (2015).
Şener, H. Ö. et al. Effects of clinical pilates exercises on patients developing lymphedema after breast cancer treatment: A randomized clinical trial. J. Breast Health 13, 16–22. https://doi.org/10.5152/tjbh.2016.3136 (2017).
Sun, H. et al. The efficacy of cognitive behavioral therapy to treat depression and anxiety and improve quality of life among early-stage breast cancer patients. Integr. Cancer Ther. 18, 1534735419829573. https://doi.org/10.1177/1534735419829573 (2019).
Dieli-Conwright, C. M. et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. 20, 124. https://doi.org/10.1186/s13058-018-1051-6 (2018).
Leach, H. J. et al. Evaluation of a community-based exercise program for breast cancer patients undergoing treatment. Cancer Nurs. 38, 417–425. https://doi.org/10.1097/NCC.0000000000000217 (2015).
Qiu, H. et al. Effects of cognitive behavioral therapy for depression on improving insomnia and quality of life in Chinese women with breast cancer: Results of a randomized, controlled, multicenter trial. Neuropsychiatr. Dis. Treat. 14, 2665–2673. https://doi.org/10.2147/NDT.S171297 (2018).
Dong, X. et al. A longitudinal study of a multicomponent exercise intervention with remote guidance among breast cancer patients. Int. J. Environ. Res. Public Health 17, 3425. https://doi.org/10.3390/ijerph17103425 (2020).
Strandberg, E. et al. Who makes it all the way? Participants vs. decliners, and completers vs. drop-outs, in a 6-month exercise trial during cancer treatment. Results from the Phys-Can RCT. Support Care Cancer 30, 1739–1748. https://doi.org/10.1007/s00520-021-06576-0 (2022).
Shang, J. et al. Who will drop out and who will drop in: Exercise adherence in a randomized clinical trial among patients receiving active cancer treatment. Cancer Nurs. 35, 312. https://doi.org/10.1097/NCC.0b013e318236a3b3 (2012).
Wolin, K. Y. et al. Implementing the exercise guidelines for cancer survivors. J. Support Oncol. 10, 171–177. https://doi.org/10.1016/j.suponc.2012.02.001 (2012).
Sadjapong, U. et al. Multicomponent exercise program reduces frailty and inflammatory biomarkers and improves physical performance in community-dwelling older adults: A randomized controlled trial. Int. J. Environ. Res. Public Health 17, E3760. https://doi.org/10.3390/ijerph17113760 (2020).
DeNysschen, C. et al. Healthy lifestyle behaviors of breast cancer survivors. Clin. Nurs. Res. 24, 504–525. https://doi.org/10.1177/1054773814553298 (2015).
Amjad, A., Kordel, P. & Fernandes, G. A review on innovation in healthcare sector (telehealth) through artificial intelligence. Sustainability 15, 6655. https://doi.org/10.3390/su15086655 (2023).
Dieli-Conwright, C. et al. Adipose tissue inflammation in breast cancer survivors: Effects of a 16-week combined aerobic and resistance exercise training intervention. Breast Cancer Res. Treat. 168, 147–157. https://doi.org/10.1007/s10549-017-4576-y (2018).
Heinrich, K. M. et al. High-intensity functional training improves functional movement and body composition among cancer survivors: A pilot study. Eur. J. Cancer Care 24, 812–817. https://doi.org/10.1111/ecc.12338 (2015).
Lin, H.-P. et al. Exercise effects on fatigue in breast cancer survivors after treatments: A systematic review and meta-analysis. Int. J. Nurs. Pract. 28, e12989. https://doi.org/10.1111/ijn.12989 (2022).
Paulo, T. R. S. et al. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: A randomized controlled trial. Health Qual. Life Outcomes 17, 17. https://doi.org/10.1186/s12955-019-1090-4 (2019).
Robinson, K. M. et al. Survivors speak: A qualitative analysis of motivational factors influencing breast cancer survivors’ participation in a sprint distance triathlon. J. Clin. Nurs. 25, 247–256. https://doi.org/10.1111/jocn.13067 (2016).
Shinde, S. B. et al. Effect of multi-component exercise program on body composition and physical, emotional and social well being in breast cancer survivors. Asian Pac. J. Cancer Prev. 25, 4397–4406. https://doi.org/10.31557/APJCP.2024.25.12.4397 (2024).
Newsome, A. et al. 2025 ACSM worldwide fitness trends: Future directions of the health and fitness industry. ACSM’s Health Fitness J. 28, 11–25. https://doi.org/10.1249/FIT.0000000000001017 (2024).
Al-Mhanna, S. B. et al. Combined aerobic and resistance training improves body composition, alters cardiometabolic risk, and ameliorates cancer-related indicators in breast cancer patients and survivors with overweight/obesity: A systematic review and meta-analysis of randomized controlled trials. J. Sports Sci. Med. 23, 366–395. https://doi.org/10.52082/jssm.2024.366 (2024).
Whipple, M. O. et al. Variability in individual response to aerobic exercise interventions among older adults. J. Aging Phys. Act. 26, 655. https://doi.org/10.1123/japa.2017-0054 (2018).
Karavirta, L. et al. Individual responses to combined endurance and strength training in older adults. Med. Sci. Sports Exer. 43, 484. https://doi.org/10.1249/MSS.0b013e3181f1bf0d (2011).
Newton, R. U. et al. Mixed-methods resistance training increases power and strength of young and older men. Med. Sci. Sports Exerc. 34, 1367 (2002).
Campbell, K. L. et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51, 2375–2390. https://doi.org/10.1249/MSS.0000000000002116 (2019).
Lopez, P. et al. Resistance training in breast cancer patients undergoing primary treatment: A systematic review and meta-regression of exercise dosage. Breast Cancer 28, 16–24. https://doi.org/10.1007/s12282-020-01147-3 (2021).
Tan, T.-W., Tan, H.-L. & Chung, Y.-C. Effectiveness of resistance training in preventing sarcopenia among breast cancer patients undergoing chemotherapy: A systematic review and meta-analysis. Worldviews Evid.-Based Nurs. 21, 687–694. https://doi.org/10.1111/wvn.12756 (2024).
Soriano-Maldonado, A. et al. Effects of a 12-week supervised resistance training program, combined with home-based physical activity, on physical fitness and quality of life in female breast cancer survivors: The EFICAN randomized controlled trial. J. Cancer Surviv. 17, 1371–1385. https://doi.org/10.1007/s11764-022-01192-1 (2023).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Routledge, 2013).
Eriksson, O. et al. Uncertainty quantification, propagation and characterization by Bayesian analysis combined with global sensitivity analysis applied to dynamical intracellular pathway models. Bioinformatics 35, 284–292. https://doi.org/10.1093/bioinformatics/bty607 (2019).
Ly, A., Verhagen, J. & Wagenmakers, E.-J. Harold Jeffreys’s default Bayes factor hypothesis tests: Explanation, extension, and application in psychology. J. Math. Psychol. 72, 19–32. https://doi.org/10.1016/j.jmp.2015.06.004 (2016).
Nuttall, F. Q. Body mass index. Nutr. Today 50, 117–128. https://doi.org/10.1097/NT.0000000000000092 (2015).
A healthy lifestyle - WHO recommendations. https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations Accessed 20 Sept 2024.
Rikli, R. E. & Jones, C. J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. Act. 7, 129–161. https://doi.org/10.1123/japa.7.2.129 (1999).
Jeffreys, H. Theory of Probability (Clarendon Press, 1939).
Koopman, B. O. Harold Jeffreys. Theory of probability. Oxford University Press, Oxford1939, vii+ 380 pp. J. Symb. Log. 8, 34–35 (1943).
Zhang, Z. et al. Hierarchical cluster analysis in clinical research with heterogeneous study population: Highlighting its visualization with R. Ann. Transl. Med. 5, 75–75. https://doi.org/10.21037/atm.2017.02.05 (2017).
Xu, R. & Wunsch, D. Survey of clustering algorithms. IEEE Trans. Neural Netw. 16, 645–678. https://doi.org/10.1109/TNN.2005.845141 (2005).
Maechler, M., Rousseeuw, P. & Struyf, A. et al. cluster: ‘Finding Groups in Data’: Cluster Analysis Extended Rousseeuw et al. (2023).
R project. R: The R Project for Statistical Computing. 2022. https://www.r-project.org/ Accessed 07 January 2023.
François, V. et al. Multi-component exercise program and improving physical performances in older inpatients: Results from a pilot interventional study. BJSTR 14, 1–5. https://doi.org/10.26717/BJSTR.2019.14.002558 (2019).
Bai, X. et al. Aerobic exercise combination intervention to improve physical performance among the elderly: A systematic review. Front. Physiol. 12, 798068. https://doi.org/10.3389/fphys.2021.798068 (2022).
Binder, J. C. et al. Multi-domain training enhances attentional control. Psychol Aging. 31, 390–408. https://doi.org/10.1037/pag0000081 (2016).
Ligibel, J. A. et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J. Clin. Oncol. 40, 2491–2507. https://doi.org/10.1200/JCO.22.00687 (2022).
Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 12, 31–46. https://doi.org/10.1158/2159-8290.CD-21-1059 (2022).
Lee, S. Y. & Gallagher, D. Assessment methods in human body composition. Curr. Opin. Clin. Nutr. Metab. Care 11, 566–572. https://doi.org/10.1097/MCO.0b013e32830b5f23 (2008).
McAndrew, N. P. et al. Effects of systemic inflammation on relapse in early breast cancer. npj Breast Cancer 7, 1–10. https://doi.org/10.1038/s41523-020-00212-6 (2021).
Pin, F., Couch, M. E. & Bonetto, A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr. Opin. Support Palliat Care 12, 420–426. https://doi.org/10.1097/SPC.0000000000000382 (2018).
Adams, S. et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. Breast Cancer Res. Treat. 158, 497–507. https://doi.org/10.1007/s10549-016-3900-2 (2016).
Weiss, E. P. et al. Effects of weight loss on lean mass, strength, bone, and aerobic capacity. Med. Sci. Sports Exerc. 49, 206–217. https://doi.org/10.1249/MSS.0000000000001074 (2017).
Anjanappa, M. et al. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support Radiat. Oncol. 16, 50–57. https://doi.org/10.1016/j.tipsro.2020.10.001 (2020).
Batrakoulis, A. et al. High intensity, circuit-type integrated neuromuscular training alters energy balance and reduces body mass and fat in obese women: A 10-month training-detraining randomized controlled trial. PLoS ONE 13, e0202390. https://doi.org/10.1371/journal.pone.0202390 (2018).
Batrakoulis, A. et al. Hybrid neuromuscular training promotes musculoskeletal adaptations in inactive overweight and obese women: A training-detraining randomized controlled trial. J. Sports Sci. 39, 503–512. https://doi.org/10.1080/02640414.2020.1830543 (2021).
Batrakoulis, A. et al. Hybrid-type, multicomponent interval training upregulates musculoskeletal fitness of adults with overweight and obesity in a volume-dependent manner: A 1-year dose-response randomised controlled trial. Eur. J. Sport Sci. 23, 432–443. https://doi.org/10.1080/17461391.2021.2025434 (2023).
Klassen, O. et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol. 53, 1356–1365. https://doi.org/10.3109/0284186X.2014.899435 (2014).
Martins, F. M. et al. Lower-body resistance training reduces interleukin-1β and transforming growth factor-β1 levels and fatigue and increases physical performance in breast cancer survivors. J. Strength Cond. Res. 37, 439. https://doi.org/10.1519/JSC.0000000000004270 (2023).
Sagarra-Romero, L. et al. Effects of an online home-based exercise intervention on breast cancer survivors during COVID-19 lockdown: A feasibility study. Support Care Cancer 30, 6287–6297. https://doi.org/10.1007/s00520-022-07069-4 (2022).
Hasenoehrl, T. et al. Resistance exercise and breast cancer–related lymphedema—a systematic review update and meta-analysis. Support Care Cancer 28, 3593–3603. https://doi.org/10.1007/s00520-020-05521-x (2020).
Klassen, O. et al. Muscle strength in breast cancer patients receiving different treatment regimes. J. Cachexia Sarcopenia Muscle 8, 305–316. https://doi.org/10.1002/jcsm.12165 (2017).
Leonard, S. T. & Droege, M. The uses and benefits of cluster analysis in pharmacy research. Res. Social Adm. Pharm. 4, 1–11. https://doi.org/10.1016/j.sapharm.2007.02.001 (2008).
Harvie, M., Howell, A. & Evans, D. G. Can diet and lifestyle prevent breast cancer: what is the evidence?. Am. Soc. Clin. Oncol. Educ. Book https://doi.org/10.14694/EdBook_AM.2015.35.e66 (2015).
Long-term benefits of adapted physical activity on upper limb performance and quality of life in breast cancer survivors. https://www.mdpi.com/2411-5142/2/4/38 Accessed 26 February 2025.
Morishita, S. et al. Differences in balance function between cancer survivors and healthy subjects: A pilot study. Integr. Cancer Ther. 17, 1144–1149. https://doi.org/10.1177/1534735418790387 (2018).
Patel, D. et al. Exercise and creatine supplementation to augment the adaptation of exercise training among breast cancer survivors completing chemotherapy: Protocol for an open-label randomized controlled trial (the THRIVE Study). JMIR Res. Protoc. 11, e26827. https://doi.org/10.2196/26827 (2022).
Rogers, L. Q. et al. Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res Treat. 159, 283–291. https://doi.org/10.1007/s10549-016-3945-2 (2016).
Funding
This work was co-financed by the European Regional Development Fund (FEDER) through the programme INTERREG VI-A Spain-Portugal (POCTEP) 2021–2027: Novas Sociedades Longevas (0137_NSL_6_E).
Author information
Authors and Affiliations
Contributions
SE—Wrote and performed the statistical analyses, prepared Figs. 3 and 4, and revised the manuscript AS—Wrote the introduction and methodology, prepared Figs. 1 and 5, and revised the manuscript RG—Wrote the discussion and revised the manuscript LB—Wrote the discussion, prepared Fig. 2, and revised the manuscript PF—Revised the manuscript HF—Revised the manuscript MM—Revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Higher Institute of Educational Sciences of the Douro (no: 2.576).
Informed consent
Informed consent to participate in this study was obtained from all subjects and/or their legal guardians prior to participation.
Consent for publication
All authors confirm that they have read, reviewed and approved the final version of the manuscript, agreeing to its submission for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
da Encarnação, S.G.A., Schneider, A., da Encarnação, R.G.A. et al. Long-term effects of multicomponent training on body composition and physical fitness in breast cancer survivors: a controlled study. Sci Rep 15, 33806 (2025). https://doi.org/10.1038/s41598-025-01702-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01702-y