Abstract
The efficacy of postoperative radiotherapy (PORT) after gross total resection (GTR) for intracranial solitary fibrous tumors (SFT) remains unclear due to the inconsistent results of previous studies, with some studies suggesting improved outcomes in progression-free survival (PFS) and overall survival (OS), while others report no significant benefit. Therefore, by evaluating and synthesizing data from relevant studies, we aimed to investigate the role of PORT, as compared with surgery alone, in survival outcomes after GTR of intracranial SFT. A systematic literature search, adhering to PRISMA guidelines and using Medline, Embase, and the Cochrane Library to identify relevant literature. The outcomes of interest included progression-free survival (PFS), overall survival (OS), and metastasis-free survival (MFS) at 3, 5, and 10 years, respectively. Differences between the two cohorts (GTR + PORT vs. GTR only) were estimated by calculating the hazard ratios. Twelve studies, including data from 419 patients (GTR + PORT, n = 225 vs. GTR, n = 194), were selected for meta-analysis. Pooled hazard ratios revealed that the PORT cohort showed sustained superiority in both PFS and OS compared with the surgery-only cohort after GTR of the tumor. These results were consistent with those of a subgroup analysis that focused on grade 2 and 3 intracranial SFT. However, no significant improvement was observed in MFS with PORT addition. This study underscores the importance of PORT in enhancing the PFS and OS of patients with intracranial SFT after GTR. These findings suggest that PORT should be considered an effective treatment strategy for all patients with intracranial SFT, irrespective of the extent of resection.
Similar content being viewed by others
Introduction
Intracranial solitary fibrous tumors (SFT) are rare extra-axial brain tumors originating from mesenchymal cells.1 Previously, intracranial SFT and hemangiopericytoma (HPC) had been recognized as distinct entities due to their dissimilar biological behaviors. However, given the evidence that both SFT and HPC carry a NAB2–STAT6 gene fusion and that STAT6 is overexpressed in the nucleus, these CNS tumors were merged in the 2016 WHO classification, and they were renamed “solitary fibrous tumor/hemangiopericytoma (SFT/HPC)”.2,3,4,5,6 In its latest (2021) edition, the term HPC has been retired to emphasize their biological similarity within tumor types. Consequently, tumors are solely referred to as SFT, aligning with soft-tissue pathology classifications.7
Intracranial SFT is extremely uncommon, accounting for < 1% of all primary CNS neoplasms and 2.5% of meningeal tumors.8,9 It is characterized by high rates of local and extracranial metastasis, unlike typical primary brain tumors.8,10,11,12 However, given its low incidence, research on its treatment and prognosis has been limited to relatively small case series.10,13,14,15,16,17 Furthermore, ongoing changes in diagnostic criteria and classifications have complicated efforts to conduct comprehensive treatment research.
We previously presented the first meta-analysis of intracranial SFT regarding the effect of extent of resection (EOR) and postoperative radiotherapy (PORT) on tumor recurrence and patient survival.18 The findings indicated that gross total resection (GTR) and PORT exert significant benefits on both progression-free survival (PFS) and overall survival (OS) in patients with intracranial SFT. However, the study was limited by the lack of subgroup analysis based on EOR, specifically examining the survival benefits of PORT in GTR or subtotal removal (STR) subgroup. There is widespread consensus that additional PORT delays tumor progression and enhances patient survival in cases of STR.19,20,21,22 However, no comprehensive study has been conducted to determine whether adding PORT to complete resection of tumors improves survival outcomes, and conflicting results have been reported.1,20,23,24 Therefore, we conducted a systematic review and meta-analysis to explore the efficacy of PORT after GTR on intracranial SFT by analyzing relevant studies.
Materials and methods
Study design
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.25 The protocol was registered at http://www.crd.york.ac.uk/PROSPERO/ (CRD42022328429).
We devised questions based on the Population, Intervention, Comparison, and Outcomes (PICO) framework. Accordingly, we conducted a systematic literature search, compiled eligible studies, and analyzed their outcomes through meta-analysis. The PICO questions were as follows: Population (P) = patients with intracranial SFT; Intervention (I) = GTR followed by PORT; Comparator (C) = GTR alone; Outcome (O) = survival outcome (PFS, OS, and metastasis-free survival [MFS]).
Criteria for considering studies and participants
We set the following inclusion criteria for relevant studies: (1) Presentation of clinical results of patients who received surgery for intracranial HPC, SFT, or HPC/SFT; (2) Clear description of the treatment modality, including EOR and PORT; and (3) Reporting of relevant outcomes, including PFS, OS, and/or MFS for each cohort. Case series, retrospective or prospective cohort studies, randomized controlled trials (RCTs), and non-randomized clinical trials were all considered.
We included patients who were pathologically confirmed to have intracranial HPC, SFT, or HPC/SFTs after surgery. Study participants were limited to newly diagnosed cases, and patients with recurrent, metastatic, spinal, or extracranial lesions were excluded.
Literature search and selection
Two independent reviewers (M.K.N. and K.S.C.) conducted a comprehensive literature search across electronic databases, including Embase, Medline, and the Cochrane Central Register of Controlled Trials, for studies published from the databases’ respective inception dates up to March 16, 2023. We did not impose any language restrictions to ensure an exhaustive search of the relevant literature. The search strategy employed for each database is provided in Supplementary Table 1.
All studies were reviewed by scanning titles and abstracts. We excluded the following studies: conference abstracts, case reports, literature reviews, technical notes, letters, editorials, irrelevant populations (recurrent, metastatic, spinal, or extracranial lesions), studies with insufficient data or a nonhomogeneous design, studies with a single cohort of treatment modality, and studies with < 20 included patients. We only included institutional cohort studies or clinical trials that clearly described clinical data. Population-based nationwide studies were excluded.
Any discrepancies between reviewers were resolved through discussion. After excluding ineligible abstracts, we retrieved the full texts of the selected articles, which were further assessed for eligibility using the same inclusion and exclusion criteria.
Data extraction and assessment of risk of bias
Two reviewers (M.K.N. and K.S.C.) independently extracted the following data from the selected studies: author and year of publication, study design, country where the study was conducted, inclusion period, number of patients, demographics, follow-up duration, version of the adopted WHO classification, definition of GTR applied, included diagnosis of each study, pathological grade, PFS, OS, and MFS (3, 5, and 10 years) of each cohort. In instances where a study only displayed the Kaplan–Meier curve, survival data were extracted from the curve using Engauge Digitizer version 12.1 (https://engauge-digitizer.software.informer.com/).26,27 When analyzing survival data, patients who died within the first month after surgery were excluded. Where necessary, we emailed the corresponding authors of each study to request the relevant data that were not adequately described.
The methodological quality of the selected articles was assessed using the Risk-of-Bias Assessment tool for Non-Randomized Studies of Interventions (ROBINS-I) tool by two independent reviewers (M.K.N and K.S.C.).28 Any discrepancies between the reviewers were resolved via discussion or by consulting a third author (S.M.K.).
Statistical analysis
Hazard ratios (HR) and 95% confidence intervals (CIs) were used to measure the effect sizes as indicators of PFS, OS, and MFS in each comparative cohort (GTR + PORT vs. GTR). In instances where the direct HR values were not provided in eligible studies, we calculated HRs using the available data. When individual patient data were presented in the literature, we used the data to derive HR. In instances where only Kaplan–Meier curves were provided, we analyzed the figures to extract cohort-specific survival data, thereby enabling the calculation of HR. HRs with 95% CIs extracted either from the text or Kaplan–Meier curves were subsequently analyzed using the methods suggested by Tierney et al.29 In order to reduce uncertainty on the pooled effect estimates, we used the generic inverse-variance method for meta-analysis, with each study weighted according to the inverse variance of each effect. Heterogeneity among studies was evaluated using I2 statistics, with values of 25%, 50%, and 75% being considered low, moderate, and high, respectively. A random effects model was adopted in all analyses, even for those without significant heterogeneity, as variations existed among studies in relation to the definition of GTR, applied version of the WHO classification, and indication or protocol of PORT. In addition, survival analysis was conducted using the log-rank test to compare data between the cohorts. The risk of publication bias was assessed using Egger’s and Begg’s tests along with funnel plots. p-values > 0.05 were considered to have no significant publication bias.
Review Manager, version 5.4 (RevMan, The Cochrane Collaboration, Odense, Denmark), Comprehensive Meta-Analysis version 3 (CMA, BioStat, Englewood, NJ, USA), and SPSS version 18.0 (SPSS, Chicago, IL, USA) were used for all statistical analyses. Statistical significance was set at p < 0.05.
Results
Literature selection
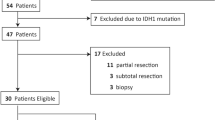
Figure 1 shows a flow diagram of our literature selection and systematic review process. The initial search yielded 2205 studies across the Embase (n = 1396), Medline (n = 779), and Cochrane Library (n = 30) databases. Subsequently, we removed 640 duplicate studies and excluded an additional 1398 studies after reviewing both titles and abstracts, leaving 167 potentially relevant studies. The full texts of these 167 articles were evaluated for eligibility, and 155 irrelevant articles were excluded based on our predefined inclusion and exclusion criteria. Additionally, a study that included 26 patients, of whom < 10 had undergone GTR, was excluded from the final analysis.7 Twelve studies were finally included in the current meta-analysis.
Study characteristics and quality assessment
Table 1 summarizes the detailed descriptions of the included studies.10,11,16,17,21,22,30,31,32,33,34,35 All studies employed a retrospective observational design. Overall, the studies included 419 patients with intracranial HPC, SFT, or SFT/HPC, categorized based on the respective WHO classification applied. Among them, 225 patients were treated with PORT after GTR of the tumor, and 194 underwent GTR alone. The definition of GTR varied across studies, with five studies employing Simpson grades I–II, one using grades I–III, and another study assessing GTR through postoperative magnetic resonance imaging. Clear descriptions of GTR were not presented in the remaining five studies.
The quality of the included studies was evaluated using the ROBINS-I tool (Supplementary Fig. 1). The studies by Champeauex et al. and Schiariti et al. were classified as high-risk, as both included a patient who underwent chemotherapy without specifying the cohort, indicating a bias due to departure from the intended intervention.30,32 Additionally, two studies were considered at high risk of bias in outcomes measurement due to the reporting of the patient follow-up period in years, which has the potential to introduce bias into the results.16,33 All included studies were identified as having a high risk of confounding bias, primarily stemming from limited information regarding confounding variables in the PORT and surgery-only cohorts, which is inherent to non-randomized studies.
PFS in the GTR + PORT versus GTR groups
PFS, OS, and MFS were assessed at 3, 5, and 10 years. Of the 12 studies included in our meta-analysis, 11 studies (219 in the GTR + PORT cohort and 189 patients in the GTR cohort) reported relevant data regarding PFS following GTR for intracranial SFT. Serial HRs for the 3-, 5-, and 10-year PFS revealed that PORT after GTR demonstrated statistically significant superiority over GTR alone, sustained up to the 10-year follow-up (Fig. 2). The actuarial 3-, 5-, and 10-year PFS rates in the GTR + PORT cohort were 90.3%, 82.2%, and 53.3%, respectively, whereas those in the GTR cohort were 77.1%, 60.6%, and 31.8%, respectively (Fig. 3A and Table 2).
Kaplan–Meier curves for (A) progression-free survival (PFS), (B) overall survival (OS), and (C) metastasis-free survival (MFS) of the gross total resection (GTR) + postoperative radiotherapy (PORT) cohort and GTR cohort. The GTR + PORT cohort showed higher 3-, 5-, and 10-year PFS and OS, while MFS showed no significant difference between the groups.
OS in the GTR + PORT versus GTR groups
Overall, 362 patients (196 in the GTR + PORT vs. 166 in the GTR cohort) from nine studies were analyzed to compare OS between the cohorts. Similarly, pooled analysis of studies showed a significant advantage of PORT over GTR alone in all OS periods (Fig. 4). The actuarial 3-, 5-, and 10-year OS rates in the GTR + PORT cohort were 98.3%, 96.6%, and 81.4%, respectively, whereas those in the GTR cohort were 93.2%, 88.5%, and 70.3%, respectively (Fig. 3B and Table 2).
MFS in the GTR + PORT versus GTR groups
Four studies comprising 105 patients who underwent PORT and 90 who underwent surgery only were included in the analysis of MFS. However, pooled HR showed no significant advantage of PORT after GTR over GTR alone for intracranial SFT (Fig. 5), although moderate heterogeneity was observed in the 10-year MFS analysis (I2 = 34%). The actuarial 3-, 5-, and 10-year MFS rates were 96.6%, 92.8%, and 65.2% in the GTR + PORT cohort and 91.8%, 88.9%, and 81.3% in the GTR cohort, respectively (Fig. 3C and Table 2).
Survival outcome in grade 2 and 3 intracranial SFT
To conduct a subgroup analysis focusing on tumor grade, we analyzed survival outcomes exclusively in pathological grade 2 and 3 intracranial SFT. In the subgroup analysis, similar to the preceding analysis, the GTR + PORT cohort consistently demonstrated significant superiority in 3-, 5-, and 10-year PFS and OS rates (Supplementary Figs. 2 and 3).
Publication bias
Regarding publication bias, as shown in Supplementary Table 2 and Fig. 4, our analysis revealed that both Egger’s regression and Begg’s rank tests yielded p-values of > 0.5. These results indicated the absence of significant evidence of publication bias in the current meta-analysis.
Discussion
Intracranial SFT is known for its propensity to recur locally after surgery, and often lead to distant metastases.36,37,38 To date, extensive surgical resection, as in the treatment of other cancers, has been the cornerstone of intracranial SFT treatment, representing a pivotal strategy for both local control and oncological outcomes.37,38,39,40 In addition, many studies have supported the use of postoperative adjuvant radiotherapy combined with surgery to enhance patient survival outcomes.12,18,37,41 Nonetheless, the impact of PORT in patients who have undergone complete tumor resection has been debated. Management strategies after GTR also vary among surgeons, primarily due to the conflicting results observed in individual studies, without the benefit of a systematic review.19,20,34 To address this, we performed a systematic review and meta-analysis of 12 eligible studies, with 225 patients in the GTR + PORT cohort and 194 in the GTR-only cohort. In the GTR + PORT cohort, superior outcomes were observed in both PFS and OS compared to the GTR cohort. These results were consistent with those of a subgroup analysis focusing on grade 2 and 3 intracranial SFT. However, we found no evidence that the addition of PORT increased MFS, for any period. We believe that the results of the current meta-analysis can contribute to the establishment of appropriate management strategies in clinical practice for patients with intracranial SFT who have undergone complete tumor resection.
In the current study, patients who underwent PORT after GTR exhibited significant increases in both PFS and OS within the entire follow-up period of up to 10 years, compared with those who underwent GTR alone. Although the effect of EOR in managing intracranial SFT is less disputed, the role of PORT, particularly after complete tumor resection, remains debatable. Several studies have suggested that combining GTR with PORT, compared to GTR alone, has the advantage of prolonging PFS, but not OS, for these patients.11,14,30,37,42 In contrast, other studies showed an improvement in OS with the addition of adjuvant radiation.1,24 Notably, a nationwide study conducted by Sonabend et al. suggested a reduced mortality rate with an HR of 0.31 (p = 0.040, 95% CI 0.10–0.95) for patients who underwent GTR plus PORT compared with those who underwent biopsy-only, with no significant reduction observed in the GTR-only group.19 Given the high propensity for recurrence and metastasis of intracranial SFT, even after complete resection, it may be advisable to consider a more aggressive initial treatment approach. The survival benefit associated with PORT after GTR, as opposed to GTR alone, implies that tumor cells that remain despite radiographically verified complete resection warrant the consideration of adjuvant treatment to maximize the survival advantage. Since the primary pattern of recurrence in intracranial SFT tends to be local rather than distant, the improved local control achieved through adjuvant radiotherapy has been associated with prolonged PFS in patients undergoing PORT, both in cases of GTR and STR, as demonstrated in our previous research.18 Therefore, adjuvant radiotherapy may be a reasonable treatment strategy for all patients diagnosed with intracranial SFT, regardless of the EOR of the tumor.
Several studies have proposed “watchful waiting” after GTR as an alternative strategy to adding adjuvant radiation therapy.20,23 A previous systematic review of the literature failed to confirm any survival advantage associated with the addition of PORT for patients who had undergone GTR.43 In another study, Wu et al. observed a PFS extension following PORT exclusively in the STR group, not in the GTR group. They advocated adjuvant radiotherapy as the primary treatment option only for patients with incomplete resection.20 In our cohort, however, the median PFS of the two cohorts was 61 and 45 months, respectively (p < 0.001), highlighting the significant advantage in preventing recurrence when PORT is used after GTR. Furthermore, the pooled outcome of prolonged OS also showed a strong statistical significance, which was absent in individual studies due to their limited sample sizes. While the log-rank analysis for OS yielded a non-significant p-value of 0.078, visual inspection of the survival curves (Fig. 3B) revealed a tendency for the survival rates to converge after the 10-year mark, which may explain the lack of statistical significance. Therefore, our results endorse the application of PORT following GTR of intracranial SFT. Currently, there are no established guidelines for this rare disease entity. While our results cannot, on their own, establish a management protocol, we believe that future clinical trials with larger sample sizes will further validate our findings and contribute to the development of comprehensive treatment guidelines for intracranial SFT.
In our subgroup analysis of pathological grade 2 and 3 SFT, PORT after GTR demonstrated a significant impact on patients’ PFS and OS, which was consistent with the abovementioned results. However, these findings necessitate cautious interpretation due to changes in the diagnostic criteria for grading intracranial SFT introduced in the 2021 CNS classification update. Under the 2016 WHO classification of CNS tumors, a grade 1 SFT was characterized as a highly collagenous, low-cellularity lesion, while grade 2 was described as a less collagenous, high-cellularity tumor with staghorn vessels. Grade 3 tumors were diagnosed when at least 5 mitoses per 1 high-power field (HPF) were observed.6 In the latest (2021) WHO classification, SFT has been reclassified into WHO grades 1, 2, and 3 based on mitotic count and the presence of necrosis. Notably, the mitotic count criterion for both grade 2 and above was adjusted to include cases with ≥ 5 mitoses per 10 HPFs.7 Consequently, individuals previously categorized as grade 3 may now be reclassified as grade 2 or 3, while those initially classified as grade 2 may be reclassified as grade 1 with the revised criteria. The current subgroup analysis excluded grade 1 intracranial SFT due to the scarcity of literature explicitly addressing this subtype. Nevertheless, given the typical benign and less invasive characteristics of grade 1 tumors relative to high-grade subtypes, PORT after GTR may not confer a significant advantage in this subset. Kinslow et al. reported that GTR followed by PORT was associated with significantly increased survival compared with GTR alone when analyzing subgroups limited to HPC or tumors with borderline/malignant code, which was not significant within the entire cohort of SFT and HPC.1 Similarly, Zheng et al. conducted separate analyses for SFT and HPC, and could not demonstrate the significance of PORT in the SFT subgroup, which is considered relatively less malignant. A significant advantage of PORT was observed exclusively in the HPC subgroup.39.
Lee et al. demonstrated a trend toward a gradual increase in the length of time to distant metastasis in the PORT group.44 In the present meta-analysis, however, we did not find any significant differences in pooled MFS between the GTR + PORT and GTR-only cohorts, which was consistent with the results reported in the included individual studies. Metastasis, either neural or extra-neural, remains a potential consequence of intracranial SFT progression, and our current analysis did not provide conclusive guidance on an optimal preventive strategy. Many studies on intracranial SFT have highlighted the frequent occurrence of metastasis regardless of various initial treatment modalities: neither EOR nor PORT has been shown to affect the time to metastasis.34,38,45,46,47 Given the literature and our current findings, which suggest that adjuvant radiotherapy does not impede the development of metastasis, we propose that it is important to maintain vigilant radiological surveillance of all patients with intracranial SFT after treatment, regardless of EOR and adjuvant radiotherapy.19.
This study did not address the potential toxicities associated with PORT. The neurotoxicity linked to adjuvant radiotherapy remains a significant concern for physicians, often leading to hesitancy in its application in patients with intracranial SFT after complete tumor resection. The incidence of neurotoxicity can vary widely (0%–16.7%), according to the tumor location, radiation dose, and modality used.27,48 Nevertheless, advancements in radiotherapy techniques, such as intensity-modulated radiotherapy and stereotactic radiosurgery, have been explored to mitigate neurotoxicity while managing intracranial SFT. These techniques aim to improve local control and potentially extend OS with a reduced risk of side effects compared to traditional radiotherapy methods. Consequently, these advancements have contributed to an improved side-effect profile, making it possible to manage most neurotoxicity effectively with appropriate interventions.48,49,50 Possibly for this reason, none of the studies included in our analysis provided results related to neurotoxicity after PORT.
The current study has some limitations. First, all studies included in the analysis were conducted retrospectively, which limits the implications of the results and introduces unmeasured biases. To address these limitations, future research should incorporate prospective studies, including RCTs, to provide more robust evidence. Second, as with all other systematic reviews, the included studies included inherent heterogeneity related to patient demographics, clinical criteria, treatment strategies and modalities, and the local expertise of each institution. Particularly, there is variability among the studies regarding the definitions of GTR, versions of WHO classification used, which may impact the reliability of the pooled results. In addition, the heterogeneity of PORT protocols among studies, as well as the lack of detailed protocol reports in some studies, presents a notable challenge that may introduce potential bias. Third, the pooled data extracted from the Kaplan–Meier curves may be less representative of the real outcome. Fourth, studies with an insufficient number of enrolled patients were excluded from the analysis, which may introduce a potential selection bias. Additionally, because of the insufficient number of studies addressing the pathological grade and/or occurrence of metastasis, we could not perform several subgroup analyses regarding tumor grade or MFS.
In conclusion, the current meta-analysis highlighted the significance of PORT in enhancing PFS and OS after surgery in patients with intracranial SFT, convincingly demonstrating that the benefits of PORT extend beyond STR cases and are applicable even when GTR has been achieved. Therefore, adjuvant radiation should be considered a viable and effective treatment strategy for all patients diagnosed with intracranial SFT, including those with complete resection of tumor. We believe that our study contributes to the growing body of evidence supporting the development of evidence-based guidelines for intracranial SFT management. Nevertheless, further large-scale clinical trials are essential to establish robust, evidence-guided guidelines that can better standardize the management of intracranial SFT.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kinslow, C. J. et al. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: A population-based study. J. Neurooncol. 138, 173–182 (2018).
Robinson, D. R. et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat. Genet. 45, 180–185 (2013).
Fritchie, K. J. et al. NAB2-STAT6 gene fusion in meningeal hemangiopericytoma and solitary fibrous tumor. J. Neuropathol. Exp. Neurol. 75, 263–271 (2016).
Yuzawa, S. et al. Analysis of NAB2-STAT6 Gene Fusion in 17 cases of meningeal solitary fibrous tumor/hemangiopericytoma: Review of the literature. Am. J. Surg. Pathol. 40, 1031–1040 (2016).
Nakada, S., Minato, H. & Nojima, T. Clinicopathological differences between variants of the NAB2-STAT6 fusion gene in solitary fibrous tumors of the meninges and extra-central nervous system. Brain Tumor Pathol. 33, 169–174 (2016).
Louis, D. N. et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 131, 803–820 (2016).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro. Oncol. 23, 1231–1251 (2021).
Lu, T., Xu, H., Dong, X., Jin, Z. & Wang, Y. Epidemiology and survival of patients with central nervous system solitary fibrous tumors: A population-based analysis. Front. Oncol. 12, 977629 (2022).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Kim, J. H. et al. Meningeal hemangiopericytomas: Long-term outcome and biological behavior. Surg Neurol 59, 47–53 (2003).
Ghia, A. J. et al. Intracranial hemangiopericytoma: Patterns of failure and the role of radiation therapy. Neurosurgery 73, 624–630 (2013).
Ghia, A. J. et al. Intracranial hemangiopericytoma and the role of radiation therapy: A population based analysis. Neurosurgery 72, 203–209 (2013).
Wu, W. et al. Hemangiopericytomas in the central nervous system. J. Clin. Neurosci. 16, 519–523 (2009).
Jeon, S. H. et al. Efficacy of adjuvant radiotherapy in the intracranial hemangiopericytoma. J. Neurooncol. 137, 567–573 (2018).
Bertero, L. et al. Pathological prognostic markers in central nervous system solitary fibrous tumour/hemangiopericytoma: Evidence from a small series. PLoS ONE 13, e0203570 (2018).
Menon, G. R., Patil, A., Pisharody, K. K. & Nair, S. N. Meningeal hemangiopericytomas: Review of an institutional series of 21 cases. Neurosurg. Q. 25, 219–227 (2015).
Gubian, A. et al. Intracranial solitary fibrous tumors: A heterogeneous entity with an uncertain clinical behavior. World Neurosurg. 126, e48–e56 (2019).
Kwon, S. M. et al. Impact of extent of resection and postoperative radiotherapy on survival outcomes in intracranial solitary fibrous tumors: A systematic review and meta-analysis. Neurosurg. Rev. 46, 138 (2023).
Sonabend, A. M. et al. The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: A surveillance, epidemiology, and end results analysis. J. Neurosurg. 120, 300–308 (2014).
Wu, Y. et al. Clinical outcomes of solitary fibrous tumors and hemangiopericytomas and risk factors related to recurrence and survival based on the 2021 WHO classification of central nervous system tumors. J. Neurosurg. 140, 69–79. https://doi.org/10.3171/2023.4.JNS23147 (2023).
Lee, J. H. et al. The role of postoperative radiotherapy in intracranial solitary fibrous Tumor/Hemangiopericytoma: A multi-institutional retrospective study (KROG 18–11). Cancer Res. Treat 54, 65–74 (2022).
Zhang, G.-J., Wu, Z., Zhang, L.-W., Li, D. & Zhang, J.-T. Surgical management and adverse factors for recurrence and long-term survival in patients with hemangiopericytoma. World Neurosurg. 104, 95–103 (2017).
Bisceglia, M. et al. Solitary fibrous tumor of the central nervous system: A 15-year literature survey of 220 cases (August 1996-July 2011). Adv. Anat. Pathol. 18, 356–392 (2011).
Stessin, A. M., Sison, C., Nieto, J., Raifu, M. & Li, B. The role of postoperative radiation therapy in the treatment of meningeal hemangiopericytoma-experience from the SEER database. Int. J. Radiat. Oncol. Biol. Phys. 85, 784–790 (2013).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 151, W65-94 (2009).
Shin, S. W. et al. Liver resection versus local ablation therapies for hepatocellular carcinoma within the Milan criteria: A systematic review and meta-analysis. Ann. Surg. 273, 656–666 (2021).
Chun, S.-W. et al. Adjuvant radiotherapy versus observation following gross total resection for atypical meningioma: A systematic review and meta-analysis. Radiat Oncol 16, 34 (2021).
Sterne, J. A. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919 (2016).
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S. & Sydes, M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16 (2007).
Schiariti, M., Goetz, P., El-Maghraby, H., Tailor, J. & Kitchen, N. Hemangiopericytoma: long-term outcome revisited. Clinical article. J. Neurosurg. 114, 747–755 (2011).
Rutkowski, M. J. et al. Intracranial hemangiopericytoma: Clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer 118, 1628–1636 (2012).
Champeaux, C., Khan, A. A., Wilson, E., Thorne, L. & Dunn, L. Meningeal haemangiopericytoma and solitary fibrous tumour: A retrospective bi centre study for outcome and prognostic factor assessment. J Neurooncol 134, 387–395 (2017).
Champeaux, C., Rousseau, P., Devaux, B., Nataf, F. & Tauziède-Espariat, A. Solitary fibrous tumours and haemangiopericytoma of the meninges. A retrospective study for outcome and prognostic factor assessment. Neurochirurgie 64, 37–43 (2018).
Shin, D.-W. et al. Intracranial solitary fibrous tumor/hemangiopericytoma: Tumor reclassification and assessment of treatment outcome via the 2016 WHO classification. J. Neurooncol. 154, 171–178 (2021).
Li, Q., Deng, W. & Sun, P. Effect of different treatments for intracranial solitary fibrous tumors: Retrospective analysis of 31 patients. World Neurosurg. 166, e60–e69 (2022).
Ghose, A., Guha, G., Kundu, R., Tew, J. & Chaudhary, R. CNS hemangiopericytoma: A systematic review of 523 patients. Am. J. Clin. Oncol 40, 223–227 (2017).
Kim, B. S. et al. Clinical outcomes of intracranial solitary fibrous tumor and hemangiopericytoma: Analysis according to the 2016 WHO classification of central nervous system tumors. J. Neurosurg. 129, 1384–1396 (2018).
Sung, K. S. et al. Solitary fibrous tumor/hemangiopericytoma: Treatment results based on the 2016 WHO classification. J. Neurosurg. 130, 1–8. https://doi.org/10.3171/2017.9.JNS171057 (2018).
Zeng, L. et al. Analyses of prognosis-related factors of intracranial solitary fibrous tumors and hemangiopericytomas help understand the relationship between the two sorts of tumors. J Neurooncol 131, 153–161 (2017).
Sonkin, D., Thomas, A. & Teicher, B. A. Cancer treatments: Past, present, and future. Cancer Genet 286–287, 18–24 (2024).
Melone, A. G. et al. Intracranial hemangiopericytoma–our experience in 30 years: A series of 43 cases and review of the literature. World Neurosurg 81, 556–562 (2014).
Zhang, G.-J., Zhang, L.-W., Li, D., Wu, Z. & Zhang, J.-T. Analysis of prognostic factors, survival rates, and treatment in anaplastic hemangiopericytoma. World Neurosurg. 104, 795–801 (2017).
Rutkowski, M. J. et al. Predictors of mortality following treatment of intracranial hemangiopericytoma. J. Neurosurg. 113, 333–339 (2010).
Lee, E. J. et al. The impact of postoperative radiation therapy on patterns of failure and survival improvement in patients with intracranial hemangiopericytoma. J. Neurooncol. 127, 181–190 (2016).
Ecker, R. D. et al. Hemangiopericytoma in the central nervous system: Treatment, pathological features, and long-term follow up in 38 patients. J. Neurosurg. 98, 1182–1187 (2003).
Fountas, K. N. et al. Management of intracranial meningeal hemangiopericytomas: Outcome and experience. Neurosurg. Rev. 29, 145–153 (2006).
Guthrie, B. L., Ebersold, M. J., Scheithauer, B. W. & Shaw, E. G. Meningeal hemangiopericytoma: Histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery 25, 514–522 (1989).
Kaur, G. et al. Adjuvant radiotherapy for atypical and malignant meningiomas: A systematic review. Neuro Oncol. 16, 628–636 (2014).
Gou, Q., Xie, Y. & Ai, P. Intracranial solitary fibrous tumor/hemangiopericytoma: Role and choice of postoperative radiotherapy techniques. Front. Oncol. 12, 994335 (2022).
Golub, D. et al. Postoperative stereotactic radiosurgery for intracranial solitary fibrous tumors: Systematic review and pooled quantitative analysis. J. Neurooncol. 165, 229–239 (2023).
Acknowledgements
None.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. RS-2024-00359028).
Author information
Authors and Affiliations
Contributions
Conceptualization: S.M.K. and M.K.N. Data curation: J.H.K. and B.H.J. Formal analysis: K.S.C. and T.H.L. Funding acquisition: S.M.K. Investigation: H.G.S. and J.L. Methodology: H.G.S., H.L., W.K. and Y.C. Resources: J.G.K. and C.A. Software: M.N. Supervision: S.M.K. Validation: H.G.S., H.L. and J.G.K. Visualization: J.L. and Y.C. Writing of manuscript: M.K.N. and K.S.C. Review and editing of manuscript: B.H.J. and S.M.K
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Na, M.K., Choi, KS., Lim, T.H. et al. A systematic review and meta-analysis on the efficacy of postoperative radiotherapy after gross total resection of intracranial solitary fibrous tumors. Sci Rep 15, 23368 (2025). https://doi.org/10.1038/s41598-025-02170-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02170-0