Abstract
Based on particle swarm optimization algorithm and first-principle calculations, the most stable structures of 10 different PuTMO3 systems are predicted according to their thermodynamical, mechanical and dynamical properties. The results indicate firstly all selected 50 structures are thermodynamically stable based on formation enthalpy results. 29 of the 50 structures are then predicted to be mechanically stable according to mechanical stability criterion for different structures. And then 10 of 29 structures are selected to be dynamically stable based on calculations of phonon frequency, that is, PuTiO3(Cc, Pca2_1), PuZnO3(Pca2_1, P2_1/m), PuGaO3(C2/m), PuMnO3(Pna2_1), PuNiO3(P4_1), PuFeO3(C222_1), PuVO3(P2_13), and PuCrO3(P2_13). Finally, the electronic properties of these 10 structures were calculated. All these results provide useful information to manufacture Pu-based MOX fuels by controlling impurities through formation of PuTMO3 compounds and the recycling of materials in recovery process.
Similar content being viewed by others
Introduction
Plutonium (Pu), which is in the special transition position between light actinides (with delocalized 5f electrons) and heavy actinides (with localized 5f electrons), has many unique properties. For example, it’s 5f electrons are located between the localized state and the delocalized state, which can cause strong correlation effects due to unfilled 5f electronic shell. Pu-based materials are confirmed to be extremely chemically active and susceptible to chemical corrosion in air1. The α-decay of 239Pu may generate energetic2 He and 235U nuclei in the normal Pu lattice. Furthermore, the self-irradiation effect of 239Pu produces displacement cascade processes resulting in formation of vacancies and interstitial atoms. Thus, the 3D He bubbles are generally observed in Pu after a long time service3. According to previous studies, Pu can exist in different phases. The low-temperature α-phase of Pu metal has low ductility and poor machinability while the high-temperature δ-phase Pu can be in metastable state at room temperature by doping with trivalent elements (Ga, Am, Al, etc.), which can be decomposed by eutectoid decomposition into ordered intermetallic compounds, such as α’ + Pu3Ga4. In addition, the phase stability of Pu-based materials is extremely sensitive to temperature, pressure, chemical composition, and time (self-irradiation effects) and never reaches a true equilibrium state. Therefore, Pu metals, alloys, and compounds exhibit many exotic properties2. To understand these properties becomes hot topics in field of Pu-based materials. Among all these materials, the mixed uranium and plutonium oxide fuel (MOX) is the most important one because of its key role for plutonium recycling and improving the utilization of uranium resources. The use of Pu-based mixed oxide (MOX) fuels, compared to traditional UO2-based materials, offers significant advantages in enhancing the sustainability and efficiency of nuclear energy systems5. By recycling plutonium derived from spent nuclear fuel, MOX fuels reduce the accumulation of high-level radioactive waste and decrease reliance on limited natural uranium resources. The higher neutron capture cross-section of 239Pu in MOX fuels enables greater fuel burnup, extending operational cycles and improving economic efficiency. Additionally, Pu-based fuels are essential for fast neutron reactors, where they support the breeding of fissile material, promoting a closed nuclear fuel cycle. The compatibility of PuO2 with UO2 ensures stable performance under extreme reactor conditions, while the utilization of plutonium in civilian reactors contributes to nuclear non-proliferation efforts by reducing stockpiles of weapons-grade material. These benefits underscore the importance of Pu-based MOX fuels in advancing sustainable nuclear energy.
In fact, during the processing, production and storage of MOX fuels, the transition metal elements such as Ga, Fe, V, Cr, Ni, etc., are inevitably introduced6,7. The doping of transition metal elements affects the performance of MOX fuels in most cases, but there are some impurity elements that have been found to be beneficial7. Therefore, it is particularly important to study the states of these impurities in MOX fuels doped with transition metal elements. Perovskite-type structure of Pu-TM-O, as an important class of plutonium-based compounds, has been confirmed to be the dominated structure and plays a key role in the recycling of weapon-grade plutonium and plutonium in the nuclear reactor fuels (MOX), which can be used to remove the effects of impurity elements on the fuels8,9. Thus, considering the difficulty to perform experiments, to explore the possible stable state of these structures and the related basic physical properties of Pu-TM-O systems formed by plutonium oxides and impurity elements is of great theoretical significance and application value for improving the performance quality of MOX fuels as well as the efficiency and lifetime of nuclear reactors.
In literature, the research about Pu-TM-O systems mainly focuses on the following aspects. Russell et al. confirmed experimentally the existence of perovskite-type compounds in plutonium, which contains PuAlO3, PuVO3, PuCrO3, PuMnO3 and PuBaO310. Dar, Tanaka and Morss et al. investigated the fundamental physical properties of PuBaO3 experimentally and theoretically11,12,13. In experiments, they measured the enthalpy of formation of PuBaO3 and analyzed the associated stability; they also determined the thermal conductivity from 300 to 1500 K, noting that the thermal conductivity of PuBaO3 is similar to that of BaUO3. By first-principles calculations, the authors noted that PuBaO3 has a large magnetic moment (4μB) and exhibits half-metallic nature. The elastic and mechanical properties have also been predicted. Moreover, they calculated thermodynamic properties like Debye temperature, specific heat, entropy, etc. about BaPuO3. Fulalrton et al. predicted that PuCrO3 and PuAlO3 structures may be formed in nuclear fuels. The lowest energy of both compounds is found to be an orthorhombic (Pnma) GdFeO3-type perovskite structure. Relevant properties such as thermal conductivity and defects were calculated14. Relative to UO2, the thermal conductivity of PuCrO3 was calculated to be approximately three times smaller over the explored temperature range and the PuAlO3 phase had a similar thermal conductivity to UO2. Calculated defect energies suggest that defects in both PuCrO3 and PuAlO3 will be dominated by antisite defects and that their radiation tolerances are similar to that of UO2. Vigier et al. experimentally obtained the PuAlO3 (Imma) orthorhombic perovskite configuration and investigated its phase transition behavior from Imma to R \(\stackrel{\text{-}}{3}\) c between 473 and 573 K and the extrapolation of the data suggested that the plutonium cubic perovskite should become stable around 1850 K (R \(\stackrel{\text{-}}{3}\) c → Pm \(\stackrel{\text{-}}{3}\) m transition)15. The doping behavior of transition metal elements in PuO2 was investigated by Ao et al.7. They also pointed out that the adoption of transition metal elements may form a Pu-TM-O ternary phase with PuO2, e.g. PuScO3, PuYO3 and PuLaO3. Stan et al. predicted the stability of perovskite compounds in the Pu–Ga–O system9. They reported that the perovskite structures cannot be obtained in normal conditions of atmospheric oxygen pressure and the stability limits of PuGaO3 were also given to be 715 −1250 K and 1173 −1825 K for oxygen partial pressures at 10–10 atm and 10–20 atm respectively. A similar conclusion was given by Kolman et al.: PuGaO3 can be fabricated in a reducing atmosphere16. Li et al. reported the phase stability, chemical bonding, lattice dynamics, and thermodynamic properties of PuGaO317. Phase stability analyses, including total energy, formation enthalpy, mechanical stability, and lattice dynamics, indicate that the ground-state structure of PuGaO3 at ambient pressure belongs to the space group Pnma. In the experiment, Sobolev et al. gave the conclusion that PuTiO3 may be present in the research of “Phase Composition of Murataite Ceramics for Excess Weapons Plutonium Immobilization”18. In addition, for PuFeO3 and PuNiO3 structures, Balachandran et al. combined machine learning and DFT calculations to predict the possibility of their existence19. Additional work has focused on Pu–Ga alloys20,21,22,23,24,25,26,27 and research on oxygen-containing systems of Pu, mostly on Pu2O3, PuO2 and Pu–Ga-O systems9,17,28,29,30,31,32,33. From these results, it can be seen that the mixing of impurity elements with Pu oxides may form a variety of Pu-TM-O structures. However, considering the complexity of these structures, numerous structures for each a Pu-TM-O system can be possibly formed, whose stabilities should be investigated to find the most stable structure. The results are expected to be useful for development of nuclear energy and the effective use of Pu materials.
To answer the above question, the stabilities of numerous structures of different Pu-TM-O systems are explored in this work through the particle swarm optimization algorithm. The first principles calculations are used to calculate the energy, mechanical and dynamic properties, to find the most stable structures for different systems. The methodology is provided in Sect. “Methodology”. Results and discussions are given in Sect. “Results and discussion”. Conclusion is made in the last section.
Methodology
In this study, the CALYPSO structure prediction program34,35 is mainly used to search the ground and metastable states of perovskite structures of Pu-TM-O systems. In this work, TM contains 10 elements, namely, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, and Ga. In CALYPSO, the particle swarm optimization algorithm has been applied, which ensures that the searching can be performed for cases with large potential energy barriers over the entire energy surface. It also has a fast convergence rate, ensuring comprehensive and efficient structure prediction, as verified by various studies36,37,38,39,40,41. In this work, for each Pu-TM-O system, in the 1st step, 5 structures with the lowest energies are identified from numerous initial states by CALYPSO program. Further optimization is then performed for these 5 structures to find the most stable state.
The stability of selected crystal structures was analyzed based on the enthalpy of formation, elastic constants and phonon properties. In this process, the unstable structures were gradually eliminated, resulting in the thermodynamically, mechanically and dynamically stable structures for each system. For the above purpose, the first principles calculations were performed by the Vienna ab initio simulation package (VASP42) based on the projector-augmented wave (PAW) method43,44. The exchange–correlation potential employed the PBE pseudopotential in the framework of generalized gradient approximation (GGA)45. For the Brillouin zone, a k-point mesh centered at the Γ point was generated using the Monkhorst–Pack method46. All atoms were fully relaxed until the Hellmann–Feynman forces on each atom converged to below 0.01 eV Å−1 and the energy tolerance was less than 10–6 eV/atom. Electron wave function was expanded in plane waves up to a cutoff energy of 520 eV and dense k-point grids with a spacing of 2π × 0.03 Å−1 were chosen. Previous studies have shown that in order to correctly describe the f electrons of Pu, it is necessary to use the strong on-site Coulomb repulsion, which was set as Ueff = 4 eV in this work17. In the calculation of elastic constants, the strain–stress method47 was used to calculate the matrix of elastic constants, which would be used to show the mechanical stability of the materials. All these elastic properties were calculated with the VASPKIT package48. The phonon dispersion calculations were realized by using the PHONOPY code with the finite displacement method49. The elastic constants and phonon dispersion calculations were performed at 0 K without external pressure. The crystal structures were illustrated by the VESTA package50.
Results and discussion
Thermodynamical stability
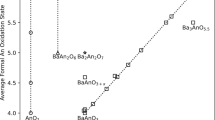
We know that for a system, the compositional phase diagram (or convex hull) can determine whether a compound of a particular composition is in a thermodynamically stable state or not at 0 K. Upon investigation, it was found that for these ten systems, the Open Quantum Materials Database (OQMD) website51,52 has listed their computationally obtained corresponding compositional phase diagrams. Calculations show that all PuTMO3 systems can be thermodynamically stabilized, and the compositional phase diagrams of Pu-V–O and Pu-Cr–O in the website are given as examples in Fig. 1. Since previous work has already been done, we will not repeat the computational work of compositional phase diagrams. However, for all the predicted PuTMO3 systems, we calculated the enthalpy of formation to further compare and judge the thermodynamic stability of all the structures. The enthalpy of formation can be calculated by the following synthetic equation according to the basic thermodynamic theory
In this reaction route, the enthalpy of formation of PuTMO3 is
where Hx is enthalpy of x which can be calculated based on DFT calculations. Table 1 lists the enthalpy of formation and volume for the structures predicted in this paper. In addition to the 5 lowest energy structures predicted by CALYPSO in this work, some results containing the same chemical composition provided by the Materials Project (hereafter MP website)53 are also included in Table 1, which are labeled with “MP”.
The results of the calculations indicate that the 5 lowest energy structures predicted by CALYPSO are all negative and exhibit thermodynamic stability. Furthermore, when a result from Materials Project is included, the energy value from the present calculation is very close to this value by using the same crystal structure. For each system, there is still a minimum energy state in these 5 structures. For example, the minimum energy states of PuCoO3, PuFeO3, PuMnO3, PuZnO3, PuTiO3, PuVO3, PuCrO3, PuNiO3, PuCuO3, PuGaO3 are Pnma, Pmm2, Pna2 \(\stackrel{\text{-}}{1}\), Pca2 \(\stackrel{\text{-}}{1}\), Cc, Cmcm, Pnma, P2 \(\stackrel{\text{-}}{1}\) 3, C2/c and Pca2 \(\stackrel{\text{-}}{1}\), respectively.
Mechanical stability
Mechanical stability is also a key factor for evaluating the stable state of a material54. Generally, the elastic constants (Cijkl) are firstly used for the above purpose. The elastic constants can be calculated as follows:
where E and V are the total energy and volume of a system. σij and εij are strain and stress applied on this system defined by i and j directions. According to definition of tensor contraction, Cijkl can be described by Cmn by following contraction of tensors.
The 1st criterion of mechanical stability evaluated by elastic constant is that all values in a matrix of elastic constant should be positive. Secondly, for different crystal structures, according to the Born stability criterion55, there some special requirements for evaluation of mechanical stability of a material, as derived in Ref.56.
For example, the space group P4 \(\stackrel{\text{-}}{1}\) with the tetragonal crystal structure has 7 independent elastic constants and the mechanical stability criteria include:
The space group Pca2 \(\stackrel{\text{-}}{1}\) with the orthorhombic crystal structure has 9 independent elastic constants and the mechanical stability criteria include:
The space group P2 \(\stackrel{\text{-}}{1}\) 3 with the cubic crystal structure has 3 independent elastic constants and the mechanical stability criteria include:
Considering numerous structures listed in Table 1, the corresponding stability criteria would not be presented in detail here, which can be found in Ref.56. According to the above criteria, the predicted mechanical stability is listed in Table 2. It can be found from this table that 29 of the 50 structures predicted in this paper exhibit mechanical stability. Furthermore, it is clear that although PuCoO3(Pnma), PuNiO3(P2 \(\stackrel{\text{-}}{1}\) 3), and PuGaO3(Pca2 \(\stackrel{\text{-}}{1}\)), have the lowest enthalpy of formation, they are mechanically unstable. In the other 7 systems, the structures with the lowest formation enthalpy exhibit the related mechanical stability. At the same time, all structures in MP website are mechanically stable.
Dynamical stability
It is well known that phonon dispersion reflects the dynamical stability of a material. When all the phonon frequencies are positive, the material is dynamically stable57. Among the 10 predicted systems, we calculate phonon dispersion for 29 mechanically stabile structures. The results without negative frequencies are shown in Fig. 2. It is clear that there are 10 structures exhibit dynamical stability: PuTiO3(Cc, Pca2 \(\stackrel{\text{-}}{1}\)), PuZnO3(Pca2 \(\stackrel{\text{-}}{1}\), P2 \(\stackrel{\text{-}}{1}\)/m), PuGaO3(C2/m), PuMnO3(Pna2 \(\stackrel{\text{-}}{1}\)), PuNiO3(P4 \(\stackrel{\text{-}}{1}\)), PuFeO3(C222 \(\stackrel{\text{-}}{1}\)), PuVO3(P2 \(\stackrel{\text{-}}{1}\) 3), and PuCrO3(P2 \(\stackrel{\text{-}}{1}\) 3). However, no structure with dynamical stability was found for PuCuO3 and PuCoO3 systems. Furthermore, we calculated the phonon frequency of 6 structures suggested in MP website but all of them are dynamically unstable.
Electronic properties
In order to further understand the ground state of the newly predicted crystal structure, the electronic properties were calculated. The band structure, densities of states (DOS) and partial densities of states (PDOS) of PuTMO3 were calculated by using GGA + U, as shown in Fig. 3. The Fermi level εF was set to 0 eV, separating the conduction and valance band. The electronic properties suggest that PuTiO3(Pca2 \(\stackrel{\text{-}}{1}\)), PuFeO3(C222 \(\stackrel{\text{-}}{1}\)), PuMnO3(Pna2 \(\stackrel{\text{-}}{1}\)), PuCrO3(P2 \(\stackrel{\text{-}}{1}\) 3), and PuVO3(P2 \(\stackrel{\text{-}}{1}\) 3) are metallic nature, PuTiO3(Cc), PuZnO3(Pca2 \(\stackrel{\text{-}}{1}\)), PuZnO3(P2 \(\stackrel{\text{-}}{1}\)/m) and PuGaO3(C2/m) are indirect band gap semiconductor, the PuNiO3(P4 \(\stackrel{\text{-}}{1}\)) shows semimetallic nature. In partial densities of states diagram, it can be observed that the contribution of O atoms is mainly distributed in the deep level and the contribution of the Pu-6d orbital is almost absent. In metallic structures, the hybridization between Pu-5f and TM are strong. In semiconductor structures, the states closest to the conduction bands (CBs) are contributed by the 5f orbitals of the Pu atom. In the same time, in all of these structures, the hybridization between Pu atoms and O atoms are weak. This means58 that more ionic character exists in the Pu–O bonds.
Additionally, we calculated the DDEC charge59 to study the charge transfer between Pu, TM and O atoms. We must stress that the DDEC charge does not equal the ultimate charge of the atoms but provides a reasonable estimate. From the DDEC charge analysis results of PuTMO3, we can see that only the O atoms have a negative charge, which indicates that there is net charge transfer from the Pu(TM)atoms to the O atoms. The results are shown in Fig. 4. The electronegativity60,61 of the TM elements is also made in the figure. It can be seen that the charge transferred by the O atom is around −1|e|, by the Pu atoms is around + 2|e|, and the TM atoms transfer a charge ranging from + 0.6–1.8|e|. A larger charge transfer of Pu than TM in Pu TMO3 suggests that ionic interactions in Pu–O bonds are stronger than those in TM–O bonds. As the electronegativity of the TM element increases, the TM atom receives less charge and the Pu atom receives more charge.
To gain detailed insights into the interactions of Pu, TM and O atoms, we plot the 3D charge-density maps in Fig. 5, which the value of isosurface is set to 0.25 e/A3. As can be seen from the figures, the charge densities between Pu–O, TM-O and Pu-TM are low for all PuTMO3 structures, while the higher density region corresponds to the core electrons. This suggests that most of the electrons are firmly bound around the atomic nuclei and only a few valence electrons can escape from their bondage. Thus, according to the current charge density study, we came to the conclusion that the Pu–O bond and TM–O bond in PuTMO3 mainly exhibit ionic character.
Discussion
In this study, the stability assessment of the predicted structure integrates thermodynamic, mechanical, and dynamical perspectives. For thermodynamic stability, the enthalpy of formation for all structures was calculated; negative values indicate that all structures can exist stably from a thermodynamic perspective. Regarding mechanical stability, the elastic constants of all structures were computed, and the Born stability criterion was applied to verify mechanical stability, leading to the elimination of some mechanically unstable structures. For dynamical stability, the phonon dispersion curves of structures satisfying both thermodynamic and mechanical stability conditions were calculated, with some dynamically unstable structures subsequently removed. Finally, we analyze the electronic properties of these stable structures.
Temperature and pressure are 0 K and 0 GPa respectively for the above calculations in this paper. Therefore, when these results are validated by experimental results, these factors should be considered. For example, the available experimental results for PuVO3, PuCrO3 and PuMnO3 systems were reported more than 60 years ago10, different to the present predictions. There are two possible reasons: the 1st one is temperature and pressure for experiments, which may lead to phase transition due to the increase of temperature and pressure14,15. The 2nd reason may be from uncertainty induced by the experimental conditions at that time, which has been proved by other predictions12. Therefore, based on all the above results, the structures with the lowest enthalpy of formation in each system that exhibits stable properties thermodynamically, mechanically and dynamically are obtained, whose structures are shown in Fig. 6. The related crystallographic data are listed in Table 3. And the lattice constants and formation enthalpy of PuVO3, PuGaO3, PuCrO3 and PuMnO3 from DFT/experiments are listed in Table 4. The enthalpy of formation was calculated under the computational conditions of this study. It can be seen that for PuVO3, PuGaO3 and PuCrO3 the enthalpy of formation of the structure is lower in DFT/experiment but for PuMnO3 the enthalpy of formation predicted in this study is lower.
Conclusion
In this paper, 10 different PuTMO3 systems are predicted based on the particle swarm optimization algorithm applied in structure prediction program CALYPSO. 5 structures with the lower energies are selected firstly for each system, that is, 50 structures for all systems. The first principles calculation is then used to evaluate the stability of the selected 50 structures in terms of thermodynamics, mechanics and dynamics. Firstly, all 50 structures exhibit thermodynamic stability as the formation enthalpy is negative. Secondly, according to Born stability criterion, 29 of the 50 structures are predicted to be mechanical stable. Thirdly, calculations of phonon frequency of the left 29 structures indicate there are 10 structures are dynamically stable: PuTiO3(Cc, Pca2 \(\stackrel{\text{-}}{1}\)), PuZnO3(Pca2 \(\stackrel{\text{-}}{1}\), P2 \(\stackrel{\text{-}}{1}\)/m), PuGaO3(C2/m), PuMnO3(Pna2 \(\stackrel{\text{-}}{1}\)), PuNiO3(P4 \(\stackrel{\text{-}}{1}\)), PuFeO3(C222 \(\stackrel{\text{-}}{1}\)), PuVO3(P2 \(\stackrel{\text{-}}{1}\) 3), and PuCrO3(P2 \(\stackrel{\text{-}}{1}\) 3). Finally, we calculated the electronic properties of these 10 structures. Among them PuTiO3(Pca2 \(\stackrel{\text{-}}{1}\)), PuFeO3(C222 \(\stackrel{\text{-}}{1}\)), PuMnO3(Pna2 \(\stackrel{\text{-}}{1}\)), PuCrO3(P2 \(\stackrel{\text{-}}{1}\) 3), and PuVO3(P2 \(\stackrel{\text{-}}{1}\) 3) are metallic nature, PuTiO3(Cc), PuZnO3(Pca2 \(\stackrel{\text{-}}{1}\)), PuZnO3(P2 \(\stackrel{\text{-}}{1}\)/m) and PuGaO3(C2/m) are indirect band gap semiconductor, the PuNiO3(P4 \(\stackrel{\text{-}}{1}\)) shows semimetallic nature. And the Pu–O bond and TM–O bond in PuTMO3 mainly exhibit ionic character. This study can provide valuable supports for preparation of Pu-based MOX fuels, the optimization and improvement of their performance in service, and the recycling of materials in recovery process.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Moore, K. T. & van der Laan, G. Nature of the 5f states in actinide metals. Rev. Mod. Phys. 81, 235–298. https://doi.org/10.1103/RevModPhys.81.235 (2009).
Terminello, L. J. Challenges in plutonium and actinide materials science. MRS Bull. 26, 667–671. https://doi.org/10.1557/mrs2001.175 (2001).
Wheeler, D. W. & Bayer, P. D. Evaluation of the nucleation and growth of helium bubbles in aged plutonium. J. Alloy. Compd. 444–445, 212–216. https://doi.org/10.1016/j.jallcom.2007.01.038 (2007).
Ellinger, F. H., Miner, W. N., O’Boyle, D. R. & Schonfeld, F. W. CONSTITUTION OF PLUTONIUM ALLOYS. (Office of Scientific and Technical Information (OSTI), 1968).
Li, X., Xie, X., Shi, M., Jiang, G. & Du, J. Structural, magnetic, mechanical, thermodynamic, and electronic properties of PuAlO3: A first-principles study. AIP Adv. 13, 065304. https://doi.org/10.1063/5.0155596 (2023).
Huff, E. A. Characterization of selected nuclear fuel cycle materials by column extraction chromatography and ICP-AES. Spectrochim. Acta Part B: Atomic Spectrosc. 42, 275–283. https://doi.org/10.1016/0584-8547(87)80069-1 (1987).
Ao, B., Tang, J., Ye, X., Tao, R. & Qiu, R. Phase segregation, transition, or new phase formation of plutonium dioxide: The roles of transition metals. Inorg. Chem. 58, 4350–4364. https://doi.org/10.1021/acs.inorgchem.8b03497 (2019).
Diwu, J., Wang, S., Good, J. J., DiStefano, V. H. & Albrecht-Schmitt, T. E. Deviation between the chemistry of Ce (IV) and Pu (IV) and routes to ordered and disordered heterobimetallic 4f/5f and 5f/5f phosphonates. Inorg. Chem. 50, 4842–4850. https://doi.org/10.1021/ic200006m (2011).
Stan, M. et al. Stability of the perovskite compounds in the Ce-Ga-O and Pu-Ga-O systems. J. Am. Ceram. Soc. 85, 2811–2816. https://doi.org/10.1111/j.1151-2916.2002.tb00533.x (2002).
Russell, L. E., Harrison, J. D. L. & Brett, N. H. Perovskite-type compounds based on plutonium. J. Nucl. Mater. 2, 310–320. https://doi.org/10.1016/0022-3115(60)90003-9 (1960).
Morss, L. R. & Eller, P. G. Enthalpy of formation of BaPuO3; Stability of perovskite as a nuclear-waste matrix for Pu4+. Radiochim. Acta. 47, 51–54. https://doi.org/10.1524/ract.1989.47.1.51 (1989).
Tanaka, K. et al. Thermal conductivity of BaPuO3 at temperatures from 300 to 1500K. J. Nucl. Mater. 414, 316–319. https://doi.org/10.1016/j.jnucmat.2011.04.057 (2011).
Dar, S. A., Srivastava, V., Sakalle, U. K. & Pagare, G. Insight into structural, electronic, magnetic, mechanical, and thermodynamic properties of actinide perovskite BaPuO3. J. Supercond. Novel Magn. 31, 3201–3208. https://doi.org/10.1007/s10948-018-4574-2 (2018).
Fullarton, M. L. et al. Structure, properties and formation of PuCrO3 and PuAlO3 of relevance to doped nuclear fuels. J. Mater. Chem. A 1, 14633–14640. https://doi.org/10.1039/C3TA12782F (2013).
Vigier, J.-F. et al. Plutonium and americium aluminate perovskites. Inorg. Chem. 58, 9118–9126. https://doi.org/10.1021/acs.inorgchem.9b00679 (2019).
Kolman, D. G., Griego, M. E., James, C. A. & Butt, D. P. Thermally induced gallium removal from plutonium dioxide for MOX fuel production. J. Nucl. Mater. 282, 245–254. https://doi.org/10.1016/S0022-3115(00)00397-4 (2000).
Li, S. et al. New insight into the structure of PuGaO3 from ab initio particle-swarm optimization methodology. J. Mater. Chem. A 6, 22798–22808. https://doi.org/10.1039/C8TA08245F (2018).
Sobolev, I. A., Stefanovsky, S. V., Myasoedov, B. F., Kullako, Y. M. & Yudintsev, S. V. Phase composition of murataite ceramics for excess weapons plutonium immobilization. AIP Conf. Proc. 532, 122–124. https://doi.org/10.1063/1.1292227 (2000).
Balachandran, P. V. et al. Predictions of new ABO3 perovskite compounds by combining machine learning and density functional theory. Phys. Rev. Mater. 2, 043802. https://doi.org/10.1103/PhysRevMaterials.2.043802 (2018).
Huang, L. & Lu, H. Collapse of quasiparticle multiplets and 5f itinerant-localized crossovers in cubic phase Pu3Ga. Phys. Rev. B 109, 205132. https://doi.org/10.1103/PhysRevB.109.205132 (2024).
Ru‐song, Li. et al. Magnetic order and valence fluctuation in a Pu-Ga intermetallic compound studied via a first principles calculation. Int. J. Quantum Chem. 120, 26105. https://doi.org/10.1002/qua.26105 (2019).
Lee, T. et al. Atomistic modeling of thermodynamic properties of Pu-Ga alloys based on the Invar mechanism. Phys. Rev. B 89, 174114. https://doi.org/10.1103/PhysRevB.89.174114 (2014).
Massalski, T. B. & Schwartz, A. J. Connections between the Pu–Ga phase diagram in the Pu-rich region and the low temperature phase transformations. J. Alloys Compounds 444–445, 98–103. https://doi.org/10.1016/j.jallcom.2006.09.132 (2007).
Turchi, P. E. A., Landa, A. I. & Söderlind, P. A. Thermodynamic assessment of the Am–Pu system with input from ab initio. J. Nucl. Mater. 418, 165–173. https://doi.org/10.1016/j.jnucmat.2011.06.034 (2011).
Beneš, O., Manara, D. & Konings, R. J. M. Thermodynamic assessment of the Th–U–Pu system. J. Nucl. Mater. 449, 15–22. https://doi.org/10.1016/j.jnucmat.2014.02.001 (2014).
Perron, A. et al. Thermodynamic assessments and inter-relationships between systems involving Al, Am, Ga, Pu, and U. J. Nucl. Mater. 482, 187–200. https://doi.org/10.1016/j.jnucmat.2016.09.012 (2016).
Wang, C. P., Li, Z. S., Fang, W. & Liu, X. J. Thermodynamic database and the phase diagrams of the (U, Th, Pu)-X binary systems. J. Phase Equilib. Diffus. 30, 535–552. https://doi.org/10.1007/s11669-009-9562-6 (2009).
Ao, B., Qiu, R., Lu, H. & Chen, P. First-principles DFT+U calculations on the energetics of Ga in Pu, Pu2O3 and PuO2. Comput. Mater. Sci. 122, 263–271. https://doi.org/10.1016/j.commatsci.2016.05.038 (2016).
Ao, B. et al. New Insights into the Formation of Hyperstoichiometric Plutonium Oxides. J. Phys. Chem. C 119, 101–108. https://doi.org/10.1021/jp5097794 (2015).
Dinh, L. N. et al. The kinetics of the PuO2 to Pu2O3 conversion. J. Chem. Phys. 158, 134703. https://doi.org/10.1063/5.0145400 (2023).
Hernandez, S. C., Venhaus, T. J. & Huda, M. N. Atomic oxygen adsorption on 3.125at.% Ga stabilized δ-Pu (111) surface. J. Alloys Compounds 643, 253–262. https://doi.org/10.1016/j.jallcom.2015.04.080 (2015).
Shi, H. & Zhang, P. First-principles study of α-Pu2O3. J. Nucl. Mater. 420, 159–163. https://doi.org/10.1016/j.jnucmat.2011.08.041 (2012).
Truphémus, T. et al. Structural studies of the phase separation in the UO2–PuO2–Pu2O3 ternary system. J. Nucl. Mater. 432, 378–387. https://doi.org/10.1016/j.jnucmat.2012.07.034 (2013).
Wang, Y., Lv, J., Zhu, L. & Ma, Y. Crystal structure prediction via particle-swarm optimization. Phys. Rev. B 82, 094116. https://doi.org/10.1103/PhysRevB.82.094116 (2010).
Wang, Y., Lv, J., Zhu, L. & Ma, Y. CALYPSO: A method for crystal structure prediction. Comput. Phys. Commun. 183, 2063–2070. https://doi.org/10.1016/j.cpc.2012.05.008 (2012).
Wang, H. et al. Seeded growth of single-crystal black phosphorus nanoribbons. Nat. Mater. 23, 470–478. https://doi.org/10.1038/s41563-024-01830-2 (2024).
Wang, X. et al. Data-driven prediction of complex crystal structures of dense lithium. Nat. Commun. 14, 2924. https://doi.org/10.1038/s41467-023-38650-y (2023).
Luo, D., Yin, K. & Dronskowski, R. Existence of BeCN2 and its first-principles phase diagram: Be and C introducing structural diversity. J. Am. Chem. Soc. 144, 5155–5162. https://doi.org/10.1021/jacs.2c00592 (2022).
Zhu, L. et al. First-principles study of novel icosahedral-based B12CN and B13CN structures. Sci. China Mater. 66, 4480–4488. https://doi.org/10.1007/s40843-023-2593-3 (2023).
Liu, L., Wang, D., Zhang, S. & Zhang, H. Pressure-stabilized GdN6 with an armchair–antiarmchair structure as a high energy density material. J. Mater. Chem. A 9, 16751–16758. https://doi.org/10.1039/D1TA03381F (2021).
Naghavi, S. S., He, J. & Wolverton, C. Crystal and electronic structures of palladium sesquichalcogenides. Chem. Mater. 33, 2298–2306. https://doi.org/10.1021/acs.chemmater.0c04227 (2021).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186. https://doi.org/10.1103/PhysRevB.54.11169 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979. https://doi.org/10.1103/PhysRevB.50.17953 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775. https://doi.org/10.1103/PhysRevB.59.1758 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192. https://doi.org/10.1103/PhysRevB.13.5188 (1976).
Le Page, Y. & Saxe, P. Symmetry-general least-squares extraction of elastic data for strained materials from ab initio calculations of stress. Phys. Rev. B 65, 104104. https://doi.org/10.1103/PhysRevB.65.104104 (2002).
Wang, V., Xu, N., Liu, J.-C., Tang, G. & Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033. https://doi.org/10.1016/j.cpc.2021.108033 (2021).
Togo, A. & Tanaka, I. First principles phonon calculations in materials science. Scripta Mater. 108, 1–5. https://doi.org/10.1016/j.scriptamat.2015.07.021 (2015).
Momma, K. & Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41, 653–658. https://doi.org/10.1107/S0021889808012016 (2008).
Saal, J. E., Kirklin, S., Aykol, M., Meredig, B. & Wolverton, C. Materials design and discovery with high-throughput density functional theory: The open quantum materials database (OQMD). JOM 65, 1501–1509. https://doi.org/10.1007/s11837-013-0755-4 (2013).
Kirklin, S. et al. The open quantum materials database (OQMD): Assessing the accuracy of DFT formation energies. npj Comput. Mater. 1, 15010. https://doi.org/10.1038/npjcompumats.2015.10 (2015).
Jain, A. et al. Commentary: The materials project: A materials genome approach to accelerating materials innovation. APL Mater. 1, 011002. https://doi.org/10.1063/1.4812323 (2013).
Wang, J. et al. Novel structural phases and the electrical properties of Si3B under high pressure. Phys. Chem. Chem. Phys. 19, 16206–16212. https://doi.org/10.1039/C7CP02450A (2017).
Born, M. On the stability of crystal lattices. I. Math. Proc. Camb. Phil. Soc. 36, 160–172. https://doi.org/10.1017/S0305004100017138 (1940).
Mouhat, F. & Coudert, F.-X. Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 90, 224104. https://doi.org/10.1103/PhysRevB.90.224104 (2014).
Gonze, X., Rignanese, G.-M. & Caracas, R. First-principle studies of the lattice dynamics of crystals, and related properties. Zeitschrift für Kristallographie – Cryst. Mater. 220, 458–472. https://doi.org/10.1524/zkri.220.5.458.65077 (2005).
Azam, S. et al. Electronic, optical and thermoelectric properties of Ce3PdIn11 and Ce5Pd2In19: An ab initio study. Intermetallics 55, 184–194. https://doi.org/10.1016/j.intermet.2014.08.001 (2014).
Manz, T. A. & Limas, N. G. Introducing DDEC6 atomic population analysis: part 1. Charge partitioning theory and methodology. RSC Adv. 6, 47771–47801. https://doi.org/10.1039/C6RA04656H (2016).
Allen, L. C. Electronegativity is the average one-electron energy of the valence-shell electrons in ground-state free atoms. J. Am. Chem. Soc. 111, 9003–9014. https://doi.org/10.1021/ja00207a003 (1989).
Mann, J. B., Meek, T. L., Knight, E. T., Capitani, J. F. & Allen, L. C. Configuration energies of the d-block elements. J. Am. Chem. Soc. 122, 5132–5137. https://doi.org/10.1021/ja9928677 (2000).
Funding
Basic Research Program for Natural Science of Shaanxi Province,2023-JC-YB-026,2023-JC-YB-026,2023-JC-YB-026,2023-JC-YB-026,2023-JC-YB-026,2023-JC-YB-026
Author information
Authors and Affiliations
Contributions
Z. Jiang : Investigation, Data curation, Visualization, Writing—original draft. J.Wang and N. Gao: Supervision, Methodology, Formal analysis, Writing—review & editing. Y. Fang, R. Li and R. Zhou: Validation, Software, Resources, Investigation. T. Wang and W. Yu: Software, Resources, Methodology, Investigation. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, Z., Wang, J., Fang, Y. et al. PuTMO3 systems crystal structures prediction and stability analysis based on first principles. Sci Rep 15, 17273 (2025). https://doi.org/10.1038/s41598-025-02046-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02046-3