Abstract
Coral reefs are increasingly threatened by marine heatwaves, which drive widespread coral bleaching and mortality. Mesophotic coral ecosystems (MCEs) have been proposed as potential thermal refuges due to their greater depth and relative isolation from surface temperature extremes. Yet their resilience to extreme heat events remains uncertain, with location specific conclusions, thus requiring further studies. Here, we investigate the effects of the 2023 marine heatwave in the Cayman Islands, which resulted in prolonged sea surface temperatures exceeding 31 °C and 17.5 DHW with extensive bleaching across shallow coral reefs. Utilizing vertical transect surveys from 10 m to 50 m, we assessed depth-related variations in bleaching prevalence and temperature profiles. Our results indicate a significant decline in bleaching with increasing depth, with a concurrent reduction in temperature. Depth-generalist species exhibited reduced bleaching at greater depths, whereas shallow-water specialists displayed severe bleaching. These findings suggest that while MCEs may provide thermal refuge for some species, their capacity to buffer against climate-driven reef degradation is species-specific. Given the increasing frequency and intensity of marine heatwaves, understanding the role of deeper reef habitats in mitigating coral loss is critical for informing conservation and management strategies. Our study underscores the importance of protecting MCEs as potential thermal refuges while emphasizing the need for continued research on species-specific thermal resilience with depth.
Similar content being viewed by others
Introduction
Over the past several decades, global coral reef ecosystems have experienced a substantial decline in coral cover, with some reefs witnessing reductions from approximately 60% to less than 20%, largely as a consequence of ocean warming1,2,3,4,5,6,7. While various local stressors—such as pollution, destructive fishing practices, and coastal tourism—contribute to coral loss, thermal stress induced by marine heatwaves remains the primary driver, leading to bleaching, disease outbreaks, and diminished calcification rates5,8,9. As scleractinian corals exist near their upper thermal limits, even minor temperature elevations can disrupt their symbiotic relationship with dinoflagellates from the family Symbiodiniaceae, a phenomenon known as coral bleaching10,11,12,13,14. Sustained bleaching events can ultimately result in mortality, as many coral species depend on photosynthetic carbon translocated from their symbionts to satisfy their metabolic needs15. With continued global warming, the frequency, severity, and duration of marine heat waves and subsequent bleaching events are expected to increase5.
Beyond the extensively studied shallow coral reef zones, reef ecosystems extend into mesophotic coral ecosystems (MCEs), which occur between approximately 30 m and the lower photic limit (~ 170 m), where light availability no longer supports photosynthesis16,17,18,19,20. A global assessment of 704 scleractinian coral species revealed that while 40% are restricted to depths shallower than 20 m, the remaining 60% of species are capable of inhabiting MCEs19,21,22. Due to their greater depth and relative isolation from direct surface influences, MCEs are hypothesized to experience reduced thermal stress compared to shallow reefs17,20,23,24,25. Shallow reef temperatures are primarily governed by atmospheric heating and wind-driven processes, leading to substantial thermal accumulation and water column stratification, where prolonged thermal exposure coupled with high light levels can exacerbate extreme thermal anomalies, ultimately triggering coral bleaching26,27,28. In contrast, increasing depth generally corresponds to reduced thermal accumulation and light driven irradiance, resulting in more stable and cooler temperatures in MCEs29,30. Nevertheless, MCEs are not immune to environmental stressors. Observations of coral bleaching at mesophotic depths have been reported in both the Pacific and Caribbean31, including multiple bleaching and disease events in MCEs of the U.S. Virgin Islands32. However, recent studies indicate that deeper reefs may provide thermal refuge during extreme heat events33,34,35,36. For example, in French Polynesia, Pérez-Rosales et al.36 found that bleaching prevalence declined with increasing depth, suggesting that depth-generalist coral species across multiple genera may benefit from deeper habitats.
In 2023, the Cayman Islands experienced an unprecedented marine heatwave37, with sea surface temperatures exceeding 31 °C for several weeks38,39. This thermal anomaly corresponded to an estimated 17.5 Degree Heating Weeks (DHW), a metric representing accumulated heat stress in an area38,40,41. The prolonged thermal stress resulted in widespread coral bleaching and mortality across the shallow fore-reef zone of Little Cayman and throughout the Caribbean39,41,42. During the peak of this marine heatwave, we conducted three vertical surveys on the highly protected reefs of Little Cayman Island to monitor the impact of the bleaching event on several coral species across their depth distribution and provide documentation of thermal refuge potential in MCEs.
Results
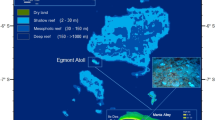
Seawater temperature at the three surveyed sites (Fig. 1) was highest at the surface (31.2 °C) and remained relatively stable between 30.8 °C and 31.0 °C from 10 m to 30 m. At 35 m, temperature declined to an average of 30.6 °C and continued to decrease by approximately 0.2 °C every 5 m, reaching a minimum of 29.9 °C at 50 m.
(a) Map of the Caribbean with Little Cayman inset showing the location of the three survey sites. The base map is from Google Earth (data sources: SIO, NOAA, U.S. Navy, NGA, GEBCO Airbus LDEO-Columbia, NSF; imagery dated 12/14/2015) and was annotated by A. Chequer using Adobe Photoshop v26.5 and Adobe Illustrator v29.4. (b) Image of Goodbody-Gringley conducting a survey on the vertical wall (photo by A. Chequer).
A GLM with a Gaussian distribution indicated covariance between temperature and depth (Supplementary Table S1). Separate GLMs for each factor showed significant effects on bleaching percentage; however, temperature yielded the lowest AIC value, indicating the best model fit (Fig. 2; Table 1).
Bleaching response across depth differed by species (Fig. 3), where significant correlations of reduced bleaching with increasing depth were found for Agaricia agaricites (R2 = 0.17, P < 0.001), Agaricia fragilis (R2 = 0.58, P < 0.001), Agaricia larmarcki (R2 = 0.66, P < 0.001), Mycetophyllia larmarckiana (R2 = 0.84, P = 0.028), Porites astreoides (R2 = 0.25, P = 0.002), Scolymia cubensis (R2 = 0.72, P < 0.001), Siderastrea siderea (R2 = 0.22, P < 0.001), and Stephanocoenia intersepta (R2 = 0.59, P < 0.001). Correlations between bleaching score and depth for other species lacked significance, possibly influenced by the low number of replicates and/or unequal sample sizes across depth for these species (Supplementary Fig. S1, P < 0.05; Supplementary Table S2).
Mean bleaching response for all species observed across depths, ranging from severely bleached in red to healthy in blue. Bleaching scores were averaged per 5 m depth bin, where bleached = −1 (red), pale = 0 (yellow), and healthy = 1 (blue). The number of colonies per species in each depth bin (n) are available in Supplementary Table S2.
Discussion
The occurrence of the extreme Caribbean heatwave in 2023 enabled opportunistic exploration of the impacts of increased temperature on coral bleaching across a vertical gradient during a naturally occurring bleaching event. Our survey results document a significant decline in coral bleaching with increasing depth or decreasing temperatures, where deeper reefs exhibited less bleaching compared to shallow reefs. This decline was found to be driven in part by temperature across the vertical gradient, suggesting that deeper reefs may serve as thermal refuges during extreme heatwave events. However, this pattern differed among species, where not all coral species expressed a uniform decrease in bleaching with increasing depth.
Mesophotic coral ecosystems (MCEs) have been proposed as potential refugia from disturbances affecting shallow-water coral reefs, including storms, sedimentation, habitat fragmentation, and rising sea surface temperatures—a concept known as the Deep Reef Refugia Hypothesis (DRRH)17,18,20,24,43,44,45,46. However, several key criteria of this hypothesis have repeatedly failed to be met, most notably the assumption of larval connectivity across depths [[e.g.,47]]. Additionally, MCEs have been documented to experience bleaching, disease, anthropogenic debris accumulation, and storm impacts31,32,48. In fact, in the Indian Ocean, bleaching was found to increase for corals below 60 m compared to shallow corals due to internal waves pushing the thermocline deeper49, suggesting that MCEs are vulnerable to temperature anomalies associated with shifting thermoclines. Yet, some studies report reduced bleaching during heatwave events, indicating that while deep reefs may not serve as universal refugia, they may provide thermal refuge for certain species in specific locations35,36,50. For example, during a moderate heatwave in French Polynesia, depth-generalist species exhibited reduced bleaching from shallow to mid-mesophotic depths (60 m), a pattern attributed primarily to changes in light-irradiance attenuation rather than temperatures, which seemed unchanging with depth36. Similarly, Baird et al.35 found a curvilinear decline in bleaching with depth, corresponding with the curvilinear attenuation of light17, suggesting that light reduction may be a key factor in mitigating bleaching at greater depths. Our model comparisons suggest that temperature, rather than depth, better explained the observed reduction in bleaching indicating that the slight thermocline at 30–35 m in the Cayman Islands during the 2023 bleaching event played a key role in moderating bleaching severity. However, since light was not measured in this study, its relative impact compared to temperature remains unknown.
While bleaching occurrence decreased significantly with increasing depth across all surveyed corals, species-specific variations were observed. Depth-generalist species, including A. fragilis, A. lamarcki, M. lamarckiana, P. astreoides, S. cubensis, S. siderea, and S. intersepta, exhibited a marked reduction in bleaching at greater depths. In contrast, MCE-specialists such as Madracis pharensis experienced minimal to no bleaching across their depth range, while corals restricted to shallow habitats, including Agaricia agaricites, Pseudodiploria strigosa, Diploria labyrinthiformis, and Porites porites, exhibited severe bleaching at all observed depths (Fig. 3; Supplementary Fig. S1). These findings align with those of Pérez-Rosales et al.36, who reported greater depth-related improvements in health status among depth-generalists than depth-specialists. However, not all depth-generalist species in the present study benefited equally. For instance, Montastraea cavernosa, Orbicella faveolata, and Madracis decactis showed no correlation between bleaching prevalence and depth. However, this lack of correlation may have been driven by low and unequal replication across depths, thereby warranting additional assessments across species depth distributions (Fig. 3; Supplementary Fig. S1; Supplementary Table S2).
Species-specific differences in thermal performance may help explain the observed variability in bleaching responses. A study on several common Caribbean coral species in Bermuda found no differences in metabolic responses to a range of temperatures among species51, yet recent research in the Pacific has identified species-specific variations in thermal performance based on photosynthetic efficiency, growth, and survival52,53. As a result, the extent to which different species will respond at molecular and organismal levels to rising temperatures across their distributions remains uncertain. However, the species-specific responses documented here during a naturally occurring heatwave suggest that depth-generalist and MCE-specialist corals may have a higher likelihood of surviving future marine heatwaves. Evidence of thermal refuge for depth generalist species is particularly important given the wide-scale mortality documented on shallow coral reefs across Little Cayman during the 2023 heatwave41. At the peak of the event, approximately 80% of corals exhibited bleaching, with 54% experiencing complete colony mortality by its conclusion. Among the most severely affected taxa were Agaricia spp., Porites porites, and Mycetophyllia spp., all of which exhibited bleaching rates exceeding 95% and high mortality. In contrast, corals of the genus Orbicella, while displaying visible bleaching, recovered following temperature declines and did not experience significant mortality. These findings underscore the role of marine heatwaves in reshaping coral reef community composition, particularly on shallow regions. As such, future coral reef community composition is likely to shift, with an increasing dominance of thermally resilient depth generalist species and a decline in species restricted to shallow reefs50.
Given that 45–54% of Atlantic shallow-water corals are currently at an elevated extinction risk54, the identification of thermal refuges is critical for sustaining coral populations. While deep reef communities have the potential to enhance shallow-reef resilience if recovery periods between MHWs are sufficient55, the extent to which these ecosystems can mitigate climate-driven coral loss remains unknown. Climate and oceanographic models suggest that most existing shallow-water thermal refuges will not persist under future warming scenarios, with few refuges at + 1.5 °C and none at + 2.0 °C above pre-industrial levels56. However, if deeper habitats continue to experience reduced thermal stress relative to shallow reefs, they may serve as long-term refuges from climate change, potentially moderating coral species losses55. The results presented here add to the growing body of evidence that MCEs can serve as thermal refuges for depth-generalist coral species. Thus, as marine heatwaves increase in frequency and intensity, the conservation and study of MCEs will be essential for informing reef management strategies, underscoring the importance of protecting MCEs as potential thermal refuges while emphasizing the need for continued research on species-specific thermal resilience.
Methods
Study site
The Cayman Islands are located 200 miles northwest of Jamaica and 150 miles south of Cuba in the Caribbean Sea and are comprised of three main islands: Grand Cayman, Cayman Brac, and Little Cayman. Little Cayman, located 80 miles northeast of Grand Cayman, is the smallest and least developed of the three islands with an area of roughly 26 km2 and a population of 160 permanent residents. Of the 45 km of shoreline, 74.2% is designated as marine protected areas with roughly 57% under full No-Take protection by the Cayman Islands Government57.
Survey methods
Three sites within the Bloody Bay Marine Protected Area considered as replicates, located on the northern side of Little Cayman Island, were surveyed on September 16, 2023: Randy’s Gazebo (19.683783; −80.082967), Marylin’s Cut (19.684733; −80.079067), and Mixing Bowl (19.684920; −80.078000). Each site features a near-vertical wall that begins at approximately 10 m and extends beyond 1,000 m in depth (Fig. 1).
At each site, a single transect was secured to the substrate at the shallowest point of the vertical wall and extended downward to a maximum depth of 50 m. Corals within 1 m on either side of the transect were identified to species level and categorized by bleaching status: “healthy” (dark in color with no visible bleaching), “pale” (lighter than conspecifics and/or exhibiting white blotching), or “bleached” (completely white).
Seawater temperature was recorded throughout each dive using HOBO ProV temperature loggers attached to the backs of two divers. Depth-specific temperature readings were determined by correlating logger timestamps with depth data from each diver’s handheld computer (Shearwater Petrel). All dives were conducted using closed-circuit rebreathers (Hollis Prism 2).
Data analyses
Temperature and bleaching status data were binned into 5 m depth intervals from < 10 m to 50 m. The effects of depth and seawater temperature on the percentage of bleached coral colonies were analyzed using Generalized Linear Models (GLMs) with a Gaussian distribution. Model assumptions were assessed using normal quantile-quantile plots, which confirmed approximate normality (Supplementary Figure S2). Due to collinearity between depth and temperature (Supplementary Table S1), separate GLMs were constructed for each variable. The best-fit model was selected using the Akaike Information Criterion (AIC)58.
To compare bleaching responses across species, a bleaching score was assigned to each coral colony: healthy = 1, pale = 0, and bleached = −1. Species-specific bleaching scores were averaged within each depth bin to facilitate visualization. Correlations of bleaching score with depth were then conducted for each species using linear regressions. The number of colonies per species at each depth is provided in Supplementary Table S2.
All statistical analyses and data visualizations were done using R version 4.2.259 and the following R packages: car v. 3.1.259,60, colorspace v. 2.1.059,60,61,62), feasts v. 0.3.263, ggcorrplot v. 0.1.4.164, ggfortify v. 0.4.1765,66, ggpmisc v. 0.5.567, hablar v. 0.3.268, janitor v. 2.2.069, knitr v. 1.4570,71,72, lme4 v. 1.1.3473, RColorBrewer v. 1.1.374, rmarkdown v. 2.2574,75,76, rstatix v. 0.7.277, tidyverse v. 2.0.078, tsibble v. 1.1.478,79, vegan v. 2.6.478,79,80. Data and code are available on Zenodo (https://doi.org/10.5281/zenodo.14889221).
Data availability
The datasets generated and/or analysed during the current study are available in the Zenodo repository, https://doi.org/10.5281/zenodo.14889221.
References
Gardner, T. A., Côté, I. M., Gill, J. A., Grant, A. & Watkinson, A. R. Long-term region-wide declines in Caribbean corals. Science 301, 958–960 (2003).
Bruno, J. F. & Selig, E. R. Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS One. 2, e711 (2007).
Schutte, V. G. W., Selig, E. R. & Bruno, J. F. Regional spatio-temporal trends in Caribbean coral reef benthic communities. Mar. Ecol. Prog Ser. 402, 115–122 (2010).
De’ath, G., Fabricius, K. E. & Sweatman, H. The 27–year decline of coral cover on the great barrier reef and its causes. Proc. Natl. Acad. Sci. U S A. 109, 17995–17999 (2012).
Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017).
Cai, C., Hammerman, N. M., Pandolfi, J. M., Duarte, C. M. & Agusti, S. Influence of global warming and industrialization on coral reefs: A 600-year record of elemental changes in the Eastern red sea. Sci. Total Environ. 914, 169984 (2024).
Edgar, G. J. et al. Continent-wide declines in shallow reef life over a decade of ocean warming. Nature 615, 858–865 (2023).
Harvell, C. D. et al. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162 (2002).
DeCarlo, T. M. et al. Mass coral mortality under local amplification of 2°C ocean warming. Sci. Rep. 7, 1–9 (2017).
Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 50, 839–866 (1999).
Jones, R. J. Coral bleaching, bleaching-induced mortality, and the adaptive significance of the bleaching response. Mar. Biol. 154, 65–80 (2008).
Bosch, T. C. G. & Miller, D. J. Bleaching as an Obvious dysbiosis in corals. Holobiont Imperative 113–125 (2016).
Brown, B., Dunne, R., Goodson, M. & Douglas, A. Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs. 21, 119–126 (2002).
Warner, M. E., Fitt, W. K. & Schmidt, G. W. Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc. Natl. Acad. Sci. U. S. A. 96, 8007–8012 (1999).
Coles, S. L. & Brown, B. E. Coral bleaching — capacity for acclimatization and adaptation. Adv. Mar. Biol. 46, 183–223 (2003).
Hinderstein, L. M. et al. Theme section on ‘mesophotic coral ecosystems: characterization, ecology, and management’. Coral Reefs. 29, 247–251 (2010).
Lesser, M. P., Slattery, M. & Leichter, J. J. Ecology of mesophotic coral reefs. J. Exp. Mar. Bio Ecol. 1–2, 1–8 (2009).
Loya, Y., Puglise, K. A. & Bridge, T. C. L. Mesophotic Coral Ecosystems (Springer International Publishing, 2019).
Rouzé, H. et al. Symbiotic associations of the deepest recorded photosynthetic scleractinian coral (172 m depth). ISME J. 15, 1564–1568 (2021).
Muir, P. R. & Pichon, M. Biodiversity of reef-building, scleractinian corals. in Coral Reefs of the World 589–620Springer International Publishing, Cham, (2019).
Carpenter, K. E. et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563 (2008).
Pérez-Rosales, G. et al. Mesophotic coral ecosystems of French Polynesia are hotspots of alpha and beta generic diversity for scleractinian assemblages. Divers. Distrib. 28, 1391–1403 (2022).
West, J. M. & Salm, R. V. Resistance and resilience to coral bleaching: implications for coral reef conservation and management. Conserv. Biol. 17, 956–967 (2003).
Kahng, S. E. et al. Community ecology of mesophotic coral reef ecosystems. Coral Reefs. 29, 255–275 (2010).
Kahng, S., Copus, J. M. & Wagner, D. Mesophotic coral ecosystems. Mar. Anim. Forests 185–206 (2016).
Wyatt, A. S. J. et al. Heat accumulation on coral reefs mitigated by internal waves. Nat. Geosci. 13, 28–34 (2020).
Wyatt, A. S. J. et al. Hidden heatwaves and severe coral bleaching linked to mesoscale eddies and thermocline dynamics. Nat. Commun. 14, 25 (2023).
Baird, A. H., Bhagooli, R., Ralph, P. J. & Takahashi, S. Coral bleaching: the role of the host. Trends Ecol. Evol. 24, 16–20 (2009).
Turner, J. A. et al. Twenty-Four key questions for mesophotic ecosystem research and conservation. in Mesophotic Coral Ecosystems (eds Loya, Y., Puglise, K. & Bridge, T.) (Springer, (2019).
Kahng, S. E. et al. Springer International Publishing, Cham,. Light, temperature, photosynthesis, heterotrophy, and the lower depth limits of mesophotic coral ecosystems. in Coral Reefs of the World 801–828 (2019).
Rocha, L. A. et al. Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361, 281–284 (2018).
Smith, T. B. et al. Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Glob Chang. Biol. 22, 2756–2765 (2016).
Baker, E. et al. Mesophotic coral ecosystems: a lifeboat for coral reefs? (2016).
Muir, P. R., Wallace, C. C., Pichon, M. & Bongaerts, P. High species richness and lineage diversity of reef corals in the mesophotic zone. Proc. Biol. Sci. 285, 20181987 (2018).
Baird, A. H. et al. A decline in bleaching suggests that depth can provide a refuge from global warming in most coral taxa. Mar. Ecol. Prog Ser. 603, 257–264 (2018).
Pérez-Rosales, G. et al. Mesophotic coral communities escape thermal coral bleaching in French Polynesia. R Soc. Open. Sci. 8, 210139 (2021).
Hobday, A. J. et al. A hierarchical approach to defining marine heatwaves. Prog Oceanogr. 141, 227–238 (2016).
NOAA Coral Reef Watch. NOAA Coral Reef Watch Version 3.1 Daily Global 5km Satellite Coral Bleaching Degree Heating Week Product. (2018).
Goreau, T. J. F. & Hayes, R. L. Record marine heat waves: coral reef bleaching hotspot maps reveal global sea surface temperature extremes, coral mortality, and ocean circulation changes. Oxf. Open. Clim. Chang. 4 https://doi.org/10.1093/oxfclm/kgae005 (2024).
Gleeson, M. & Strong, A. Applying MCSST to coral reef bleaching. Oceanogr. Lit. Rev. https://doi.org/10.1016/0273-1177(95)00396-V (1995).
Doherty, M. L., Johnson, J. V. & Goodbody-Gringley, G. Widespread coral bleaching and mass mortality during the 2023-2024 marine heatwave in Little Cayman. PLoS One. 20, e0322636 (2025).
Reimer, J. D. et al. The fourth global coral bleaching event: where do we go from here? Coral Reefs. 43, 1121–1125 (2024).
Huston, M. A. Patterns of species diversity on coral reefs. Annu. Rev. Ecol. Syst. 16, 149–177 (1985).
Hoegh-Guldberg, O. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007).
Lesser, M. P. et al. Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: light, food, and genetics. Ecology 91, 990–1003 (2010).
Bongaerts, P., Ridgway, T. & Sampayo, E. M. Hoegh-Guldberg, O. Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs. 29, 309–327 (2010).
Bongaerts, P. et al. Deep reefs are not universal refuges: reseeding potential varies among coral species. Sci. Adv. 3, e1602373 (2017).
Bongaerts, P., Muir, P., Englebert, N. & Bridge, T. C. L. Hoegh-Guldberg, O. Cyclone damage at mesophotic depths on myrmidon reef (GBR). Coral Reefs. 32, 935–935 (2013).
Diaz, C. et al. Mesophotic coral bleaching associated with changes in thermocline depth. Nat. Commun. 14, 6528 (2023).
Muir, P. R., Marshall, P. A., Abdulla, A. & Aguirre, J. D. Species identity and depth predict bleaching severity in reef-building corals: shall the deep inherit the reef? Proc. Biol. Sci. 284, (2017).
Gould, K., Bruno, J. B., Ju, R. & Goodbody-Gringley, G. Upper-mesophotic and shallow reef corals exhibit similar thermal tolerance, sensitivity and Optima. Coral Reefs. 40, 907–920 (2021).
Álvarez-Noriega, M., Marrable, I., H Noonan, S., R Barneche, D. & C Ortiz, J. Highly conserved thermal performance strategies May limit adaptive potential in corals. Proc. Biol. Sci. 290, 20221703 (2023).
García, F. C. et al. Seasonal changes in coral thermal threshold suggest species-specific strategies for coping with temperature variations. Commun. Biol. 7, 1680 (2024).
Gutierrez, L. et al. Half of Atlantic reef-building corals at elevated risk of extinction due to climate change and other threats. PLoS One. 19, e0309354 (2024).
Giraldo-Ospina, A., Kendrick, G. A. & Hovey, R. K. Depth moderates loss of marine foundation species after an extreme marine heatwave: could deep temperate reefs act as a refuge? Proc. Biol. Sci. 287, 20200709 (2020).
Dixon, A. M., Forster, P. M., Heron, S. F., Stoner, A. M. K. & Beger, M. Future loss of local-scale thermal refugia in coral reef ecosystems. PLOS Clim. 1, e0000004 (2022).
UNESCO World Heritage Centre. (2023).
Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 19, 716–723 (1974).
R Core Team. A language and environment for statistical computing. (2022).
Fox, J. & Sanford, W. An R Companion To Applied Regression (SAGE, 2019).
Zeileis, A., Hornik, K. & Murrell, P. Escaping RGBland: selecting colors for statistical graphics. Comput. Stat. Data Anal. 53, 3259–3270 (2009).
Zeileis, A. et al. Colorspace: A toolbox for manipulating and assessing colors and palettes. J. Stat. Softw. 96, (2020).
O’Hara-Wild, M., Hyndman, R. & Wang, E. feasts: Feature Extraction and Statistics for Time Series. (2024).
Kassambara, A. ggcorrplot: Visualization of a Correlation Matrix Using ‘ggplot2’. (2023).
Tang, Y., Horikoshi, M., Li, W. & Ggfortify Unified interface to visualize statistical results of popular R packages. R J. 8, 474 (2016).
Horikoshi, M. et al. ggfortify: Data Visualization Tools for Statistical Analysis Results. (2018).
Aphalo, P. J. & ggpmisc Miscellaneous extensions to ‘ggplot2’. CRAN: Contributed Packages R Foundation. https://doi.org/10.32614/cran.package.ggpmisc (2016).
Sjoberg, D. Non-Astonishing Results in R. CRAN: Contributed Packages The R Foundation (2018). https://doi.org/10.32614/cran.package.hablar
Firke, S. janitor: Simple Tools for Examining and Cleaning Dirty Data. (2023).
Xie, Y. Dynamic Documents with R and Knitr (Productivity, 2015).
Xie, Y., Allaire, J. J. & Grolemund, G. R. Markdown: The definitive guide. (2023).
Xie, Y., Dervieux, C. & Riederer, E. R Markdown Cookbook. (2024).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models Usinglme4. J. Stat. Softw. 67, (2015).
Neuwirth, E. RColorBrewer: ColorBrewer Palettes. (2022).
Xie, Y. & Knitr A comprehensive tool for reproducible research in R. in Implementing Reproducible Research 3–31 (Chapman and Hall/CRC, (2018).
Allaire, J. J. et al. Rmarkdown: Dynamic Documents for R. (2023). https://github.com/rstudio/rmarkdown
Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. (2023).
Wickham, H. et al. Welcome to the tidyverse. J. Open. Source Softw. 4, 1686 (2019).
Wang, E., Cook, D. & Hyndman, R. J. A new tidy data structure to support exploration and modeling of Temporal data. J. Comput. Graph Stat. 29, 466–478 (2020).
Oksanen, J. et al. vegan: Community Ecology Package. (2025).
Acknowledgements
The authors thank Dr. Jack Johnson and Balt von Huene for their assistance on the boat. This work was supported by the National Science Foundation (NSF #1937770), the Heising Simons Foundation, and the Darwin Plus Initiative (Ref: DPlus 162). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
GG-G and AC developed the project and conducted all data collection. GG-G analyzed the data and wrote the manuscript, AC contributed imagery, and both authors edited the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Goodbody-Gringley, G., Chequer, A.D. Mesophotic reefs offer thermal refuge to the 2023 Caribbean mass bleaching event in the Cayman Islands. Sci Rep 15, 16496 (2025). https://doi.org/10.1038/s41598-025-01813-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01813-6