Abstract
To investigate the effect of intravenous lidocaine on postoperative fatigue syndrome (POFS) in laparoscopic radical colorectal cancer surgery patients, a randomized controlled trial enrolled 86 patients aged over 18 with preoperative Christensen score ≤ 4 at Xuzhou Central Hospital from September 2023 to June 2024. The lidocaine group (group L) received an intravenous infusion of 1.5 mg·kg−1 of lidocaine for 15 min, 30 min prior to anesthetic induction, followed by sustained infusion at 1.5 mg·kg− 1·h− 1 until surgical closure. The control group (group C) received an equal volume of normal saline in the same manner. Compared with the group C, the time-weighted average (TWA) of Christensen score in the group L decreased by 0.42 (95% CI, 0.12 ~ 0.73, P < 0.05). Compared with the group C, the VAS at 1,3 and 5 days after surgery in the group L were lower (P < 0.05), the levels of IL-6 and TNF-α immediately after surgery and 24 h after surgery were lower (P < 0.05), and the time to first flatus and defecation was shorter (P < 0.05). No significant differences between the two groups in extubation time, PACU stay duration, incidence of postoperative nausea and vomiting (PONV), or length of postoperative hospital stay (P > 0.05). Results indicate that intravenous lidocaine effectively improved POFS in patients undergoing laparoscopic radical resection of colorectal cancer, which might be achieved by inhibiting the postoperative inflammatory response and reducing postoperative pain.
Similar content being viewed by others
Introduction
Colorectal cancer, a prevalent gastrointestinal malignancy, has exhibited a steadily increasing incidence across East Asia in recent years1. While laparoscopic radical resection has emerged as a cornerstone of treatment due to its minimally invasive nature, enhanced safety profile, and accelerated postoperative recovery2,3,4, postoperative complications such as postoperative fatigue syndrome (POFS) remain a persistent clinical challenge5. Characterized by debilitating fatigue, muscle weakness, sleep disturbances, cognitive impairment, and anxiety, POFS affects 34–87% of patients following abdominal surgery6,7. This syndrome not only impedes functional recovery and prolongs hospitalization but also exacerbates psychological distress, deteriorates quality of life, and undermines patient satisfaction8. Against the backdrop of widespread adoption of Enhanced Recovery After Surgery (ERAS) protocols, developing targeted strategies to mitigate POFS has become an urgent priority in surgical care.
The pathophysiology of POFS is multifactorial and incompletely understood. Emerging evidence highlights the role of systemic inflammatory responses triggered by surgical trauma, which disrupt cytokine homeostasis (e.g., elevated IL-6, TNF-α, and CRP) and induce metabolic dysregulation9,10. For instance, Liu et al.11demonstrated that ginsenoside Rb1 alleviated POFS in rats by suppressing pro-inflammatory cytokines, underscoring inflammation as a therapeutic target. Pain is another key contributor: postoperative pain amplifies stress responses, exacerbates inflammatory cascades, and disrupts neuroendocrine-immune interactions, further perpetuating fatigue12,13. A meta-analysis by Whibley et al.14 confirmed bidirectional relationships between pain, sleep disruption, and fatigue, creating a vicious cycle that hinders recovery. Current management strategies for POFS include reducing surgical trauma, using anesthetic drugs, exercise intervention, and ERAS, but these approaches often provide incomplete relief and lack mechanistic specificity8,15,16,17.
Lidocaine, an amide local anesthetic, has garnered renewed interest for its dual anti-inflammatory and analgesic mechanisms when administered intravenously18,19. Preclinical studies demonstrate its capacity to suppress neutrophil activation, downregulate pro-inflammatory cytokines (e.g., IL-6, TNF-α), and reduce the excitability of spinal dorsal horn neurons, inhibit pain sensitization20,21. Clinically, perioperative intravenous lidocaine can accelerate the recovery of gastrointestinal function, reduce the use of opioids, and alleviate postoperative pain, which is in line with the ERAS protocols22,23. The improvement effect of lidocaine intramuscular injection on the fatigue symptoms of patients has been shown in a previous study24, but there are still few reports on whether its intravenous infusion can alleviate postoperative fatigue syndrome (POFS). We hypothesize that intravenous lidocaine may alleviates POFS in laparoscopic colorectal cancer surgery patients through synergistic modulation of systemic inflammation and pain pathways. This prospective, double-blind, randomized controlled trial evaluates lidocaine versus placebo, with the primary endpoint defined as POFS severity (assessed by the TWA of Christensen score within 7 days after surgery). the secondary endpoints include postoperative inflammatory markers (serum IL-6 and TNF-α), VAS at 1, 3, 5, and 7 days after surgery, and time to first flatus and defecation.
Method
Study design and patient enrollment
This single-center, randomized, double-blind, controlled clinical trial included patients scheduled for elective laparoscopic radical colorectal cancer surgery, who were randomly assigned to the lidocaine group (Group L) or the control group (Group C). The study adhered to the principles of the Declaration of Helsinki. The trial was registered at 03/09/2023 with the Chinese Clinical Trial Registry (ChiCTR2300075372) before patient enrollment. All subjects signed an informed consent form before participating in the study. This study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines25.
A total of 86 patients who underwent elective laparoscopic radical resection of colorectal cancer at Xuzhou Central Hospital from September 2023 to June 2024 were selected. The patients were ≥ 18 years old, with ASA grades II - III, body mass index (BMI) from 18.5 to 27.9 kg·m− 2, Christensen score ≤ 4 one day before surgery, and surgery time ≤ 4 h. Patients with the following conditions will not be included in this trial: (1) impaired cognition, psychiatric/neurological disorders; (2) severe hepatic/renal insufficiency (Child-Pugh C or eGFR < 30 mL/min/1.73 m2); (3) cardiac disease (NYHA class III-IV, LVEF < 40%); (4) preoperative chemotherapy/radiotherapy within 6 months; (5) regular NSAID/opioid use (> 3 times/week); (6) lidocaine allergy; (7) inability to provide informed consent. Withdrawal criteria included: (1) severe adverse events occurred during the surgery such as cardiac arrest; (2) conversion to open surgery; (3) postoperative admission to the ICU; (4) voluntary withdrawal of the subjects.
Ethics approval
The study was approved by the Institutional Ethics Committee of Xuzhou Central Hospital (No. XZXY-LK-20230801-0119).
Randomization and blinding
A research personnel not involved in patient recruitment, coordination, data collection, or outcome assessment generated the random sequence (1:1, block sizes of 2 and 4) using the Sealed Envelope online randomization tool (https://www.sealedenvelope.com/simple-randomiser/v1/lists). The random results were concealed in sequentially numbered sealed opaque envelopes. Shortly before anesthesia induction, a researcher who was unaware of the randomization procedure opened the envelopes and assigned patients to either the group L or the group C. Afterwards, she loaded lidocaine or 0.9% sodium chloride solution into syringes of the same appearance and handed them to the anesthesiologist. The surgeons, anesthesiologist, patients, data collectors, and data analysts were all blinded to the study group assignments. On the same time (15:00 p.m.) of the 1st, 3rd, 5th, and 7th days after the surgery, the Christensen score and VAS of the patients were evaluated by two researchers who were unaware of the trial groupings.
Research protocol
All patients underwent preoperative education on Christensen and VAS scoring systems 24 h before surgery. A venous access was established under aseptic technique on upper limb. Standard monitoring included electrocardiography, non-invasive blood pressure, pulse oximetry, bispectral index (BIS), and core temperature. Invasive arterial monitoring was achieved via radial artery catheterization with local anesthesia.
Study participants were stratified into two experimental cohorts. The lidocaine intervention group received an intravenous infusion of 1.5 mg·kg−1 of lidocaine (Hebei Tiancheng Pharmaceutical Co., Ltd.) for 15 min, at 30 min prior to anesthetic induction, followed by sustained infusion at 1.5 mg·kg− 1·h− 1 until surgical closure. The control cohort underwent identical procedural protocols with volume-matched 0.9% sodium chloride solution (Shandong Qidu Pharmaceutical Co., Ltd.) administration. Total intravenous anesthesia was uniformly administered. Induction comprised propofol (1–2 mg·kg− 1, Liaoning Haisco Pharmaceutical Co., Ltd.), cisatracurium (0.15 mg·kg− 1, Zhejiang Zhenyuan Pharmaceutical Co., Ltd.), and sufentanil (0.3–0.6 µg·kg− 1, Yichang Humanwell Pharmaceutical Co., Ltd.). Orotracheal intubation followed confirmed mandibular relaxation and loss of corneal reflexes. Ventilation maintained end-tidal CO₂ at 35–45 mmHg. Maintenance utilized propofol (4–12 mg·kg− 1·h− 1) and remifentanil (0.05–2 µg·kg− 1·min− 1, Jiangsu Nhwa Pharmaceutical Co., Ltd.), titrated to sustain BIS 40–60. Hemodynamic parameters were controlled within ± 20% of baseline. Intermittent cisatracurium maintained muscle relaxation. All anesthetic agents were discontinued upon completion of dermal closure.
Postoperatively, patients received PCIA with sufentanil (0.015 µg·kg− 1·h− 1) and tropisetron (10 mg, Shandong Luoxin Pharmaceutical Co., Ltd.) in 100 mL saline: continuous infusion at 2 mL·h− 1 for 48 h, supplemented by 1.5 mL patient-controlled boluses (15-minute lockout). Rescue analgesia (intravenous flurbiprofen axetil 50 mg, Beijing Tide Pharmaceutical Co., Ltd.) was administered for VAS ≥ 4.
Outcomes
The primary outcome measure was the TWA of Christensen score at 1, 3, 5, and 7 days after surgery. The TWA of Christensen score is equal to the sum of the portion of each time interval in-between two adjacent Christensen score measurements multiplied by the average of the corresponding two Christensen score divided by the time interval between the first and the last Christensen score measurements. Compared with the scores at fixed time points, the TWA score contains information on intensity and temporal changes and is considered a more relevant indicator of clinical effects and has been applied in perioperative studies26. The Christensen score is a commonly used international method for assessing the degree of fatigue. It can better describe the subjective feelings of patients with postoperative fatigue. This scale is simple to operate and easy to use, and is currently the mainstream fatigue scoring scale27. The Christensen score28 criteria: 1–2 points, feeling normal, fatigue during excessive activity, normal sleep; 3–5 points, able to perform normal daily activities, and occasionally able to perform slightly strenuous activities; 6–8 points, only able to maintain some daily activities, strenuous when walking or climbing stairs, and sleep is required; 9–10 points, unable to perform daily activities and extremely in need of sleep.
Secondary outcome measures included VAS at 1, 3, 5, and 7 days after surgery, the level of IL-6 and TNF-α, the extubation time, the PACU stay time, the incidence of postoperative nausea and vomiting (PONV), the time to first flatus and defecation, and the postoperative hospital stay. Peripheral venous blood was drawn at 10 min before anesthesia induction(T0), immediately after surgery(T1), and 24 h after surgery(T2), and the levels of IL-6 and TNF-α were determined by ELISA kit (Shanghai Hengyuan Biotechnology Co., Ltd.).
Data collection
All patients received a standardized preoperative anesthetic assessment 24 h prior to the procedure. Baseline demographic and clinical characteristics were prospectively collected, including age, gender, BMI, ASA physical status classification, tumor TNM staging system, preoperative VAS, and preoperative Christensen score. Intraoperative parameters encompassing total surgical duration, cumulative doses of propofol, remifentanil, and cisatracurium besylate were systematically recorded during the operative period. After surgery, the level of IL − 6 and TNF – α in both groups of patients were collected, along with the extubation time, the PACU stay time, the incidence of PONV, the time to first flatus and defecation, and the length of postoperative hospital stay. The patients were followed up (including the assessment of Christensen score and VAS) by two researchers (Chunyan Zhou and Weihua LI) who were unaware of the trial groupings after surgery.
Statistical methods and sample size calculation
According to our unpublished pilot study, the TWA of the Christensen score within 7 days after surgery in the Group L was 6.10 ± 0.41, and that in the Group C was 5.70 ± 0.62. The sample size was calculated by using the Two-Sample T-Tests Allowing Unequal Variance via PASS 11.0 software (NCSS LLC., Kaysville, U.T., USA), with the test power defined as 90% and the test level as 0.05, 38 cases were needed in each group. Considering a 10% dropout rate, 43 cases were finally included in each group, with a total of 86 cases.
The Kolmogorov-Smirnov test was used to assess normality. Normally distributed continuous variables were expressed as mean ± standard deviation (SD) and compared using the two-tailed independent samples t-test. Non-normally distributed continuous variables were expressed as the median (25th-75th percentiles) and compared using the Mann-Whitney U test. Categorical variables were expressed as frequency (percentage, %) and analyzed using the χ2 test. Repeated measurement data were analyzed using repeated measures ANOVA. P < 0.05 was considered statistically significant. Statistical analyses were carried out using the SPSS 26.0 software (IBM, Armonk, NY, USA), GraphPad Prism version 10.1 for Windows (GraphPad Software, San Diego, California, USA) was utilized to generate graphs.
Results
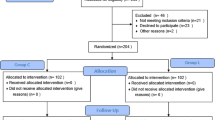
A total of 118 patients were screened between September 2023 to June 2024, of whom 32 patients were excluded. The remaining 86 patients were randomly assigned to Group L and Group C. All randomized patients underwent their surgical procedures with designated anesthesia regimens. During the postoperative follow-up period, 1 patient in Group L and 2 patients in Group C were converted to open surgery, 1 patient in Group L and 1 patient in Group C were admitted to the ICU after surgery and 1 patient in Group L voluntarily withdrawal from the study midway. Finally, 40 patients in each group were included in the analysis (Fig. 1). Clinical characteristics were comparable between the two groups, with no differences observed in the baseline data (Table 1).
The Christensen score of group L on postoperative day 1(6.88 ± 0.83 vs. 7.37 ± 0.71, P = 0.006), postoperative day 3(5.83 ± 0.69 vs. 6.24 ± 0.76, P = 0.014), postoperative day 5(4.74 ± 0.83 vs. 5.14 ± 0.79, P = 0.029), postoperative day 7(3.98 ± 0.60 vs. 4.41 ± 0.61, P = 0.002) were all significantly lower than those of group C (Fig. 2). And the TWA of the Christensen score in Group L was significantly lower than that in Group C (5.33 ± 0.69 vs. 5.76 ± 0.69, P = 0.008) (Fig. 3). Compared with Group C, the TWA of the Christensen score in Group L decreased by an average of 0.42, and the 95% CI was (0.12, 0.73).
The VAS of patients in Group L on postoperative day 1(6.26 ± 0.95 vs. 7.28 ± 0.84, P < 0.001), postoperative day 3(3.24 ± 0.60 vs. 3.73 ± 0.64, P = 0.001), postoperative day 5(2.50 ± 0.50 vs. 2.75 ± 0.43, P = 0.023) were all significantly lower than those of patients in Group C. There was no statistically significant difference in the VAS on postoperative day 7 between the two groups (1.82 ± 0.29 vs. 1.93 ± 0.39, P = 0.162). (Fig. 4)
There was no statistically significant difference in the levels of IL-6 (17.17 ± 4.24 pg·ml− 1 vs. 18.55 ± 6.59 pg·ml− 1, P = 0.269) and TNF-α (22l05 ± 5.77 pg·ml− 1 vs. 22.47 ± 4.49 pg·ml− 1, P = 0.718) at T0 between the two groups. The levels of IL-6 was lower in Group L than Group C at T1 (88.67 ± 7.39 pg·ml− 1 vs. 100.98 ± 9.36 pg·ml− 1, P < 0.001) and T2 (39.94 ± 4.12 pg·ml− 1 vs. 48.13 ± 5.07 pg·ml− 1, P < 0.001). And the levels of TNF-α was similarly lower in Group L than Group C at T1 (71.62 ± 9.83 pg·ml− 1 vs. 84.15 ± 8.22 pg·ml− 1, P < 0.001) and T2 (40.72 ± 5.81 pg·ml− 1 vs. 48.64 ± 6.81 pg·ml− 1, P < 0.001). (Table 2).
There were no significant differences between the Group L and Group C in terms of extubation time (15.30 ± 2.28 min vs. 14.83 ± 2.24 min, P = 0.35), PACU stay duration (43.60 ± 3.82 min vs. 43.90 ± 3.44 min, P = 0.713), incidence of PONV (22.5% vs. 27.5%, P = 0.606), or length of postoperative hospital stay (10.41 ± 1.02 d vs. 10.57 ± 1.13 d, P = 0.509). Additionally, the time to first flatus (28.63 ± 4.13 h vs. 30.83 ± 3.49 h, P = 0.012) and defecation (50.53 ± 4.36 h vs. 53.90 ± 6.20 h, P = 0.006) was significantly shorter in Group L than Group C (Table 3).
Discussion
This study shows that intravenous injection of lidocaine can significantly improve POFS in patients undergoing laparoscopic radical resection of colorectal cancer and has a positive effect on the inflammatory response and pain management in the early postoperative period.
POFS, as a common complication after surgery, seriously affects the postoperative rehabilitation process of patients. Its main symptoms include fatigue, muscle weakness, inattention, etc. And it is particularly common in patients undergoing abdominal surgery29,30. However, due to the complex pathogenesis of POFS, no single effective intervention measure has been found so far31. Some studies in recent years have found that the occurrence of POFS may be closely related to the postoperative inflammatory response32,33,and the inflammatory factors may aggravate the fatigue of patients by acting on the central nervous system34. Postoperative pain is another important factor that may affect the occurrence and development of POFS. Previous studies35,36 have shown that postoperative pain aggravates the symptoms of POFS and delays the recovery of patients.
Lidocaine is an amide local anesthetic, first synthesized in 1942, and its intravenous injection was initially used for anti-arrhythmia37. Postoperative formal clinical evaluations in the perioperative setting were conducted in the late 1950s where intravenous lidocaine was demonstrated to have a postoperative analgesic effect and can help patients into a rapid course of post-surgical recovery38. Since then, with an increasing number of research reports on intravenous lidocaine, it has been found that intravenous lidocaine can also play more roles, such as anti-inflammation and analgesia, thereby accelerating the perioperative recovery of patients.
The results in this study are consistent with the study by Sammour et al.39, that is, the level of postoperative inflammatory factors is positively correlated with the severity of POFS. In this study, compared with patients in Group C, the TWA of the Christensen score within 7 days after surgery in patients in Group L was significantly reduced, and the postoperative inflammatory factor levels were also significantly reduced. We speculate that intravenous injection of lidocaine may achieve the effect of reducing postoperative fatigue by inhibiting the release of inflammatory factors such as TNF-α and IL-6.
This study also found that the postoperative VAS of patients in the Group L was significantly lower than that of the Group C, and the difference was statistically significant. We observed a difference of 1.02 points in postoperative day 1 VAS scores between the two groups. Previous literature40 indicates that the conventional minimal clinically important difference (MCID) for VAS is ≥ 1 point, while another study41 suggest that VAS score differences of 0.8–1.0 points may still hold clinical significance. Therefore, we consider that intravenous lidocaine infusion improved postoperative pain at 24 h. On postoperative day 3 and day 5, the VAS score differences were 0.49 and 0.24 points. Although these values fall below the conventional MCID threshold, we found that the intervention group simultaneously exhibited reductions in rescue analgesia rates by 22.5% (POD3) and 10% (POD5), showing certain clinical significance. Considering the influence of postoperative pain on fatigue, we speculate that the good analgesic effect brought by intravenous infusion of lidocaine might indirectly reduce the sense of fatigue. In addition, lidocaine may further reduce the aggravation of fatigue caused by pain by blocking sodium channels in the dorsal root ganglion of the spinal cord and reducing the excitability of neurons42.
Moreover, the time to first flatus and defecation after surgery in the Group L was significantly shorter than that in the Group C, indicating that intravenous lidocaine helps accelerate the recovery of postoperative gastrointestinal function, which was similar to the results of previous studies43,44. However, there was no significant difference in the incidence of PONV between the two groups. Some studies22,45,46 have shown that intravenous lidocaine can reduce PONV, but the dose in those studies (mostly 2 mg·kg− 1·h− 1) was higher than that in this study (1.5 mg·kg− 1·h− 1). Moreover, lidocaine may dose-dependently reduce the risk of PONV46. This might be one of the reasons why there was no significant difference in incidence of PONV between the two groups in this study. Furthermore, PONV is more common in female patients47. But most patients in this study were male (60% in Group C and 65% in Group L), and tropisetron was used in PCIA for all. The low incidence of PONV might also explain the lack of difference.
Regarding the safety of lidocaine, the results of this study show that there was no statistically significant difference between the two groups in terms of extubation time, PACU stay time and incidence of PONV, and no significant adverse events were observed in the Group L. This is consistent with the reports in existing studies48,49, indicating that the use of intravenous lidocaine at the recommended dose is safe and does not significantly increase the risk of postoperative complications.
Therefore, intravenous lidocaine has a good application prospect in the clinical prevention and treatment of POFS.
Limitations
Firstly, this study was a single-center, small-sample study, and the external generalization of the results was limited. Secondly, the follow-up time of this study was short, only evaluating fatigue and pain within 7 days after surgery, and failing to fully explore the long-term effects of intravenous lidocaine on postoperative chronic fatigue. Furthermore, based on the research results of this trial, we speculated that intravenous lidocaine inhibiting the postoperative inflammatory response might be one of the mechanisms for improving POFS. However, we did not conduct further studies to verify this speculation. Therefore, larger-scale multicenter randomized controlled trials are needed in the future to further verify these conclusions and evaluate the potential role of intravenous lidocaine in the management of long-term POFS.
Conclusion
Intravenous lidocaine effectively improved POFS in patients undergoing laparoscopic radical resection of colorectal cancer, which might be achieved by inhibiting the postoperative inflammatory response and reducing postoperative pain.
Data availability
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request.
References
Huang, J. et al. Updated epidemiology of Gastrointestinal cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 20, 271–287. https://doi.org/10.1038/s41575-022-00726-3 (2023).
Janež, J., Korać, T., Kodre, A. R., Jelenc, F. & Ihan, A. Laparoscopically assisted colorectal surgery provides better short-term clinical and inflammatory outcomes compared to open colorectal surgery. Archives Med. Sci. 6, 1217–1226. https://doi.org/10.5114/aoms.2015.56348 (2015).
Li, Q. et al. Clinical application of enhanced recovery after surgery in perioperative period of laparoscopic colorectal Cancer surgery. J. Laparoendosc Adv. Surg. Tech. A 29, 178–183. https://doi.org/10.1089/lap.2018.0708 (2019).
Zhang, Y., Liu, C., Nistala, K. R. Y. & Chong, C. S. Open versus laparoscopic Hartmann’s procedure: A systematic review and meta-analysis. Int. J. Colorectal Dis. 37, 2421–2430. https://doi.org/10.1007/s00384-022-04285-6 (2022).
Pallan, A. et al. Postoperative complications of colorectal cancer. Clin. Radiol. 76, 896–907. https://doi.org/10.1016/j.crad.2021.06.002 (2021).
Zhao, L., Zhang, H. & Cheng, H. Effect of a single sub-dose of ketamine on postoperative fatigue syndrome in colorectal cancer patients undergoing radical laparoscopic surgery: A double-blind, pilot study. J. Affect. Disord. 312, 146–151. https://doi.org/10.1016/j.jad.2022.06.029 (2022).
Zheng, Q., Wang, R., Shi, Y. & Sun, Q. Effects of acupoint massage combined with relaxation therapy on patients with postoperative fatigue syndrome after lumbar surgery. Med. (Baltim). 100, e25849. https://doi.org/10.1097/MD.0000000000025849 (2021).
Lin, X. et al. Effects of Esketamine on postoperative fatigue syndrome in patients after laparoscopic resection of gastric carcinoma: A randomized controlled trial. BMC Anesthesiol. 24 https://doi.org/10.1186/s12871-024-02513-w (2024).
Bautmans, I., Njemini, R., De Backer, J., De Waele, E. & Mets, T. Surgery-Induced inflammation in relation to age, muscle endurance, and Self-Perceived fatigue. Journals Gerontol. Ser. A: Biol. Sci. Med. Sci. 65A, 266–273. https://doi.org/10.1093/gerona/glp145 (2009).
Himbert, C. et al. Inflammation- and angiogenesis‐related biomarkers are correlated with cancer‐related fatigue in colorectal cancer patients: Results from the ColoCare study. Eur. J. Cancer Care. 28 https://doi.org/10.1111/ecc.13055 (2019).
Liu, S. et al. Inflammation disturbed the Tryptophan catabolites in Hippocampus of Post-operative fatigue syndrome rats via indoleamine 2,3-Dioxygenas enzyme and the improvement effect of ginsenoside Rb1. Front. Neurosci. 15, 652817. https://doi.org/10.3389/fnins.2021.652817 (2021).
Zhu, G. et al. The characteristics and related factors of insomnia among postoperative patients with gastric cancer: A cross-sectional survey. Support. Care Cancer 29, 7315–7322. https://doi.org/10.1007/s00520-021-06295-6 (2021).
Kehlet, H. Enhanced postoperative recovery: Good from Afar, but Far from good? Anaesthesia 75 https://doi.org/10.1111/anae.14860 (2020).
Whibley, D. et al. Sleep and pain. Clin. J. Pain 35, 544–558. https://doi.org/10.1097/ajp.0000000000000697 (2019).
Yu, J. et al. Risk factors for postoperative fatigue after Gastrointestinal surgery. J. Surg. Res. 194, 114–119. https://doi.org/10.1016/j.jss.2014.09.041 (2015).
Machado, P. et al. Effect of exercise training on quality of life after colorectal and lung cancer surgery: A meta-analysis. Cancers 13 https://doi.org/10.3390/cancers13194975 (2021).
Bartlett, E. L., Zavlin, D., Friedman, J. D., Abdollahi, A. & Rappaport, N. H. Enhanced recovery after surgery: The plastic surgery paradigm shift. Aesthetic Surg. J. 38, 676–685. https://doi.org/10.1093/asj/sjx217 (2018).
Castro, I., Carvalho, P., Vale, N., Monjardino, T. & Mourão, J. Systemic Anti-Inflammatory effects of intravenous Lidocaine in surgical patients: A systematic review and Meta-Analysis. J. Clin. Med. 12 https://doi.org/10.3390/jcm12113772 (2023).
Licina, A. & Silvers, A. Perioperative intravenous Lidocaine infusion for postoperative analgesia in patients undergoing surgery of the spine: Systematic review and Meta-Analysis. Pain Med. (Malden Mass) 23, 45–56. https://doi.org/10.1093/pm/pnab210 (2022).
Hollmann, M. W. & Durieux, M. E. Local anesthetics and the inflammatory response: A new therapeutic indication? Anesthesiology 93, 858–875, (2000). https://doi.org/10.1097/00000542-200009000-00038
Kurabe, M., Furue, H. & Kohno, T. Intravenous administration of Lidocaine directly acts on spinal dorsal Horn and produces analgesic effect: An in vivo patch-clamp analysis. Sci. Rep. 6, 26253. https://doi.org/10.1038/srep26253 (2016).
Wei, S., Yu-Han, Z., Wei-Wei, J. & Hai, Y. The effects of intravenous Lidocaine on wound pain and Gastrointestinal function recovery after laparoscopic colorectal surgery. Int. Wound J. 17, 351–362. https://doi.org/10.1111/iwj.13279 (2020).
Li, J., Wang, G., Xu, W., Ding, M. & Yu, W. Efficacy of intravenous Lidocaine on pain relief in patients undergoing laparoscopic cholecystectomy: A meta-analysis from randomized controlled trials. Int. J. Surg. (London England). 50, 137–145. https://doi.org/10.1016/j.ijsu.2018.01.001 (2018).
Staud, R., Kizer, T. & Robinson, M. E. Muscle injections with Lidocaine improve resting fatigue and pain in patients with chronic fatigue syndrome. J. Pain Res. 10, 1477–1486. https://doi.org/10.2147/jpr.S139466 (2017).
Schulz, K. F., Altman, D. G. & Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ (Clinical Res. ed.). 340, c332. https://doi.org/10.1136/bmj.c332 (2010).
Ma, J. H. et al. Effect of acute pain on the association between preoperative cognitive impairment and postoperative delirium: A secondary analysis of three trials. Br. J. Anaesth. 130, e272–e280. https://doi.org/10.1016/j.bja.2022.06.033 (2023).
Nostdahl, T., Bernklev, T., Fredheim, O. M., Paddison, J. S. & Raeder, J. Defining the cut-off point of clinically significant postoperative fatigue in three common fatigue scales. Qual. Life Res. 28, 991–1003. https://doi.org/10.1007/s11136-018-2068-0 (2019).
Christensen, T., Bendix, T. & Kehlet, H. Fatigue and cardiorespiratory function following abdominal surgery. Br. J. Surg. 69, 417–419. https://doi.org/10.1002/bjs.1800690721 (1982).
Lin, X. H. et al. Fatigue and its associated factors in liver transplant recipients in Beijing: A cross-sectional study. BMJ Open. 7 https://doi.org/10.1136/bmjopen-2016-011840 (2017).
Xu, X. Y. et al. Risk factors and the utility of three different kinds of prediction models for postoperative fatigue after Gastrointestinal tumor surgery. Supportive Care Cancer: Official J. Multinational Association Supportive Care Cancer. 29, 203–211. https://doi.org/10.1007/s00520-020-05483-0 (2021).
Lin, X. et al. Effects of Esketamine on postoperative fatigue syndrome in patients after laparoscopic resection of gastric carcinoma: a randomized controlled trial. BMC Anesthesiol. 24, 185. https://doi.org/10.1186/s12871-024-02513-w (2024).
Chen, W. Z. et al. Prevention of postoperative fatigue syndrome in rat model by ginsenoside Rb1 via down-regulation of inflammation along the NMDA receptor pathway in the hippocampus. Biol. Pharm. Bull. 38, 239–247. https://doi.org/10.1248/bpb.b14-00599 (2015).
Mo, Y., Wang, C., Yin, D. & Li, F. Efficacy of dexamethasone in reducing pain and inflammation and accelerating total hip arthroplasty postoperative recovery: A randomized controlled trial. J. Perianesthesia Nursing: Official J. Am. Soc. PeriAnesthesia Nurses. 39, 589–595. https://doi.org/10.1016/j.jopan.2023.10.022 (2024).
Wang, X. S. et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with Gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav. Immun. 26, 699–705. https://doi.org/10.1016/j.bbi.2011.12.007 (2012).
Hodges, A. et al. Prevalence and determinants of fatigue following total knee replacement: A longitudinal cohort study. Arthritis Care Res. (Hoboken). 68, 1434–1442. https://doi.org/10.1002/acr.22861 (2016).
Mendy, N. et al. Postoperative fatigue after day surgery: prevalence and risk factors. A prospective observational study. Minerva Anestesiol. 86, 1269–1276. https://doi.org/10.23736/S0375-9393.20.14358-X (2020).
Weinberg, L. Pharmacokinetics and pharmacodynamics of Lignocaine: A review. World J. Anesthesiol. 4 https://doi.org/10.5313/wja.v4.i2.17 (2015).
Beaussier, M., Delbos, A., Maurice-Szamburski, A., Ecoffey, C. & Mercadal, L. Perioperative use of intravenous Lidocaine. Drugs 78, 1229–1246. https://doi.org/10.1007/s40265-018-0955-x (2018).
Sammour, T., Kahokehr, A., Chan, S., Booth, R. J. & Hill, A. G. The humoral response after laparoscopic versus open colorectal surgery: A meta-analysis. J. Surg. Res. 164, 28–37. https://doi.org/10.1016/j.jss.2010.05.046 (2010).
Huang, K., Giddins, G. & Wu, L. D. Platelet-Rich plasma versus corticosteroid injections in the management of elbow epicondylitis and plantar fasciitis: An updated systematic review and Meta-analysis. Am. J. Sports Med. 48, 2572–2585. https://doi.org/10.1177/0363546519888450 (2020).
Webster, L. R., Johnson, F. K., Stauffer, J., Setnik, B. & Ciric, S. Impact of intravenous Naltrexone on intravenous morphine-induced high, drug liking, and euphoric effects in experienced, nondependent male opioid users. Drugs R&D. 11, 259–275. https://doi.org/10.2165/11593390-000000000-00000 (2011).
Hermanns, H. et al. Molecular mechanisms of action of systemic Lidocaine in acute and chronic pain: A narrative review. Br. J. Anaesth. 123, 335–349. https://doi.org/10.1016/j.bja.2019.06.014 (2019).
Song, X., Sun, Y., Zhang, X., Li, T. & Yang, B. Effect of perioperative intravenous Lidocaine infusion on postoperative recovery following laparoscopic Cholecystectomy-A randomized controlled trial. Int. J. Surg. (London England). 45, 8–13. https://doi.org/10.1016/j.ijsu.2017.07.042 (2017).
Cooke, C. et al. Meta-analysis of the effect of perioperative intravenous Lidocaine on return of Gastrointestinal function after colorectal surgery. Tech. Coloproctol. 23, 15–24. https://doi.org/10.1007/s10151-019-1927-1 (2019).
Wang, T., Liu, H., Sun, J. H., Wang, L. & Zhang, J. Y. Efficacy of intravenous Lidocaine in improving post-operative nausea, vomiting and early recovery after laparoscopic gynaecological surgery. Experimental Therapeutic Med. 17, 4723–4729. https://doi.org/10.3892/etm.2019.7497 (2019).
Hu, Y. et al. Lidocaine and risk of postoperative vomiting in children undergoing tonsillectomy: A randomised clinical trial. Sci. Rep. 14, 19752. https://doi.org/10.1038/s41598-024-70804-w (2024).
Apfel, C. C. et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 109, 742–753. https://doi.org/10.1093/bja/aes276 (2012).
Marret, E., Rolin, M., Beaussier, M. & Bonnet, F. Meta-analysis of intravenous Lidocaine and postoperative recovery after abdominal surgery. Br. J. Surg. 95, 1331–1338. https://doi.org/10.1002/bjs.6375 (2008).
Foo, I. et al. The use of intravenous Lidocaine for postoperative pain and recovery: International consensus statement on efficacy and safety. Anaesthesia 76, 238–250. https://doi.org/10.1111/anae.15270 (2021).
Acknowledgements
The authors thank all the participants enrolled into in this study and the researchers involved in the study design, recruitment of patients, treatment, and data collection.
Funding
This work was supported by the Xuzhou Medical Key Talent Training Project (No. XWRCHT20210033), grant (Dr. Liwei Wang), Jiangsu Province’s Key Discipline / Laboratory of Medicine (No. JSDW202231), grant (Dr. Liwei Wang) and the Key Research and Development Program of Scientific and Technological Innovation in Xuzhou City (No. KC23146), grant (Dr. Liwei Wang).
Author information
Authors and Affiliations
Contributions
Fan Conghai , Wang Liwei and Sun Jia: have contributed equally to this work and share senior authorship; Guo Songhai, Sun Bin and Wang Xinghe: are co-first authors and contributed equally to this work. All authors contributed to the manuscript and approved the final version. Wang Liwei: obtained funding; Guo Songhai, Sun Bin and Wang Xinghe: drafting manuscript and statistical analysis; Li Weihua and Zhou Chunyan: acquisition, analysis, or interpretation of data; Fan Conghai , Wang Liwei and Sun Jia: critical revision of the manuscript for important intellectual content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, S., Sun, B., Wang, X. et al. Effect of intravenous lidocaine on postoperative fatigue syndrome in patients undergoing laparoscopic radical colorectal cancer surgery: a randomized clinical trial. Sci Rep 15, 18146 (2025). https://doi.org/10.1038/s41598-025-01892-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01892-5