Abstract
The utilization of organic amendments has been considered as the best nonchemical management strategy for controlling the soil borne pathogens and hence can act as a potential tool to manage different soil borne diseases of tomato. Keeping this in view, several organic amendments were used against sclerotium root rot disease of tomato which is soil borne in nature. Effects of these amendments on the plant growth parameters as well as on the soil mycoflora were also studied. The experimental result revealed that among different organic amendments the maximum plant height and leaf number/plant were observed in neem cake treated plot representing the values 54.61 cm and 122.08 per plant respectively at 50 days after transplanting, against 40.86 cm and 71.03 per plant in case of control plots. The least sclerotium root rot disease incidence (14.75%) and maximum fruit yield (266.33q ha−1) were also observed in neem cake treated plot (T1). Quantitative changes in the rhizosphere fungal population were also noticed due to the application of organic amendments in soil. It was observed that the fungal population peaked at 60 DAT and declined thereafter irrespective of the treatment. Minimum population was recorded in neem cake treated plot (T1) and maximum population was recorded in farmyard manure treated plot (T5) at all stages of plant growth except initial population (before transplanting). Soil beneficial fungi like Penicillium chrysogenum, Trichoderma asperellum were also observed in neem cake treated plot (T1) which can play an important role in sustainable management of the disease.
Similar content being viewed by others
Introduction
Tomato (Solanum lycopersicum L.) is one of the most widely cultivated vegetable crops1. Tomatoes are also called as edible berry, the major dietary source of the antioxidant lycopene, which has been linked to many health benefits, including reduced risk of cardiovascular disorder, cellular aging and cancer2,3,4. In India, tomato is one of the economically important horticultural crops, grown on 8.5 lakh hectares producing 208.19 lakh tons whereas in Odisha 14.34 lakh tons from 1.07 lakh hectare area was produced during the year 2023-24 (www.agricoop.nic.in). Tomato is subjected to be attacked by various soil-borne diseases among which root rot diseases are devastating in nature due to their effects on yield5,6,7. Root rot diseases in tomato are caused by different soil borne fungi namely Fusarium solani, Rhizoctonia solani and Sclerotium rolfsii8,9 among which root rot caused by Sclerotium rolfsii is of utmost importance as it directly affects the crop stand ultimately reducing the yield of tomato10. Management of soil-borne diseases is very difficult as they have a wide host range, and the pathogens remain in the soil for a long time by producing different surviving structures. Use of different organic amendments can suppress a wide range of soil borne pathogens due to induction of physicochemical and biological changes in soil and significantly improves the soil health by supplying essential nutrients to the soil11. Moreover, cultivation of vegetable crops by using organic manures is getting more importance because of less chemical residues, better taste as well as their beneficial effect on soil health and environment12,13. Application of organic amendments to the soil not only reduces the dependence on chemical fertilizers but also improves soil structure, encourages the growth and activity of beneficial organisms in the soil and sustains higher productivity due to improved soil health14,15,16. The indiscriminate use of chemical pesticides has created huge negative impact on environment; pesticide residues are contaminating food and feed, causing harm to non-target organisms, environmental degradation, pest resurgence and have negative effect on biodiversity17,18,19. Therefore, there is a need to reduce the use of inorganic fertilizers as well as chemical pesticides to manage the disease (s) in a sustainable way. It will eventually increase the crop yield, reduce the production cost, stimulate plant growth and restore soil fertility. Keeping these points in view, the present investigation was carried out to know the impact of organic amendments for sustainable management of sclerotium root rot disease of tomato.
Materials and methods
Experimental site
Field experiments were conducted for two consecutive crop seasons viz., Rabi, 2020-21 and 2021-22 at the Research farm of Regional Research and Technology Transfer Station (RRTTS), Odisha University of Agriculture and Technology, Chiplima, Sambalpur, Odisha. The station is situated at 20021’N latitude and 80055’E longitude in Dhankauda block of Sambalpur district at an altitude of 178.8 m above mean sea level.
Soil
The experiment was conducted on sandy loam soil (pH 6.7) with organic carbon content (0.60%), content of available nitrogen (453 kg ha−1), content of plant available phosphorus (297 kg ha−1) and content of plant available potassium (580 kg ha−1).
Lay out of the experiment
The experiment was laid out in a plot size of 5 m × 2 m following randomized block design (RBD) with three replications. Five treatments of organic amendment, one chemical check and an untreated control constituted a total of seven different treatments of the experiment (Table 1).
Raising the crop
Tomato seeds (cultivar hybrid Ritu) were sown at 10 cm apart, covered with thin layer of soil and mulched with straw. The healthy 30 days old seedlings were transplanted in the main experimental field after final land preparation. The recommended dose of fertilizers @100:50:50 (N: P2O5: K2O) kg ha−1 were applied. All the organic amendments were applied at the time of final land preparation in respective treatments of the experiment with their respective doses. Proper soil moisture was maintained in the experimental field for decomposition of the organic amendments. One month old seedlings were transplanted 7 days after final land preparation with spacing of 60 cm × 45 cm. Standard agronomic practices were followed as and when necessary to raise the crop.
Recording of field data
Observations on plant growth parameters (Plant height and number of leaves/plant) were recorded at 50 DAT. Ten numbers of plant were selected randomly from each plot for taking observation excluding the border rows. Root rot incidence (%) was calculated by using the following formulae.
Collection of soil sample and enumeration of fungal population
Soil samples from the rhizosphere zone adhering to the roots of tomato plant were collected from different treatments separately. The plants were carefully dug up at 0–40 cm soil depth. The composite soil samples were collected at 15 days interval and brought to Plant Pathology laboratory, RRTTS, Chiplima. Soil dilution plate method20 was used for enumeration of fungi from soil samples. Soil dilutions were made by suspending 1 g of soil of each sample in 10 ml of sterile distilled water. Then dilutions of 10−3, 10−4 and 10−5 were made to enumerate the fungal population to avoid over-crowding of the fungal colonies. 1 ml suspension of each concentration was added to sterile petri dishes in triplicates of each dilution, containing sterilized Rose Bengal agar medium amended with streptomycin (1%) solution for preventing bacterial growth, before pouring into petri dishes. The plates were then incubated at 28 ± 2℃ for 3–5 days. Fungal colonies appeared and were easily counted as they formed surface colonies that were well dispersed, particularly at higher dilutions. Total fungal population (CFU g−1 dry soil) was counted before transplanting (initial) and at 30, 45, 60, 75 and 90 DAT.
Identification of fungi
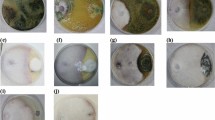
Rhizosphere soil mycoflora were identified to the genus level and the species level based on their morphological characters and microscopic observation by using suitable media. The specimen was observed under the microscope for identification. Identification was done by using monographs and relevant literature21.
Relative frequency and abundance of soil fungi isolated from experimental field
The frequency and relative abundance of soil fungi isolated from different treatments were determined by using a formula described as22.
Results
Effect on plant growth
Effects of different organic amendments on plant growth parameters, disease incidence and fruit yield of tomato were recorded for two consecutive years and pooled data is presented in Table 2. Plant height is one of the key growth parameters that ultimately influence the total growth and output of the plant. From the pooled data (Table 2) it was found that, at 50 DAT treatment T1 recorded highest plant height (54.61 cm) followed by T2 (52.01 cm) and T5 (50.81 cm). A rise of 33.7% in plant height was observed in T1 treatment over untreated control. The highest number of leaves was found at 50 DAT in treatment T1 (122.08) followed by T2 (108.08) and T5 (96.98).
Effect on disease incidence
The root rot incidence (%) was recorded during both the years of experiment and pooled mean of two years data is presented (Table 2) and conclusions are drawn on the basis of the pooled mean. The pooled mean revealed that percent disease incidence was significantly reduced in all the treatments in comparison to untreated control. Although the least root rot disease incidence (12.64%) was observed in chemical check (T6) but it was statistically at par with treatment T1. Highest disease control i.e. 64.8% was achieved by the same treatment (chemical check plot). Among the organic treatments the least disease incidence was observed in T1 treatment. From the two-year pooled data, the percent reduction of disease incidence over control was also calculated and it was found that among the organic treatments the maximum disease control i.e. 58.9% was achieved in T1 treated plots.
Effect on fruit yield
The effect of various treatments on the yield of tomato was also evaluated during both the years of study and it was found that, highest fruit yield of 276.50 q ha−1was obtained from the chemical check plot. Among the organic treatments the maximum fruit yield was recorded in T1 treatment (266.33q ha−1) i.e. soil application of neem cake @ 5 q ha−1and gave 50.2% yield increase over untreated control.
Effect on soil mycofloral population
Effect of various organic amendments on quantitative soil mycofloral population of tomato plants was studied during both the years and pooled data is presented in Table 3. Soil mycoflora population was estimated before transplanting (initial) and at 30, 45, 60, 75 and 90 DAT through soil dilution plate method. Data (Table 3) revealed that initial mycofloral population (before transplanting) and application of different treatments did not differ significantly with each other. Highest mycofloral population was found in farmyard manure treated plot (T5) irrespective of the crop ages. The lowest fungal population was found in T1 i.e. neem cake treated plot in all the ages of crop plants except chemical check plot.
Relative frequency of fungi isolated from different treatments of the experiment
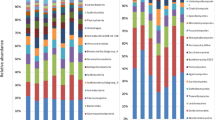
Qualitative nature of major rhizosphere mycofloral population were identified through soil Dilution plating from different treatments and their relative frequencies were presented in Tables 4; Fig. 1. Ten fungal species under eight genera were isolated from the different treatments of the study site. They were Aspergillus flavus, Aspergillus Niger, Aspergillus nidulans, penicillium chrysogenum, Trichoderma asperellum, rhizopus stolonifer, fusarium oxysporum, Mucor Mucedo, sclerotium rolfsii and Verticillium dahliae. It was found that the relative frequencies of isolated soil fungi varied in different treatments of experimental site. Among the soil fungi isolated from treatment T1, the highest frequency of fungi was in Penicillium chrysogenum (100%) followed by Trichoderma asperellum (83.3%), Aspergillus flavus (66.7%) and Aspergillus Niger (66.7%). The least frequency was found in Rhizopus stolonifer (16.6%) whereas Fusarium oxysporum, Mucor Mucedo, Sclerotium rolfsii, Verticillium dahliae were absent. In case of treatment T5, the highest frequency (66.7%) was found in Aspergillus flavus, Aspergillus Niger, Aspergillus nidulans, Penicillium chrysogenum and Rhizopus stolonifer. In case of treatment T6, the highest frequency (50%) was found in Aspergillus flavus, Penicillium chrysogenum, and Rhizopus stolonifer. In this treatment three fungi namely Trichoderma asperellum, Mucor Mucedo and Verticillium dahliae were absent. In case of treatment T7, the highest frequency (66.7%) was found in Aspergillus flavus, Rhizopus stolonifera and Sclerotium rolfsii whereas Trichoderma asperellum was absent.
Relative abundance of fungi isolated from different treatments of the experiment
The relative abundance of rhizospheric fungi of tomato plant were calculated and presented in Table 5; Fig. 2. It was found that relative abundance of isolated rhizospheric soil fungi significantly varies among the different treatments used in the experiment. In treatment T1 the relative abundance of soil fungi was Penicillium chrysogenum (42.9%), Aspergillus niger (34.3%), Trichoderma asperellum (28.6%), Aspergillus flavus (20.0%), Aspergillus nidulans (14.3%) and Rhizopus stolonifer (11.4%) whereas the pathogenic soil fungi Fusarium oxysporum, Mucor mucedo, Sclerotium rolfsii and Verticillium dahliae were absent. In case of treatment T7, highest abundance of soil fungi was found in Penicillium chrysogenum (31.3%) and the abundance of pathogenic soil fungi was reported in Fusarium oxysporum (12.5%), Mucor mucedo (9.4%), Sclerotium rolfsii (15.6%) and Verticillium dahliae (12.5%).
In Fig. 3 it has been clearly found that in all the treatments, rhizosphere mycofloral population increased gradually up to 60 DAT and then gradually decreased. Results regarding total mycofloral population indicated that application of neem cake reduced the mycofloral population effectively at all the ages of plant growth in comparison to other treatments including untreated control except chemical check plot.
Discussion
The results obtained from the study revealed that higher growth performance of tomato plant was observed in neem cake treated plots (T1) as compared to other treatments and untreated control. This finding is supported by many previous workers23,24 who reported that neem cake was quick acting, provide slow and steady nourishment and improve yield and quality of crops. Other previous reports25,26,27,28 also confirmed that shoot length and leaf number increased due to the use of neem cake. It improved the growth and yield of crops because it contains essential nutrients necessary for growth of crops11. According to a previous report29 significantly higher yield (10.42 q/ha) was registered in red chilli by application of neem cake @ 250 kg ha−1 followed by vermicompost @ 1 ton ha−1. In another experiment30 application of neem cake @ 5 kg plant−1 was found to give better yield performance than FYM @ 34.7 kg plant−1 in guava plant.
It is also evident from the result that least disease incidence was recorded in fungicide treated plots followed by neem cake treated plots. As environmentally safe disease management technologies are gaining importance in recent years, emphasis must be given to non-chemical disease management technologies. On the basis of the above fact, neem-based products are on the approved list for organic mode of disease management. In a previous study also, addition of neem cake to the soil resulted minimum root rot disease incidence in mungbean31. The effects of neemcake in disease management might be attributed to the production of azadirachtin which reduced the fungal population32. Similar results were also obtained by many workers33,34,35.
Results regarding total rhizosphere mycofloral population indicated that the applications of neem cake reduced the mycofloral population effectively in all the cases in comparison to untreated control. Minimum mycofloral population was recorded in neem cake treated plots at all the stages of plant growth except initial population. Results are in agreement with many previous workers36,37. The possible reason behind this might be ammonia toxicity as neem is a highly nitrogen containing product and degradation of nitrogen finally releases ammonia which is toxic to mycofloral population38. Similar type of finding was also observed in past28 which reported that soil application of neem cake reduced the fungal population in brinjal plant.
It was also observed that total rhizosphere mycofloral population counts of various treatments gradually increased with age of the crop up to 60 DAT and then decreased with increase in crop age. This may be due to initial increase of nutrient supply to the mycofloral population which gradually depleted with great vigour of plants that lead to luxuriant uptake of nutrients and secretion of some toxic metabolites39,40.
The four fungal species viz. Penicillium chrysogenum, Aspergillus flavus, Aspergillus niger and Aspergillus nidulans were found commonly in all the treatments. Moreover, beneficial soil fungi viz. Penicillium chrysogenum and Trichoderma asperellum were more dominant in neem cake, vermicompost, mustard cake and groundnut cake treated plot. This may be due to the fact that the organic amendment treated plots promotes the growth of beneficial soil fungi. Whereas pathogenic fungi like Fusarium oxysporum, Sclerotium rolfsii and Verticillium dahliae were more dominant in untreated control plot. According to a previous report41 the variation in fungal colonization, abundance and distribution in the rhizosphere soil may depend on host plant species, soil properties or treatment, local climate and environmental factors.
Conclusion
Therefore, it can be concluded that the use of organic amendments in the form of oil cakes especially neem cake significantly reduced sclerotium root rot disease incidence in tomato and the nutrient content of this amendment may have a possible role in improving the plant growth and ultimately yield of the tomato plant. This will also improve beneficial microorganisms of soil which play an important role in management of the disease in an eco-friendly way. This strategy will be helpful for sustainable management of sclerotium root rot disease of tomato as well as enhancement of growth and yield of the crop.
Data availability
The datasets used and/or analysed during the current study will be available from the corresponding author on reasonable request.
References
Serio, F., Ayala, O., Bonasia, A. & Santamaria, P. Antioxidant properties and health benefits of tomato. In Recent Progress in Medicinal Plants Vol. 13 (Studium, 2006).
Abdel-Monaim, M. F. Induced systemic resistance in tomato plants against fusarium wilt disease. Int. Res. J. Microbiol. 3, 14–23 (2012).
Di Cesare, L. F. et al. Effects of irrigation fertilization and irrigation-mycorrhization on the alimentary and nutraceutical properties of tomatoes. In Irrigation Systems and Practices in Challenging Environments (eds Lee, T. S. et al.) 207–233 (Tech, 2012).
Giovannucci, E. Tomatoes, tomato-based products, lycopene and cancer: review of the epidemiologic literature. J. N C. .1, 317–331 (1999).
Ma, M., Taylor, P. W. J., Chen, D., Vaghefi, N. & He, J. Z. Major soilborne pathogens of field processing tomatoes and management strategies. Microorganisms 11, 263. https://doi.org/10.3390/microorganisms11020263 (2023).
Saad, M. M. Destruction of Rhizoctonia Solani and Phytophthora capsici causing tomato root-rot by Pseudomonas fluorescens lytic enzymes. Res. J. Agric. Biol. Sci. 2, 274–281 (2006).
Taheri, P., Hosseini-Zahani, F. & Tarigh Saeed. Binucleate Rhizoctonia induced tomato resistance against Rhizoctonia Solani via affecting antioxidants and cell wall reinforcement. Heliyon 10, e27881. https://doi.org/10.1016/j.heliyon.2024.e27881 (2024).
El-Mohamedy, R. S. R., Jabnoun-Khiareddine, H. & Daami-Remadi, M. Control of root rot diseases of tomato plants caused by Fusarium Solani, Rhizoctonia Solani and Sclerotium rolfsii using different chemical plant resistance inducers. Tunis J. Plant. Prot. 9, 45–55 (2014).
Sachdev, S. & Singh, R. P. Sustainable management of soil borne pathogens of tomato. Int. J. Sci. Tech. Soc. 3 (2), 36–40. https://doi.org/10.18091/ijsts.v3i02.11409 (2017).
Mahato, A., Biswas, M. K. & Patra, S. Prevalence of collar rot of tomato caused by sclerotium rolfsii (Sacc.) under the red and lateritic zone of West Bengal, India. Int. J. Curr. Microbiol. App Sci. 6 (11), 3231–3236. https://doi.org/10.20546/ijcmas.2017.611.378 (2017).
Reddy, J. K. & Umesha, C. Effect of organic nutrients and Neemcake on growth and yield of wheat. Environ. Ecol. 41 (4), 2292–2296. https://doi.org/10.60151/envec/PGVF6815 (2023).
Gao, F. et al. Effects of organic fertilizer application on tomato yield and quality: A Meta-Analysis. Appl. Sci. 13, 2184. https://doi.org/10.3390/app13042184 (2023).
Sunanda Rani, N. & Mallareddy, M. Effects of different organic manures and inorganic fertilizers on growth yield and quality of Carrot. Karnataka J. Agric. Sci. 20, 686–688 (2007). http://14.139.155.167/test5/index.php/kjas/article/viewFile/1009/1002
Mohamed, A. S., Shohba, N. E. A., Abou-Taleb, S. A., Abbas, M. S. & Soliman, A. S. Beneficial effects of Bio-Organic fertilizers as a partial replacement of chemical fertilizers on productivity and fruit quality of pomegranate trees. Biosci. Res. 15, 4603–4616 (2018).
Singh, Y., Singh, C. S., Singh, T. K. & Singh, J. P. Effect of fortified and unfortified rice-straw compost with NPK fertilizers on productivity, nutrient uptake and economics of rice (Oryza sativa). Indian J. Agron. 51, 297–300 (2006). JAST49771361565000.pdf.
Tiwari, K. N. Nutrient management for sustainable agriculture. J. Ind. Soc. Soil. Sci. 50, 374–377 (2002).
Fountain, E. D. & Wratten, S. D. Conservation biological control and biopesticides in agricultural. Encycl Ecol. 1, 377–381. https://doi.org/10.1016/B978-0-12-409548-9.00539-X (2013).
Kumar, S. & Biopesticides A need for food and environmental safety. J. Biofertil. Biopestici. 3, 4. https://doi.org/10.4172/2155-6202.1000e107 (2012).
Kumar, S., Sindhu, D., Satyavir, S. & Kumar, R. Biofertilizers: an ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 3, 100094. https://doi.org/10.1016/j.crmicr.2021.100094 (2022).
Waksman, S. A. Principle of Soil Microbiology (Williams and Wilkins Co. Baltimore, 1927).
Wantanabe, T. Pictorial atlas of soil and seed fungi. In: Morphologies of cultured fungi and key tospecies, 2nd edn. CRC Press. Boca Raton. Florida. USA, 504 (2002).
Mc Lean, V. & Cook, W. R. Practical Field Ecology (George Allen &Unwin, 1957).
Gaur, A. C., Neelakanthan, S. & Dargah, K. S. Organic Manurespp157 (Indian Council of Agricultural Research, 1992).
Mandal, D. & Pal, R. Ecology of rhizosphere mycoflora in organic matter amended soil: A review. J. Pharm. Innov. 11(7), 3677–3679 (2022).
Agbenin, J. O., Agbaji, E. O., Suleiman, I. & Agbaji, A. S. Assessment of nitrogen mineralization potential and availability from Neem seed residues in a savanna soil. Biol. Fertil. Soils. 29, 408–412 (1999).
Ketkar, C. M. & Ketkar, M. S. Neem seeds crush and deoiled cake as manure and nitrification inhibitors. In The Neem Tree (ed. Schumutterer, H.) 531–540 (VHC, 1995).
Oyinlola, E. Y., Magajil, E. A., Garba, J. & Mohammed, K. O. Effect of Neem seed cake and inorganic fertilizer application on soil properties, and on growth, nutrient concentrations and uptake of tomato (Lycopersicon esculentum Mill). Nigerian J. Soil. Environ. Res. 12, 91–100 (2014).
Singh, A. K., Dwivedi, M. C., Singh, Vaibhav, K., Singh, V. K. & Williams, P. Effect of Neem products on rhizosphere Mycoflora and growth of Brinjal (Solanum Melangena L.) plants. J. Mycopathol Res. 52 (1), 145–147 (2014).
Veena, S. K., Giraddi, R. S., Bhemmanna, M. & Kandpal, K. Effect of Neem cake and vermicompost on growth and yield parameter of Chilli. J. Entomol. Zool. Stud. 5 (5), 1042–1044 (2017).
Maity, P. K., Das, B. C. & Kundu, S. Effect of different sources of nutrients on yield and quality of guava cv. L-49. J. Crop Weed 2(2), 17–19 (2006).
Gahlot, N., Ahir, R. R., Choudhary, S. & Abhinav. & Evaluation of bio-control agents and soil amendments against root rot incidence of Mungbean incited by Macrophomina Phaseolina (Tassi.) goid. J. Pharm. Innov. 11 (4), 701–705 (2022).
Gopal, M., Gupta, A., Arunachalam, V. & Magu, S. P. Impact of Azadirachtin, an insecticidal allelochemical from Neem on soil microflora, enzyme and respiratory activities. Bioresour Technol. 98, 3154–3158. https://doi.org/10.1016/j.biortech.2006.10.010 (2007).
Dhingani, J. C., Solanky, K. U. & Kansara, S. S. Management of root rot disease [Macrophomina phaseolina (Tassi.) Goid] of chickpea through botanicals and oil cakes. Bioscan 8 (3), 739–742 (2013).
Dubey, S. C. Efficacy of some of oil cake and plant extracts against web blight of urd and Mungbean caused by Thanatephorus Cucumeris. J. Mycol. Plant. Pathol. 32 (2), 158–161 (2002).
Tiyagi, S. A. & Alam, M. M. Efficacy of oil-seed cakes against plant-parasitic nematodes and soil-inhabiting fungi on Mungbean and Chickpea. Bioresour Technol. 51 (2–3), 233–239. https://doi.org/10.1016/0960-8524(94)00128-N (1995).
Chandel, S. & Tomar, M. Effectiveness of bioagents and Neem formulations against fusarium wilt of carnation. Indian Phytopathol. 61, 152–154 (2008). https://epubs.icar.org.in/index.php/IPPJ/article/view/12781
Singh, N. & Singh, R. S. Inhibition of Fusarium Udum (pigeon pea wilt pathogen) by other distillate of Margosa cake amended soil. Indian J. Mycol. Plant. Pathol. 23, 329–333 (1983).
Tenuta, M. & Lazarovits, G. Ammonia and nitrous acid from nitrogenous amendments kill the microsclerotia of Verticillium dahliae. Phytopathol 92, 255–264 (2002).
Gangwane, L. V. & Deshpande, K. B. Agronomic treatments and changes in the rhizosphere Mycoflora of groundnut. Acta Agron. Academiae Scientiarum Hung., p26 (1977).
McDonald, D. The influence of the developing groundnut fruit on soil Mycoflora. Trans. Br. Mycol. Soc. 53, 393–403 (1969).
Srivastava, V. & Kumar, K. Biodiversity of Mycoflora in rhizosphere and rhizoplane of some Indian herbs. Biol. Forum. 5, 123–125 (2013).
Acknowledgements
The authors are thankful to Dean of Research, OUAT, Bhubaneswar, Odisha, India for providing funds to carry out this research work.
Author information
Authors and Affiliations
Contributions
DM, RP & SM planned the research experiment. DM, AS and SNB implemented the field experiment and recorded field data. DM, RP and AS planned laboratory work and recorded data. DM and RP wrote the manuscript. DM, PS and SB drafted and made critical revisions to the manuscript. DM and JS analyzed data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mandal, D., Pal, R., Mohapatra, S. et al. Use of organic amendments for sustainable management of root rot disease of tomato (Solanum lycopersicum L.) caused by Sclerotium rolfsii. Sci Rep 15, 35772 (2025). https://doi.org/10.1038/s41598-025-01706-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01706-8