Abstract
Alzheimer’s disease (AD) is a leading cause of dementia, underscoring the need for early and accurate diagnostic methods. This cross-sectional study examines the thickness of individual retinal layers and vessel density (VD) in 23 patients with AD and 22 healthy controls (HC). All participants underwent comprehensive ophthalmologic assessments and cognitive evaluations. AD patients exhibited significantly reduced mean macular retinal thickness compared to HC in foveal and parafoveal areas (p < 0.05). The most significant differences were noted in specific sectors of the Inner Nuclear Layer (INL) and Outer Nuclear Layer (ONL) (p < 0.05), while no significant changes were found in the Outer Retinal Layer (p > 0.05). Additionally, AD patients showed decreased VD in the deep vascular plexus (DVP) (p = 0.030) and an increased foveal avascular zone (FAZ) (p = 0.021). The areas under the receiver operating characteristic curves (AUC) analysis revealed the highest diagnostic accuracy for ONL thickness in the S2 sector (0.79) and for mean macular thickness in C0 and T1 sectors (0.76). Positive correlations were observed in some areas between individual retinal layers and VD in their corresponding vascular plexuses in the AD group. The findings suggest that alterations in retinal layer thickness and VD may serve as potential non-invasive diagnostic markers for AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder and the most common cause of dementia among the elderly1. First identified in the early 20th century, it remains without an effective treatment, highlighting the need for early detection and accurate diagnosis to slow the progression of neurodegenerative processes2,3.
In 2018, the National Institute on Aging and the Alzheimer’s Association (NIA-AA) revised the diagnostic framework for AD, proposing a biomarker classification system known as A/T/N. This system integrates three types of biomarkers, β-amyloid (Aβ) accumulation, pathological tau (T), and neurodegeneration or neuronal injury markers (N), to enhance diagnostic accuracy4. Notably, these biomarkers may show abnormalities in the preclinical phase of AD, which precedes the onset of behavioral symptoms5. Current diagnostic methods—such as positron emission tomography (PET), tau-PET, and lumbar puncture for assessing Aβ or tau levels in cerebrospinal fluid—are frequently expensive, invasive, and time-consuming6,7. These limitations drive the search for alternative diagnostic strategies that confirm AD faster and less invasively.
The interconnection between the brain and the retina has significant implications for the diagnosis of neurodegenerative diseases, including AD8. The accumulation of Aβ in the retina may serve as an early indicator of brain pathology. Retinal ganglion cells (RGC), which contribute to the optic nerve, are known to transport amyloid precursor protein, suggesting that retinal changes may reflect underlying brain processes9. This relationship opens avenues for the development of non-invasive diagnostic methods that may facilitate early detection of neurodegenerative diseases through retinal assessment. Moreover, understanding this relationship may pave the way for new therapeutic options for diseases affecting the nervous system.
Since the introduction of optical coherence tomography (OCT) in the 1990s, measurements of the peripapillary retinal nerve fiber layer (pRNFL) thickness has become a widely accepted parameter in the diagnosis and monitoring of various conditions, especially those affecting the optic nerve, such as glaucoma10,11. In particular, thinning of the pRNFL and the ganglion cell layer (GCL) due to retinal ganglion cells (RGC) damage has been observed in neurodegenerative diseases, including Parkinson’s disease12, multiple sclerosis13, dementia with Lewy bodies14, and especially AD15.
OCT angiography (OCTA) extends the capabilities of OCT by providing a non-invasive method for both quantitative and qualitative assessment of vascular status in the macula and optic nerve head (ONH). Postmortem studies of AD patients have shown that neuronal apoptosis in the central nervous system correlates with vascular changes, specifically amyloid angiopathy16. These pathological alterations affect not only neuronal cells but also the vascular system, highlighting the potential of OCTA as a valuable diagnostic tool for AD17.
Recent studies using spectral-domain OCT (SD-OCT) have identified significant changes in retinal thickness surrounding the macula, particularly in specific layers such as the pRNFL and the ganglion cell layer (GCL)18,19. Additionally, a decrease in vascular density (VD), particularly in the deep vascular plexus (DVP), has been documented through OCTA20. However, the individual retinal layers with the most pronounced thickness changes have not been thoroughly investigated.
The aim of this study was to investigate the thickness of individual retinal layers in the macula and analyze the correlations between these measurements and vascular density in the corresponding superficial vascular plexus (SVP) and DVP among patients with AD. Additionally, the study evaluated the diagnostic capability of these measurements—including both retinal layer thickness and VD—in discriminating AD from a healthy control group (HC).
Results
Nine eyes were initially excluded from the analyses due to poor image quality caused by vitreous floaters, motion artifacts, and incorrect segmentation in OCTA or SD-OCT examinations. A total of 55 eyes were included in the final evaluation and statistical analyses, comprising 23 eyes from the AD group and 22 eyes from the HC group. No significant differences in age, gender, or BCVA (best corrected visual acuity) were observed between the groups (p < 0.05). The AD group exhibited a significantly lower MMSE (mini-mental state examination) score compared to the HC group (p < 0.001). The demographic and clinical characteristics are presented in Table 1.
Macular retinal thickness
In the analysis of the macular retina, patients with AD showed a significant reduction in mean macular thickness compared to HC participants. The most notable differences between the study groups were found in the following areas: C0 (p = 0.003), T1 (p = 0.03), N1 (p = 0.09), I1 (p = 0.014), and S1 (p = 0.038). No significant differences were observed in the perifoveal area across any of the assessed quadrants (p > 0.05). Regarding the inner retinal layer (IRL) thickness, significant changes were noted between the examined groups, except for two sectors: S2 (p = 0.363) and N2 (p = 0.195). The largest differences occurred in the C0 sector (p = 0.003) and the T1 sector (p = 0.005). In contrast, the outer retinal layer (ORL) values were comparable between the AD and HC groups across all examined sectors (p > 0.05) (Table 2).
Individual macular layer thicknesses in segmentation analysis
Thickness values for all individual layers across the 9 Early Treatment Diabetic Retinopathy Study (ETDRS) sectors were obtained and are presented in Table 3. The most significant differences between the studied groups were observed in the inner nuclear layer (INL) for the following sectors: C0, S1, T1, I1, N1, and I2, as well as in the outer nuclear layer (ONL) for sectors C0, S1, T1, S2, and T2 (p < 0.39). Significant changes were also noted in the inner plexiform layer (IPL) only in sectors C0 and T2 (p < 0.048), as well as in the macular RNFL (mRNFL) and GCL in sector C0 (p < 0.039). Insignificant differences in thickness for the entire analyzed area between studied groups were observed in the OPL (p > 0.05). The most frequent changes were observed in the C0 sector across each analyzed layer (excluding the OPL); however, the most significant change was observed in the T2 sector of the ONL (p < 0.001).
Vessel density in the macular area
The AD group showed a decreased VD in the macular area compared to the HC group, along with an increased foveal avascular zone (FAZ) (p = 0.021). Significant changes in VD were observed in the DVP of the whole enface image (p = 0.030), as well as in the fovea (p = 0.004) and the perifoveal area (p = 0.012). Although no statistically significant changes were identified in SVP, the p-value for SVP in the fovea approached the significance threshold (p = 0.05) (Table 4).
Areas under the receiver operating characteristic curves
The areas under the receiver operating characteristic curves (AUC) were calculated to evaluate the diagnostic capacity of these results in discriminating between AD and HC using the parameters with the most significant differences (AUC > 0.7). The highest AUC for individual macular layer thicknesses in the segmentation analysis were 0.79 (0.65–0.93) for the ONL in the S2 sector, 0.76 (0.62–0.88) for mean macular retinal thickness in the C0 sector, and 0.76 (0.62–0.90) in the T1 sector. For VD in individual plexuses, a value of 0.75 (0.61–0.89) was obtained for the foveal DVP (Fig. 1) (Supplementary Table 1).
Spearman correlations
Table 5 presents the Spearman correlations between VD and the thickness of the corresponding retinal layers across the same study areas. For this analysis, VD in the SVP was correlated with the thickness of the retinal layers between the ILM and the outer boundary of the IPL, while VD in the DVP was correlated with the thickness of the retinal layers between the INL and the outer boundary of the OPL. Positive correlations were observed between the ILM-IPL in the foveal area and the VD measured in the corresponding SVP (R = 0.421; p = 0.005), between the INL-OPL in the foveal area and the VD measured in the corresponding DVP (R = 0.396; p = 0.007), and between the ILM-IPL in the perifoveal area and the VD measured in the corresponding SVP (R = 0.388; p = 0.008).
Discussion
The results of our study offer valuable insights into the thickness of individual retinal layers and vascular changes in the macula, highlighting their potential as diagnostic markers for AD. Although some studies have investigated the thickness of retinal layers in the macula of patients with AD, further research is necessary to assess individual retinal layers and their corresponding vascular plexus.
The strength of this study comes from its focus on the analysis of individual retinal layers in the macula, allowing for a precise assessment of areas affected by pathological processes in eyes with AD. In our previous study utilizing the Avanti SD-OCT device, we demonstrated that the most significant changes in retinal thickness in AD patients were observed in the ORL, which extend from the INL to the outer RPE18 Building on this knowledge, our current study employing Spectralis SD-OCT technology reveals that the most pronounced changes occur in the INL and ONL, reinforcing our earlier observations that classified the retina solely into IRL and ORL. Additionally, we quantitatively assessed VD in specific macular regions by analyzing both the SVP and the DVP. This approach enabled us to correlate the thickness of individual retinal layers with their corresponding vascular plexuses in specific areas of the macula.
We confirmed that AD patients have a thinner mean retinal thickness in the macula compared to the HC group, and the greatest differences were observed in the central retinal thickness (C0) and in individual sectors (T1, S1, I1, N1) where the highest number of retinal ganglion cells (RGCs) are located. The changes in the retina may reflect the underlying neurodegenerative processes associated with AD. These results are consistent with previous studies that have indicated RGCs degeneration as an important marker of AD pathology21,22.
The degeneration of ganglion cells and their axonal and dendritic projections to the retina may be the result of premature apoptotic processes, probably related to the accumulation of Aβ and tau proteins23,24. As AD progresses, it can be assumed that the structural abnormalities of the retina, especially its IRL, are thought to reflect pathological changes occurring in the brain, which may facilitate the identification of AD in its early stages25.
In contrast to the IRL, the results regarding the thickness of the ORL did not show significant differences between the groups (p > 0.05). This observation suggests that retinal changes in AD patients may be more pronounced in the IRL, reflecting more advanced degenerative processes. Such differences may be consistent with other studies that have shown that the thickness of the ORL is less sensitive to AD-related changes, in contrast to the IRL26. The decrease in IRL thickness may reflect more critical pathophysiological processes related to neuronal damage and degenerative dynamics in the brain27.
Another important finding was the change in INL thickness. The mean values of the INL thickness in sectors C0, S1, T1, I1, N1, and I2 revealed significant differences between the groups, which may indicate severe damage to this layer as a result of neurodegeneration. This is particularly important as the INL is an area of the neuron in the retina that is associated with the synaptic network, which may be crucial in the context of signal transmission and overall retinal function28,29.
An equally important finding was the change in ONL thickness. The mean values of the ONL thickness showed significant differences between the groups, indicating severe damage as a result of neurodegeneration. The ONL layer is crucial for the function of the photoreceptor cells, which are responsible for vision and image processing29. In the context of AD, Santos et al. suggests that the loss of the ONL may indicate retrograde transsynaptic degeneration and that changes in the thickness of this layer may be important for the overall function of the retina and its ability to process visual information30.
In line with our findings concerning the most pronounced changes in the IRL of the retina, recent electrophysiological studies have shown impairments in the function of RGCs and subtle alterations in photoreceptors in patients with AD. Reduced amplitudes of electroretinograms (ERG) have been linked to dysfunction in the IRL particularly in RGCs, suggesting a potential loss of function that may correlate with disease severity31,32. These electrophysiological changes provide additional support for the hypothesis that retinal degeneration in AD may reflect the neurodegenerative processes occurring in the brain. Furthermore, histological analyses have confirmed these findings, revealing significant loss of both RGCs and photoreceptors, as well as bipolar cells, in the retinas of individuals with AD33. Such histological evidence strengthens the notion that the retina can serve as a valuable window into the neurodegenerative processes affecting the central nervous system.
In addition to assessing retinal layer thickness, our study confirmed significant reductions in VD, particularly in the DVP, and an increased FAZ in patients with AD compared to HC, which is consistent with previous studies20,34. These findings are particularly intriguing and suggest that vascular changes may occur concomitantly with neurodegenerative changes35. Reduced VD and integrity may reflect underlying brain microvascular dysfunction in the brain, which has been implicated in the pathophysiology of AD. Vascular dysregulation, characterized by amyloid angiopathy and neuronal cell death, may contribute to the progressive nature of AD36,37. Previous studies have emphasized the importance of ocular perfusion in maintaining retinal and nerve fibre health, and our results support the importance of vascular assessment in diagnostic procedures for suspected AD38,39.
Spearman correlation results reveal positive associations between retinal layer thickness and VD. This suggests that structural changes in the retina may be related to the overall vascular status, underlining the importance of retinal assessment as a potential indicator of pathophysiological changes in AD. These correlations suggest a reciprocal relationship between neurodegeneration and vascular health, reinforcing the need for comprehensive assessments that include both retinal thickness and VD measurements to improve diagnostic capabilities. Such correlations may point to mechanisms that protect neuronal cells in the context of neurodegenerative diseases.
The AUC analysis confirms that retinal layer thickness and changes in VD may be useful as diagnostic biomarkers in AD. The highest AUC values were obtained for ONL thickness in the S2 sector and macular retinal thickness in the central sector, raising hopes for the use of non-invasive methods such as SD-OCT and OCTA in clinical practice.
Limitations
Despite the promising results, our study has limitations. The sample size was modest and, although we observed significant differences, future studies with larger cohorts are needed to confirm our results and to clarify the potential of these retinal markers in the clinical setting. Furthermore, longitudinal studies are necessary to clarify the relationship between changes in retinal parameters and the progression of AD symptoms over time. It is also important to consider other potential confounding factors that affect retinal morphology and vascular status. For example, changes in overall vascular health may affect retinal outcomes and need to be included in future studies. Additionally, while we acknowledge the importance of vascular and metabolic factors, the lack of detailed data on blood pressure and blood glucose levels should be recognized as a limitation.
Conclusions
In conclusion, our study demonstrates that evaluation of changes in specific layers of macular retinal thickness and VD may be significant diagnostic indicators for AD. These non-invasive measurements may improve early detection and monitoring of the disease and may be a valuable adjunct to conventional diagnostic methods. Further research is needed to understand the mechanisms behind these changes and to develop standardized protocols for incorporating retinal assessments into the clinical diagnosis of AD.
Materials and methods
Study subjects
This cross-sectional study was conducted from 2017 to 2022 at the Oftalmika Eye Hospital in Bydgoszcz, Poland. The protocol of the study was approved by the Bioethical Commission of Nicolaus Copernicus University in Torun, Collegium Medicum in Bydgoszcz, and all participants provided signed informed consent. The study was conducted in accordance with the Helsinki Declaration, and participants were fully informed about the nature of the study and its potential consequences before providing their consent.
Each individual included in the study underwent a cognitive function assessment by a psychologist using the MMSE screening test. All patients underwent a comprehensive ophthalmological examination, including measurement of BCVA with Snellen charts, tonometry (Icare TAO1i, Finland Oy, Vantaa, Finland), fundus evaluation with a Volk lens, biomicroscopy, measurement of retinal structures using SD-OCT, and evaluation of VD with OCTA. The visual acuity measured with Snellen charts was subsequently converted into the logarithm of the minimum angle of resolution (logMAR) for statistical analysis. These examinations were conducted by an ophthalmologist in a single session.
A group of AD patients were referred from the Center for Psychoneurology of the Elderly in Bydgoszcz and the Department of Psychiatry of the Collegium Medicum of the Nicolaus Copernicus University in Bydgoszcz. A diagnosis of AD was made by a psychiatrist based on the guidelines of the National Institute on Aging and the Alzheimer’s Association and the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). The diagnosis was confirmed by neuroimaging, which showed the presence of amyloid plaques in the brain using positron emission tomography (PET) with the radioligand Florbetapir (18 F). Patients were classified as amyloid-positive if their neocortex standardized uptake value ratio was greater than 1.5. Patients with mild to moderate dementia (MMSE scores between 10 and 23) were eligible for inclusion in the study. Other criteria included normal intraocular pressure (IOP) ≤ 21 mmHg and the absence of fundus changes suggestive of retinal or optic nerve diseases (e.g., AMD or glaucoma).
Members of the Senior Club in Bydgoszcz formed the HC group. After giving their consent, they underwent comprehensive ophthalmological and psychological assessments. To be included in the control group, participants had to have a normal MMSE score (≥ 27 points), no evidence of any eye or neurodegenerative diseases.
Exclusion criteria included those younger than 55 years or older than 87 years, those with BCVA less than 0.6, refractive errors greater than + 3.0 Dsph or less than − 3.0 Dsph, and a history of eye surgery other than uncomplicated cataract phacoemulsification. The study also excluded individuals with any eye diseases (defined as those identified through a comprehensive ophthalmological examination, SD-OCT assessment, and patient interview) or neurodegenerative diseases (except for Alzheimer’s disease confirmed by PET scan), which were defined based on psychological assessment and patient interview. Additionally, individuals with a history of head or eye trauma, increased IOP (>21 mmHg), diabetes, uncontrolled hypertension (greater than 150/90 mm Hg), obesity (body mass index over 30 kg/m²), and smoking were excluded from the study.
Optical coherence tomography angiography
OCTA imaging was conducted using the Avanti RTVue XR (Optovue, Inc., Fremont, CA, USA), which scans at a rate of 70.000 A-scans per second and provides an axial resolution of 5 μm with a light source wavelength of 840 ± 10 nm. A system (software version 2017.1.0.151) with featuring three-dimensional Projection Artifact Removal (3D PAR) was used, which effectively reduces projection artifacts in all deeper layers while preserving their authentic layout and improving the FAZ parameters.
The macula was analyzed using B-scans covering a area of 6 × 6 mm², scanned both vertically and horizontally. Each B-scan consisted of 400 A-scans centered on the fixation point. Macular VD was assessed across the 6.0 × 6.0 mm² area, within the 1-mm diameter foveal annulus, the parafoveal area between the 1-mm and 3-mm annuli, and the perifoveal area. Data were processed using commercial software that automatically segmented the SVP and DVP and measured the VD in both plexuses. The SVP comprised the area between the ILM and the outer boundary of the IPL, while the DVP comprised the area between the outer boundary of the IPL and the outer boundary of the OPL.
Both eyes of all participants were examined on the same day between 8:00 AM and 12:00 PM following pupil dilation. Only measurements of high technical standard, with a scan quality (SQ) of 6 or above on a 10-point scale (as indicated by the commercial camera), were included in further analysis. Measurements showing motion artifacts on the en face images, such as irregular vessel patterns or blurred images, were excluded.
Spectral-domain optical coherence tomography
The retinal structure analysis was conducted using the Spectralis SD-OCT device (Heidelberg Engineering GmbH, Heidelberg, Germany), equipped with real-time eye-tracking software (Heidelberg Eye Explorer®, version 1.10.4.0). Each participant was scanned in IR + OCT mode with a 30° area setting. The acquired volumes consisted of 61 images, each measuring of 768 × 496 pixels (width × height). The axial resolution was 3.9 microns, and the transversal resolution ranged from 10 to 12 microns. Importantly, high-resolution (HR) scans were utilized to ensure optimal image clarity and precision. The spacing between B-scans varied from 120 to 140 microns. To reduce speckle noise and improve image contrast, each B-scan was averaged from five aligned images using TruTrack active eye tracking technology.
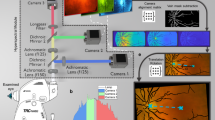
The Spectralis software provided thickness measurements for several retinal layers, including: (1) mean macular retinal thickness, (2) mRNFL, (3) GCL, (4) IPL, (5) INL, (6) OPL, (7) ONL, (8) retinal pigment epithelium (RPE), (9) IRL—defined as the area from the ILM to the external limiting membrane (ELM), and (10) ORL—defined as the area from the ELM to Bruch’s membrane (Fig. 2).
Sample results of the study for the right eye of a 78-year-old patient with Alzheimer’s disease. (A) Retinal thickness maps divided into nine subfields as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS), along with retinal layer segmentation in spectral domain optical coherence tomography. Layers: internal limiting membrane (ILM), retina nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), external limiting membrane (ELM), photoreceptor layer (PR), retina pigment epithelium (RPE), Bruch membrane (BM). (B) Superficial vascular plexus with qualitative and quantitative assessments of vessel density, divided into nine subfields as defined by the ETDRS. (C) Deep vascular plexus with qualitative and quantitative assessments of vessel density, divided into nine subfields as defined by the ETDRS.
After the automatic segmentation of each slice, the Spectralis software provides thickness maps divided into nine subfields as defined by the ETDRS. Inner, intermediate, and outer rings with diameters of 1, 3, and 6 mm respectively, were considered for the analysis. The average of all points within the inner 1-mm radius circle was defined as central foveal thickness (C0). The intermediate ring was divided into four sectors designated as inner superior (S1), inner nasal (N1), inner inferior (I1), and inner temporal (T1); and so was the outer ring, with four sectors designated as outer superior (S2), outer nasal (N2), outer inferior (I2), and outer temporal (T2). The numerical values recorded for each of the nine zones for every layer were used in the analysis.
The same experienced operator performed all scans, and no manual correction was applied to the SD-OCT output. The quality of the scans was assessed on a scale ranging from 0 (poor) to 40 (high), and scans scoring lower than 25, as well as those exhibiting incorrect segmentation, were discarded.
Statistical analysis
Statistical analyses were performed using Statistica Software (ver. 14; TIBCO Software Inc.). First of all, the data were compared between the eyes as no significant difference was found, further analyses were performed on the one eye (the right eye) of each participant. The Shapiro-Wilk test showed that the data sets were far from a normal distribution, so nonparametric tests were used for farther analyses. The median, minimum (Min), and maximum (Max) values, along with the lower (Q1) and upper (Q2) quartiles, were calculated. Among the groups, OCTA and SD-OCT data were compared by using the Mann-Whitney U Test. Correlations between measured parameters were assessed using the Spearman rang order correlations test.
The AUC was used to determine the diagnostic accuracy of the measured parameters in discriminating between AD and HD groups. The AUC of 1.0 represents perfect discrimination, a value of 0.7 or higher represents acceptable discrimination, whereas an AUC of 0.5 represents chance discrimination. Differences were considered significant if the p-value was equal to 0.05 or less.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Scheltens, P. et al. Alzheimer’s disease. Lancet 397, 1577–1590 (2021).
Hippius, H. & Neundörfer, G. The discovery of Alzheimer’s disease. Dialog Clin. Neurosci. 5, 101–108 (2003).
Juganavar, A., Joshi, A. & Shegekar, T. Navigating early Alzheimer’s diagnosis: a comprehensive review of diagnostic innovations. Cureus 15, 9 (2023).
Jack, C. R. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Dubois, B. et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 12, 292–323 (2016).
Van Oostveen, W. M. & de Lange, E. C. Imaging techniques in Alzheimer’s disease: a review of applications in early diagnosis and longitudinal monitoring. Int. J. Mol. Sci. 22, 2110 (2021).
Brier, M. R. et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci. Transl Med. 8, 338ra66 (2016).
Ausó, E., Gómez-Vicente, V. & Esquiva, G. Biomarkers for Alzheimer’s disease early diagnosis. J. Pers. Med. 10, 114 (2020).
Wang, L. & Mao, X. Role of retinal amyloid-β in neurodegenerative diseases: overlapping mechanisms and emerging clinical applications. Int. J. Mol. Sci. 22, 2360 (2021).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Bussel, I. I., Wollstein, W., Schuman, J. S. & G. & OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br. J. Ophthalmol. 98, ii15–ii19 (2014).
Satue, M. et al. Retinal thinning and correlation with functional disability in patients with Parkinson’s disease. Br. J. Ophthalmol. 98, 350–355 (2014).
Birkeldh, U. et al. Retinal nerve fiber layer thickness associates with cognitive impairment and physical disability in multiple sclerosis. Mult Scler. Relat. Disord. 36, 101414 (2019).
Moreno-Ramos, T. et al. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with lewy bodies, and Alzheimer’s disease. J. Alzheimers Dis. 34, 659–664 (2013).
Blanks, J. C., Hinton, D. R., Sadun, A. A. & Miller, C. A. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res. 501, 364–372 (1989).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Patton, N. et al. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease. J. Anat. 206, 318–348 (2005).
Zabel, P. et al. Quantitative assessment of retinal thickness and vessel density using optical coherence tomography angiography in patients with Alzheimer’s disease and glaucoma. PLoS ONE. 16, e0248284 (2021).
Mei, X. et al. Changes in retinal multilayer thickness and vascular network of patients with Alzheimer’s disease. BioMed. Eng. OnLine. 20, 1–13 (2021).
Zabel, P. et al. Comparison of retinal microvasculature in patients with Alzheimer’s disease and primary open-angle glaucoma by optical coherence tomography angiography. Investig Ophthalmol. Vis. Sci. 60, 3447–3455 (2019).
Davis, M. R. et al. Retinal ganglion cell vulnerability to pathogenic Tau in Alzheimer’s disease. BioRxiv (2024).
Kim, J. I. & Kang, B. H. Decreased retinal thickness in patients with Alzheimer’s disease is correlated with disease severity. PLoS ONE. 14, e0224180 (2019).
Gupta, V. et al. One protein, multiple pathologies: multifaceted involvement of amyloid Β in neurodegenerative disorders of the brain and retina. Cell. Mol. Life Sci. 73, 4279–4297 (2016).
Gupta, V. B. et al. Retinal changes in Alzheimer’s disease—integrated prospects of imaging, functional and molecular advances. Prog Retin Eye Res. 82, 100899 (2021).
Shi, Z. et al. Retinal nerve fiber layer thickness is associated with hippocampus and lingual gyrus volumes in nondemented older adults. Prog Neuro Psychopharmacol. Biol. Psychiatry. 99, 109824 (2020).
Cunha, L. P. et al. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer’s disease. PLoS ONE. 11, e0153830 (2016).
Chiquita, S. et al. The retina as a window or mirror of the brain changes detected in Alzheimer’s disease: critical aspects to unravel. Mol. Neurobiol. 56, 5416–5435 (2019).
López-Cuenca, I. et al. The relationship between retinal layers and brain areas in asymptomatic first-degree relatives of sporadic forms of Alzheimer’s disease: an exploratory analysis. Alzheimers Res. Ther. 14, 79 (2022).
Robinson, E. K. et al. Retinal neurodegeneration and synaptic loss in relations to core-proteinopathies and cognitive scores in MCI and AD patients. Alzheimers Dement. 20, e087799 (2025).
Santos, C. Y. et al. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement. Diagn. Assess. Dis. Monit. 10, 196–209 (2018).
Liao, C. et al. Retinal dysfunction in Alzheimer’s disease and implications for biomarkers. Biomolecules 11, 1215 (2021).
Asanad, S. et al. Retinal ganglion cell dysfunction in preclinical Alzheimer’s disease: an electrophysiologic biomarker signature. Sci. Rep. 11, 6344 (2021).
Asanad, S. et al. The retina in Alzheimer’s disease: histomorphometric analysis of an ophthalmologic biomarker. Investig Ophthalmol. Vis. Sci. 60, 1491–1500 (2019).
Yeh, T. C. et al. Retinal microvascular changes in mild cognitive impairment and Alzheimer’s disease: a systematic review, meta-analysis, and meta-regression. Front. Aging Neurosci. 14, 860759 (2022).
Gaire, B. P. et al. Alzheimer’s disease pathophysiology in the retina. Prog Retin Eye Res. 101, 101273 (2024).
Shi, H. et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 139, 813–836 (2020).
Ashraf, G. et al. Retinal imaging biomarkers of Alzheimer’s disease: A systematic review and meta-analysis of studies using brain amyloid beta status for case definition. Alzheimers Dement. Diagn. Assess. Dis. Monit. 15, e12421 (2023).
Romaus-Sanjurjo, D. et al. Alzheimer’s disease seen through the eye: ocular alterations and neurodegeneration. Int. J. Mol. Sci. 23, 2486 (2022).
Cabrera DeBuc, D., Somfai, G. M. & Koller, A. Retinal microvascular network alterations: potential biomarkers of cerebrovascular and neural diseases. Am. J. Physiol. Heart Circ. Physiol. 312, H201–H212 (2017).
Author information
Authors and Affiliations
Contributions
Conception and design: P.Z., K.Z., J.J.K. Development of methodology: P.Z., J.J.K., R.K. Data acquisition: D.J., K.S., M.G-T. Analysis and interpretation of data: P.Z., A.P-K. Statistic Review: A.P-K. Writing, review of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zabel, P., Przekoracka-Krawczyk, A., Jaworski, D. et al. Assessment of individual retinal layer thickness and vascular changes in Alzheimer’s disease. Sci Rep 15, 17287 (2025). https://doi.org/10.1038/s41598-025-02377-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02377-1
Keywords
This article is cited by
-
Combining OCT and OCT angiography improves differentiation of mild cognitive impairment and Alzheimer’s disease
Alzheimer's Research & Therapy (2026)