Abstract
Screening for metabolic dysfunction-associated steatotic liver disease (MASLD) across the entire general population is not currently a recommended strategy. However, it is not uncommon to receive a medical check-up or health check-up for a various of reasons. We tried to investigate whether advanced fibrosis screening in MASLD patients is cost-effective for adults aged 40–49 years during medical or health check-up. The target group for analysis was adults who received medical check-ups for various reasons in the United States. We constructed a hybrid model of the decision tree model and Markov model to compare expected costs and quality-adjusted life-years (QALYs) between ‘screening’ and ‘no screening’ groups from healthcare system perspectives. Patients diagnosed MASLD with advanced fibrosis by FIB4 and VCTE were given intensive lifestyle intervention (ILI). The incremental cost-effectiveness ratio (ICER) was calculated for a 30-year horizon. Assuming effect of ILI is limited to regression of liver fibrosis, ICER of the FIB-4-based two steps algorithm was $103,405 per QALY in adults aged 40–49 years, which was slightly above the threshold value ($100,000/QALY). And in those in adults aged 50–59 and 60–69 years, the ICER was $137,593 and $197,901 per QALY, respectively. If we assume the effect of ILI can improve liver fibrosis as well as cardiovascular disease events, ICERs of screening in aged 40–49 and 50–59 years were $74,596, and $95,974 per QALY, respectively. In an analysis that included additional positive effect on extrahepatic cancer by ILI, estimated ICERs were below the threshold in those in aged 40–49 and 50–59 years. Advanced fibrosis screening in MASLD patients using the FIB-4-based two-step algorithm and ILI was cost-effective for adults aged 40–49 years, taking into account both liver fibrosis and cardiovascular disease.
Similar content being viewed by others
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) leading to advanced hepatic fibrosis is a common and growing public health problem worldwide1 Recent study based on the National Health and Nutrition Examination Survey (NHANES) data set from 2017 to 2020 in United States shows prevalence of MASLD reaches 32.4% in the general population2,3 With the obesity epidemic, steatosis liver disease (SLD) is the major cause of chronic liver disease or hepatic fibrosis in the general population in the world4 The prevalence of suspected fibrosis in the US general population was 13.8%5 and advanced fibrosis in the general population of Western and Asian countries is significant at 2.2 ~ 5.5%,6,7,8 raising concerns for future incidence of cirrhosis and its complications. Advanced fibrosis is associated with increased risk of morbidity and mortality, making it crucial to identify subjects with hepatic fibrosis9,10.
Currently, most guidelines recommend targeted screening for advanced fibrosis in ‘at risk groups’ with type 2 diabetes mellitus (T2DM), metabolic syndrome, or elevated liver enzyme level in MASLD patients, emphasizing that this approach is cost-effective11. Early recognition of such at-risk group facilitates timely interventions, potentially preventing future hepatic complications12 Recently, Crossan et al. suggested that it is cost-effective with sequentially use of FIB4 followed by vibration-controlled transient elastography (VCTE), FibroTest, or enhanced liver fibrosis (ELF) in screening patients with NAFLD at primary care centers13.
Although the prevalence of MASLD is high, the proportion of advanced hepatic fibrosis is low in the general population, so routine MASLD-associated fibrosis screening in the entire general population is not currently recommended. However, the number of people receiving medical check-ups for various reasons or health check-ups without any symptoms has been increasing14. In general, during a health or medical check-up, liver enzyme and platelet count tests for fibrosis-4 (FIB-4) calculation are usually performed15. More active MASLD screening strategy is needed for people who undergo medical check-up or health check-up. Early intervention facilitated by appropriate screening tests in groups with a high disease prevalence has the potential to mitigate the social burden associated with the disease.
As MASLD-associated fibrosis tend to be progress over time without treatment, it is important to identify such patients early. It is well known that prevalence of hepatic fibrosis increased with age4,6. The prevalence of advanced hepatic fibrosis is particularly high in the elderly population, reaching 7.7%4. Advanced hepatic fibrosis is a problem in itself, the pathophysiological effects of MASLD extend beyond the liver, demonstrating that MASLD is independently associated with cardiovascular disease (CVD) and extrahepatic malignancies16,17 Although a new drug for MASLD was approved by the FDA, the gold standard for SLD treatment is still intensive lifestyle intervention (ILI). ILI, including dietary changes, increased physical activity, and gradual weight loss, is consistently emphasized as the primary treatment for MASLD18. ILI appears to have beneficial effects beyond reducing hepatic fibrosis, including the potential to decrease cardiovascular disease and extrahepatic cancers. More active MASLD screening followed by ILI is needed in communities aged 40 years and above to reduce disease and socioeconomic costs.
Therefore, the aim of this study is to evaluate the cost-effectiveness of a screening MASLD utilizing a sequential combination of FIB-4 and VCTE during medical check-ups or health check-ups for adults aged 40–49 years, considering hepatic fibrosis, CVD and extrahepatic malignancies.
Materials and methods
Study population
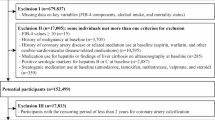
The target group for analysis was adults who received a medical check-ups or health check-ups for various reasons in the US. Subjects with a clear risk for liver diseases such as viral hepatitis or significant alcohol consumption were excluded. All subjects had their FIB-4 calculated, and if it was higher than 1.3, VCTE test was sequentially performed. If the liver stiffness value was higher than 8.8kpa, and there was no evidence of chronic viral hepatitis, and there was no history of significant alcohol consumption (intake greater than 210 g for male and 140 g for female), the ILI applied to all subjects.
To identify the burden of fibrosis in the US population, we estimated the prevalence of advanced hepatic fibrosis from data of VCTE of the US National Health and Nutrition Examination Survey (NHANES) from 2017-March 2020. According to previous study, the liver stiffness cut off based on VCTE results of 6.2 kPa, 7.6 kPa, 8.8 kPa and 11.8 kPa were used for F0, F1, F2, F3 and F4, respectively19,20,21. Stratifying by age groups ranging from the 30–39 years to the 60–69 years, the prevalence of advanced fibrosis was 6.3–10.7% (Table 1).
Construction of a decision analytic model
A cost-utility analysis was conducted to evaluate the cost-effectiveness of advanced fibrosis screening for long-term clinical outcomes. We constructed a hybrid decision tree and Markov model using Excel 2019 (Microsoft Corp) to assess the difference in quality-adjusted life-year (QALY) and expected cost between the’ screening’ and ‘no screening’ groups.
Decision tree model: screening strategy
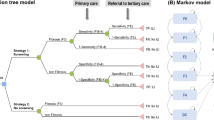
The decision tree represents the detailed structure of the events and outcomes associated with the two comparison strategies (Fig. 1A). Those with a high FIB-4 (> 1.3) were referred to a referral center and VCTE testing. The sensitivity and specificity of FIB-4 was obtained from previous studies22. In the aged 40–49 years, FIB-4 sensitivity and specificity were 56.6% and 86.2%, respectively22 For the performance of VCTE, sensitivity was 80.0%, specificity was 74.0%, respectively23 After screening, patients diagnosed with advanced fibrosis (both true positive (TP) and false positive (FP) cases) participated in an intensive lifestyle intervention (ILI) program. On the other hand, in the case of the no screening strategy, the simulation cohort with the same risk as the screening group did not receive ILI.
Overview of decision analytic model (A) decision tree model, (B) Markov model. CVD, cardiovascular disease; DC, decompensated cirrhosis; FN, false negative; FP, false positive; HCC, hepatocellular carcinoma; ILI, intensive lifestyle intervention; MRE, magnetic resonance elastography; NIT, non-invasive test; TN, true negative; TP, true positive; VCTE, vibration controlled transient elastography.
* It represents three states of CVD in H0, F0-F2, F3, and F4.
Markov model
The population classified according to the diagnostic results was subdivided from F0 to F4 according to the distribution of fibrosis. We assumed half of the F0 population as healthy population (H0) who do not have the risk of fibrosis. And then the classified population entered into the Markov model to estimate long-term cost and obtain life-year (LY) and QALY (Fig. 1B). The model included H0 (healthy population), fibrosis stages; F0 to F4 (and each pair condition undergoing ILI), cardiovascular disease (CVD) complications (and each pair condition undergoing ILI), decompensated cirrhosis (DC), and hepatocellular carcinoma (HCC), extrahepatic malignancy and death. The CVD status was subdivided into three stages (CVD with F0-F2/F3/F4) according to the stage of fibrosis. HCC can occur in F3, F4 and DC. The disease-specific mortality (F0-F4, CVD, HCC, and extrahepatic malignancies) and age-specific all-cause mortality were applied for all health conditions. Details of parameters related on disease progression risk and mortalities applied to the model are described in Supplementary Table 1, Supplementary Tables 2, and Supplementary Methods. The cycle length of the model was 1 year, and the time horizon was set to 30 years.
Assumption
The following assumptions were applied to this analytical model. (i) The population classified by screening results was subdivided into H0 and F0-F4 according to the ratio of fibrosis stage. Patients with H0 (healthy population who do not have the risk of fibrosis) maintain a healthy state that does not progress to fibrosis, but they have a risk of CVD and incurred all-cause mortality. (ii) In the H0 and F0-F4, all-cause mortality in the general population was applied; mortality due to liver disease was not additively applied. There was no HCC risk in the H0 and F0-F2 population. (iii) All patients diagnosed with advanced fibrosis received the ILI program. While receiving ILI, patients were monitored for advanced fibrosis regardless of TP or FP. (iv) The effect of ILI was reflected in the newly development of CVD and extrahepatic malignancies, and hepatic fibrosis regression. The regression rate of hepatic fibrosis (F1-F3) due to ILI was applied at 17.0%, and it was assumed that hepatic fibrosis regression would occur at 7.4% even in the control group24. The difference of hepatic fibrosis regression rate between in the ILI group and control group was 9.6%12,25,26 (v). The gap in regression rate by ILI between ‘screening’ and ‘no-screening’ groups lasted only for 10 years, the duration of receiving ILI. And after stopping the ILI program, the population of screening group had the same risk as no-screening group.
Cost and quality of life
We performed the analysis from the healthcare system perspective, and all the cost parameters were adjusted to the 2022 US dollars. An annual discount rate of 3% was applied to all costs and outcomes. Detailed cost parameters and utility weights of health states to calculate QALY were described in Supplementary Tables 3 and Supplementary Methods section.
Analysis
The primary outcome was the incremental cost effectiveness ratio (ICER) between each strategy, which was calculated by dividing the incremental cost by the incremental QALY (or LY) between comparison strategies. We simulated our model by applying different screening ages between 30 and 39, 40–49, 50–59 and 60–69 years. According to their age group, distribution of fibrosis and performance of FIB-4 were applied differently (Table 1). The results were interpreted as cost-effective when the expected ICER was less than $100,000/QALY, which was implicitly accepted as the willingness-to-pay (WTP) threshold in the US27.
Deterministic and probabilistic sensitivity analyzes (PSA) were performed to explore the impact of uncertainty in parameter estimates and assumptions applied to the analytic model. One-way sensitivity analyses were conducted by varying values over clinically relevant ranges (Supplementary Tables 1 and Supplementary Table 3). We also performed a two-way sensitivity analysis to investigate the impact of simultaneous variation by the ILI effect (regression rate) and duration on cost-effectiveness. And PSA using second-order Monte Carlo simulations was performed to evaluate the overall impact of uncertainty. Beta distributions were applied for transition probabilities and utility weights, and gamma distributions for costs.
Results
Cost-effective analysis of MASLD screening according to age group in aspect of liver disease of ILI
ICERs calculated according to age group. Assuming that the effect of ILI is limited to regression of liver fibrosis, the ICER of the FIB-4-based two steps algorithm was $103,405 per QALY in people in aged 40–49 years (Table 2; Fig. 2A), which was slightly exceeded the willingness-to-pay threshold value ($100,000). However, in those in aged 50–59 and 60–69 years, the ICER was $147,914 and $214,821 per QALY, respectively, which were considerable higher than the threshold, indicating that screening strategy was not cost-effective (Supplementary Table 4).
Cost-effective analysis of MASLD screening and ILI according to age group in aspect of liver disease and cardiovascular disease
If we assume the effect of ILI can improve liver fibrosis as well as decrease CVD events, ICERs of screening for MASLD using FIB-4 based two-step algorithm in health check-up population aged 40–49 years (base-case) was $74,596 per QALY, and aged 50–59 years was $95,974 per QALY, respectively (Table 2; Fig. 2B). Both age groups were cost-effective. However, the estimated ICER for aged 30–39 and 60–69 years were $117,859 and $118,166, which still exceeded the threshold. We also showed clinical outcomes through simulating our model. It showed that clinical events related the liver diseases and CVD were reduced in the screening group compared to the no screening group (Supplementary Table 5).
Cost-effective analysis in aspect of including beneficial effects of ILI on extrahepatic malignancy
Additionally, considering the positive effects on extrahepatic cancer of ILI, similar to the analysis in the aspect of liver disease and CVD above, the screening was cost-effective in those in aged 40–49 and 50–59 years, however the ICER was estimated 0.9 times lower ($67,135 and $84,951/QALY, respectively). And in aged 30–39 and 60–69 years, the estimated ICERs were slightly exceeded the willingness-to-pay threshold value ($104,513 and $103,081/QALY, respectively, Table 2; Fig. 2C and Supplementary Table 4).
Sensitivity analysis
One-way sensitivity analyses are presented in Table 3 and Fig. 3. The most influential parameters on cost-effectiveness were the regression rate of hepatic fibrosis after ILI, screening age, and duration of ILI. If the ILI regression rate of hepatic fibrosis dropped to 15% (regression rate of hepatic fibrosis in control group is fixed as 7.4%: net difference between two groups is 7.6%), the ICER increased to $94,782.
Tornado diagram for one-way sensitivity analysis. CVD, cardiovascular disease; EHM, extrahepatic malignancy; FIB-4, fibrosis-4; ICER, incremental cost-effectiveness ratio; ILI, intensive lifestyle intervention; MRE, magnetic resonance elastography; QALY, quality-adjusted life-year; VCTE, vibration controlled transient elastography.
Shortening the ILI duration led to a decrease in the effect of preventing the occurrence of important clinical outcomes such as CVD or HCC, resulting in an increase in ICER and exceeding the threshold. The change of other variables showed a small impact on ICER. Supplementary Table 6 presents a two-way sensitivity analysis for two parameters that significantly impact ICER estimation: the regression rate and duration of ILI. If an ILI duration was shortened to 6 years, the regression rate from ILI had to be at least 17.5% for screening to be cost-effective. The ILI impact required a minimum of 16% and 15% regression rates at 8 and 10 years of ILI duration, respectively, for screening to be deemed cost-effective. When ILI duration was extended beyond 12 years, screening was cost-effective, even though the regression rate decreased to 14% (net difference between the two groups, 6.6%).
The results of a PSA simulated 1,000 times are illustrated in the cost-effectiveness acceptability curve (Supplementary Fig. 1). The curve shows the probabilities of being cost-effective associated with different levels of willingness-to-pay thresholds. Screening was a cost-effective option compared to no screening if the willingness-to-pay threshold was higher than $70,000, and the probability based on the threshold of $100,000 was 70.9%.
Discussion
Our data revealed that more active screening for MASLD and ILI during health check-up examinations in patients aged 40–49 years remained below the accepted threshold, regardless of the effects of ILI on extrahepatic cancer, given the decrease in CVD events. If the beneficial effects of ILI extend to CVD and extrahepatic malignancy, ICERs decrease more in those aged 40–49 and 50–59 years. The strengths of our study encompass not only the evaluation of the impact of ILI induced reduction of liver fibrosis following screening in the general population but also the comprehensive analysis of ILI’s broader effects on extrahepatic outcomes, specifically CVD and extrahepatic-related events. Interestingly, the target population of our analysis has much lower risk for MASLD compare to previous ‘at risk’ population with type 2 diabetes mellitus (T2DM), metabolic syndrome, or elevated liver enzyme level28,29 And we also considered the effect of ILI on reducing cardiovascular events and extrahepatic malignancy in addition to hepatic fibrosis regression. However, the results of this study do not mean that MASLD screening is effective for the entire general population. The analysis target of this study is limited to situations in which FIB-4 calculation is possible, such as health or medical check-up, for various reasons. The clinical significance of this study is that MASLD screening and ILI through FIB-4 calculation and following VCTE is cost-effective when health check-up is performed for various reasons in the primary care setting.
Recently, a new drug for MASLD was approved by the FDA. But ILI is still considered the gold standard of treatment30 ILI, including dietary changes, increased physical activity, and gradual weight loss, is consistently emphasized as the primary and initial treatment approach for MASLD18. Previous literature demonstrated the impact of ILI on the progression of liver fibrosis, as evidenced by significant improvements in serial VCTE values within the intervention groups compared to the control groups31. ILI has been shown to not only reduce hepatic fibrosis and reverse steatohepatitis but also have positive effects on CVD associated with SLD32 However, cost-effectiveness studies have been underestimated the extrahepatic effects of ILI28,29 Studies have demonstrated that weight loss achieved through ILI can decrease the risk of CVD by 45% and reduce CVD-related mortality by 59%.33,34 It is worth noting that these cardiovascular outcomes become more prevalent and significant as individuals enter middle age. Sanyal et al. also suggests that advanced hepatic fibrosis associated with increasing extrahepatic malignancy and cardiovascular events, and surpassed liver related events. Specifically, the annual incidence of hepatocellular carcinoma in NAFLD with bridging fibrosis was reported to be 0.34%. In contrast, patients with advanced hepatic fibrosis exhibit a higher annual CVD events of 1.39%, and the annual incidence rate of extrahepatic malignancy is 1.03%.35 In our Markov model, during our simulation of major clinical events over a 30-year period in the general population in aged 40–49 years, CVD-related events was also estimated at 0.88% per year, surpassing the annual incidence of hepatocellular carcinoma, 0.11% (Supplementary Table 5).
ILI is still a cornerstone of treatment in the SLD26 However, there are concerns regarding the limited effects of ILI, as well as the short duration of its effectiveness. There is a scarcity of studies investigating the impact of ILI, specifically in SLD. A study by Vilar-Gomez et al., ILI conducted for 52 months showed that rate of fibrosis regression was 19%, and resolution of steatohepatitis was 25%.26 The sensitivity analysis of our study showed that regression rate of the ILI as the primary factor influencing ICER. In a previous study reported weight loss achieved through ILI in 30% of individuals26 In our study, we assume the hepatic effectiveness of ILI was considered as fibrosis regression rate, which was 17.0%. When the regression rate of ILI was 19% or higher, ICER was deemed cost-effective for all age groups, however, when it decreased under 14.6%, ICER rose (exceeded the threshold), resulting that screening & ILI strategy may not be cost-effective for all individuals.
There are several limitations in this study. First, the effectiveness and duration of ILI may be over-estimated. However, a study by Liu et al., found that patients who achieved significant weight loss by ILI, and it was maintained for more than 4 years34 It is challenging to maintain ILI in the long term, and studies had found many patients with initial weight loss regain weight within 2 years36. Second, the diagnosis and distribution of advanced fibrosis was not performed through liver biopsy. However, it is not ethically possible to perform the liver biopsy, therefore VCTE was used which is the most widely validated noninvasive method for diagnosing advanced liver fibrosis.
In conclusion, due to the prevalence of advanced hepatic fibrosis and the diagnostic performance of FIB-4, age is a very important factor to decide the cost-effectiveness of screening for advanced hepatic fibrosis in the general population. Prevalence of advanced hepatic fibrosis was low in aged 30–39 yeas and diagnostic performance of FIB-4 was low in aged 60–69 years in health check-up. Our study provides compelling evidence supporting the more active screening strategy for individuals aged 40–49 years during health check-up system.
Data availability
The generated datasets are available by request to the corresponding author.
Abbreviations
- CVD:
-
Cardiovascular disease
- DC:
-
Decompensated cirrhosis
- FIB-4:
-
Fibrosis-4 index
- FN:
-
False negative
- FP:
-
False positive
- HCC:
-
Hepatocellular carcinoma
- ICER:
-
Incremental cost-effectiveness ratio
- ILI:
-
Intensive lifestyle intervention
- LYs:
-
Life-years
- MASLD:
-
Metabolic dysfunction-associated steatotic liver disease
- MRE:
-
Magnetic resonance elastography
- NHANES:
-
National health and nutrition examination survey
- NIT:
-
Non-invasive test
- QALYs:
-
Quality-adjusted life-years
- TN:
-
True negative
- TP:
-
True positive
- VCTE:
-
Vibration-controlled transient elastography
- WTP:
-
Willingness-to-pay
References
Yoon, E. L. & Jun, D. W. Waiting for the changes after the adoption of steatotic liver disease. Clin. Mol. Hepatol. 29, 844 (2023).
Kalligeros, M. et al. Prevalence of steatotic liver disease (MASLD, MetALD, and ALD) in the united States: the National health and nutrition examination survey 2017–2020. Clin. Gastroenterol. Hepatology: Official Clin. Pract. J. Am. Gastroenterol.Assoc. S1542-3565(1523), 00914–X (2023).
Lee, C. M. et al. Prevalence, distribution, and hepatic fibrosis burden of the different subtypes of steatotic liver disease in primary care settings. Hepatology https://doi.org/10.1097/HEP.0000000000000664 (2023).
Caballería, L. et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin. Gastroenterol. Hepatol. 16, 1138–1145 (2018).
Kim, D., Cholankeril, G., Loomba, R. & Ahmed, A. Prevalence of fatty liver disease and fibrosis detected by transient elastography in adults in the united States, 2017–2018. Clin. Gastroenterol. Hepatol. 19, 1499–1501 (2021). e1492.
Kim, M. et al. Prevalence of advanced hepatic fibrosis and comorbidity in metabolic dysfunction-associated fatty liver disease in Korea. Liver Int. 42, 1536–1544 (2022).
Ciardullo, S., Monti, T., Grassi, G., Mancia, G. & Perseghin, G. Blood pressure, glycemic status and advanced liver fibrosis assessed by transient elastography in the general United States population. J. Hypertens. 39, 1621 (2021).
Kang, K. A., Jun, D. W., Kim, M. S., Kwon, H. J. & Nguyen, M. H. Prevalence of significant hepatic fibrosis using magnetic resonance elastography in a health check-up clinic population. Aliment. Pharmacol. Ther. 51, 388–396. https://doi.org/10.1111/apt.15626 (2020).
Le, M. H. et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the united States. PloS One. 12, e0173499 (2017).
Park, H. et al. Comparative evaluation of non-invasive tests for risk stratification for cause specific mortality in at-risk population of hepatic fibrosis. Sci. Rep. 14, 7189. https://doi.org/10.1038/s41598-024-56085-3 (2024).
Kang, S. H. et al. KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 27, 363–401. https://doi.org/10.3350/cmh.2021.0178 (2021).
Noureddin, M. et al. Screening for nonalcoholic fatty liver disease in persons with type 2 diabetes in the United States is cost-effective: A comprehensive cost-utility analysis. Gastroenterology 159, 1985–1987.https://doi.org/10.1053/j.gastro.2020.07.050 (2020).
Crossan, C. et al. Referral pathways for patients with NAFLD based on non-invasive fibrosis tests: diagnostic accuracy and cost analysis. Liver Int. 39, 2052–2060 (2019).
Park, B. H. et al. Association of participation in health check-ups with risk factors for cardiovascular diseases. J. Korean Med. Sci. 36 (2021).
Nagakura, Y., Kato, H., Asano, S., Jinno, Y. & Tanei, S. The significant association between health examination results and population health: a cross-sectional ecological study using a nation-wide health checkup database in Japan. Int. J. Environ. Res. Public. Health. 18, 836 (2021).
Thomas, J. A., Kendall, B. J., Dalais, C., Macdonald, G. A. & Thrift, A. P. Hepatocellular and extrahepatic cancers in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Cancer 173, 250–262 (2022).
Adams, L. A., Anstee, Q. M., Tilg, H. & Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 66, 1138–1153 (2017).
Younossi, Z. M., Corey, K. E. & Lim, J. K. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology 160, 912–918 (2021).
Abeysekera, K. W. et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol. Hepatol. 5, 295–305 (2020).
Kanwal, F. et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterol 161, 1657–1669 (2021).
Hsu, C. et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: A systematic review and pooled analysis of individual participants. Clin. Gastroenterol. Hepatol. 17, 630–637e638. https://doi.org/10.1016/j.cgh.2018.05.059 (2019).
McPherson, S. et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. J. Gastroenterol. 112, 740–751. https://doi.org/10.1038/ajg.2016.453 (2017).
Siddiqui, M. S. et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 17, 156–163e152. https://doi.org/10.1016/j.cgh.2018.04.043 (2019).
Park, H. et al. Cost-effectiveness study of FIB‐4 followed by transient elastography screening strategy for advanced hepatic fibrosis in a NAFLD at‐risk population. Liver Int. 44, 944–954 (2024).
Park, H. et al. Cost-effectiveness study of FIB-4 followed by transient elastography screening strategy for advanced hepatic fibrosis in a NAFLD at-risk population. Liver Int. 44, 944–954. https://doi.org/10.1111/liv.15838 (2024).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378. https://doi.org/10.1053/j.gastro.2015.04.005 (2015).
Neumann, P. J., Cohen, J. T. & Weinstein, M. C. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl. J. Med. 371, 796–797. https://doi.org/10.1056/NEJMp1405158 (2014).
Tapper, E. B., Sengupta, N., Hunink, M. G., Afdhal, N. H. & Lai, M. Cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration controlled transient elastography. Am. J. Gastroenterol. 110, 1298–1304. https://doi.org/10.1038/ajg.2015.241 (2015).
Tanajewski, L. et al. Economic evaluation of a community-based diagnostic pathway to stratify adults for non-alcoholic fatty liver disease: A Markov model informed by a feasibility study. BMJ Open. 7, e015659. https://doi.org/10.1136/bmjopen-2016-015659 (2017).
Ko, E., Yoon, E. L. & Jun, D. W. Risk factors in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 29, S79–S85. https://doi.org/10.3350/cmh.2022.0398 (2023).
Paul, J. et al. Effects of lifestyle modification on liver enzyme and fibroscan in Indian patients with non-alcoholic fatty liver disease. Gastroenterol. Rep. 6, 49–53 (2018).
Katsagoni, C. N. et al. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 120, 164–175 (2018).
Wing, R. R. et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 34, 1481–1486 (2011).
Liu, M. et al. Cardiovascular effects of intensive lifestyle intervention in adults with overweight/obesity and type 2 diabetes according to body weight time in range. EClinicalMedicine 49 (2022).
Sanyal, A. J. et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl. J. Med. 385, 1559–1569 (2021).
Hall, K. D. & Kahan, S. Maintenance of lost weight and long-term management of obesity. Med. Clin. 102, 183–197 (2018).
Author information
Authors and Affiliations
Contributions
Conceptualization: H.K., D.W.J.Data curation: M.K., H.P.Investigation: E.L.Y.Methodology: H.K., M.K., H.P., E.L.Y., D.W.J.Supervision: R.C., D.K. Writing—original draft: H.K., M.K., H.P., E.L.Y., D.W.J Writing—review & editing: D.W.J., R.C., D.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, M., Park, H., Yoon, E.L. et al. Cost-effectiveness analysis of MASLD screening using FIB-4 based two-step algorithm in the medical check-up. Sci Rep 15, 19336 (2025). https://doi.org/10.1038/s41598-025-01740-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01740-6