Abstract
This study analyzed adverse events (AEs) of GLP-1RA and metformin combination therapy using FAERS data (2004–2024). A retrospective disproportionality analysis was conducted using the reporting odds ratio (ROR) on FAERS data (2004–2024), with Bayesian confidence propagation neural network (BCPNN) employed for sensitivity analysis. Adverse reactions were compared between combination therapy and monotherapy (GLP-1 RAs or metformin alone), followed by stratified analyses by gender, age, and body weight. Among 48,214 reports (57.5% female), common AEs included nausea and weight loss. Unexpected signals included kidney injury and pancreatic cancer. Monotherapy showed higher AE rates. Males reported more renal calculi and early-onset AEs (≤ 30 days), while females had delayed AEs (> 360 days). Weight loss was consistent across all demographics. The combination therapy of GLP-1 receptor agonists and metformin demonstrates superior safety over monotherapy, while revealing novel adverse events and demographic-specific risk patterns, thereby offering reliable evidence to inform clinical practice.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) represents a chronic metabolic disorder characterized by insulin resistance and β-cell dysfunction, with its escalating global prevalence posing substantial challenges to public health systems worldwide1. Epidemiological data from the International Diabetes Federation (IDF) indicate that the global burden of diabetes affected approximately 463 million adults in 2019, with projections estimating a dramatic increase to 700 million cases by 20452. Current therapeutic strategies for T2DM management prioritize metformin as the first-line pharmacological intervention, primarily through its dual mechanisms of suppressing hepatic gluconeogenesis and enhancing peripheral glucose utilization in skeletal muscle tissue3,4. Beyond its glucose-lowering effects, metformin demonstrates additional metabolic benefits by improving insulin sensitivity and ameliorating insulin resistance5. In parallel, glucagon-like peptide-1 (GLP-1) receptor agonists have emerged as a novel therapeutic class, exerting their glucose-regulatory effects through multiple mechanisms. These agents activate the cAMP-PKA signaling cascade in pancreatic β-cells6, resulting in glucose-dependent insulin secretion, suppression of pancreatic α-cell glucagon release, and modulation of gastrointestinal motility through delayed gastric emptying7. Furthermore, GLP-1 receptor agonists demonstrate pleiotropic effects, including significant improvements in glycemic control and lipid metabolism parameters8,9.
Despite their therapeutic efficacy, the clinical application of GLP-1 receptor agonists is frequently associated with gastrointestinal complications, including nausea, vomiting, and diarrhea, with rare but serious adverse events such as pancreatitis and cholelithiasis being documented in clinical reports10,11. Similarly, metformin administration commonly induces gastrointestinal disturbances, manifesting as diarrhea, abdominal distension, and nausea12, with potentially severe complications including lactic acidosis in rare instances13. The safety profile of concomitant GLP-1 receptor agonist and metformin therapy remains controversial in current literature. While some clinical investigations indicate that combination therapy primarily exhibits gastrointestinal-related adverse effects without significant escalation in overall adverse event incidence14,15, contrasting expert opinions advocate for metformin dose reduction or discontinuation during GLP-1 receptor agonist treatment16. This discrepancy in clinical recommendations underscores the critical need for comprehensive real-world evidence studies to elucidate the safety profile and adverse reaction patterns associated with combination therapy.
The FDA Adverse Event Reporting System (FAERS), a pharmacovigilance database maintained by the U.S. Food and Drug Administration, serves as a comprehensive repository for post-marketing surveillance data, systematically collecting and analyzing spontaneous adverse drug reaction reports from global sources. This investigation utilizes the FAERS database to conduct a large-scale pharmacoepidemiologic analysis, aiming to generate robust real-world evidence regarding the safety profile of GLP-1 receptor agonist and metformin combination therapy through systematic evaluation of reported adverse events.
Materials and methods
Data source
The FDA Adverse Event Reporting System (FAERS), maintained by the U.S. Food and Drug Administration, serves as a critical pharmacovigilance tool for post-marketing surveillance of pharmaceutical products and therapeutic biologics17. This comprehensive database systematically collects and analyzes spontaneous reports of adverse drug events and medication errors, enabling the identification and evaluation of potential drug safety signals. The FAERS database undergoes quarterly updates, with all data sets publicly accessible through the FDA’s Open Data portal (https://open.fda.gov/data/downloads/).
Data extraction and processing
The FAERS database architecture incorporates seven distinct data tables, each serving specific pharmacovigilance functions: (1) DEMO (patient demographic and administrative information), (2) DRUG (medication-related data), (3) REAC (adverse event reports), (4) OUTC (patient outcomes), (5) RPSR (report source information), (6) THER (temporal drug administration data), and (7) INDI (therapeutic indications). Within the DRUG table, medications are systematically classified into four causality categories: Primary Suspect (PS), Secondary Suspect (SS), Concomitant (C), and Interacting (I). Serious adverse outcomes are categorized according to FDA-defined criteria, including: mortality (DE), life-threatening conditions (LT), hospitalization (HO), disability (DS), congenital anomalies (CA), and other medically significant events (OT). Case identification was performed through drug name queries in the DRUG table, with subsequent filtering restricted to Primary Suspect (PS) cases. Adverse event terminology standardization was achieved through implementation of the Medical Dictionary for Regulatory Activities (MedDRA), which employs a five-tier hierarchical classification system: System Organ Class (SOC), High-Level Group Term (HLGT), High-Level Term (HLT), Preferred Term (PT), and Lower-Level Term (LLT).
Data preprocessing and quality control
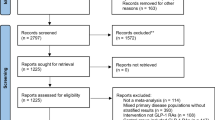
This study employed the FDA-recommended data extraction protocol to retrieve all case reports associated with GLP-1 receptor agonist and metformin combination therapy from the first quarter of 2004 (2004 Q1) to the third quarter of 2024 (2024 Q3). To ensure data integrity and minimize potential biases in disproportionality analysis, rigorous quality control measures were implemented, including systematic removal of duplicate reports using FDA-endorsed deduplication algorithms. According to FDA’s recommended method for removing duplicate reports, the PRIMARYID, CASEID, and FDA_DT fields of the DEMO table were selected and sorted in the order of CASEID, FDA_DT, and PRIMARYID, and for reports with the same CASEID, the one with the largest FDA_DT value was retained; followed by the one with the largest PRIMARYID value for reports with the same CASEID and FDA_DT. For reports with the same CASEID, the one with the largest PRIMARYID value is retained. The integrated workflow encompassing data extraction, preprocessing, and analytical procedures is illustrated in Fig. 1.
Disproportionality analysis
Disproportionality analysis represents a fundamental methodology in pharmacovigilance and drug safety surveillance, designed to identify potential safety signals through the detection of statistical imbalances in adverse drug reaction (ADR) reporting patterns. This analytical framework encompasses several established measures, including the Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS). In the current investigation, we specifically implemented the Reporting Odds Ratio (ROR) methodology to detect adverse reaction signals, utilizing both the System Organ Class (SOC) and Preferred Term (PT) classifications for the target pharmacological agents. The ROR metric serves as a quantitative indicator of the strength of association between the target drug and specific adverse events (AEs), with elevated ROR values denoting stronger statistical associations. The computational algorithm for ROR determination is detailed in Supplementary Table 1. All statistical analyses were conducted using R software (version 4.2.3, R Foundation for Statistical Computing, Vienna, Austria).
Sensitivity analysis
In pharmacovigilance studies using the FDA Adverse Event Reporting System (FAERS) database, sensitivity analysis serves to validate the robustness and reliability of drug safety signal detection. We implemented the Bayesian Confidence Propagation Neural Network (BCPNN) for sensitivity analysis, with its specific computational formula detailed in Supplementary Table 1. All analyses were conducted using R software (version 4.2.3).
Time-to-onset analysis
The analysis commenced with the exclusion of reports containing data inconsistencies, specifically those where the event date (EVENT_DT) preceded the medication initiation date (START_DT) or where essential temporal information was absent. The time-to-onset interval was subsequently computed as the temporal difference between EVENT_DT (the recorded date of adverse event manifestation) and START_DT (the documented date of therapeutic intervention initiation). For the characterization of time-to-onset distribution patterns, we employed robust statistical descriptors, specifically the median value accompanied by the interquartile range (IQR), to account for potential distributional skewness and outlier effects.
Data visualization
We used R 4.3.3 along with the ggplot2 package in R and WPS for plotting.
Ethical statement
The data from the FAERS database is freely available for download and use. This study does not require ethical review or approval but must comply with relevant laws and regulations.
Results
Descriptive analysis
The pharmacovigilance database analysis identified 48,214 adverse drug reaction (ADR) reports associated with concomitant administration of GLP-1 receptor agonists and metformin from 2004 through the third quarter of 2024. Table 1 presents the demographic and clinical characteristics of the reported cases, stratified by gender distribution, age groups, body weight categories, reporter profiles, geographical origin, and clinical outcomes.
The gender distribution revealed that 57.5% of reports involved female patients, while male patients accounted for 40.5%, with the remaining 2.0% lacking gender specification. Body weight analysis demonstrated that 26.4% of patients fell within the 50–100 kg range, while 17.9% exceeded 100 kg and 0.3% were below 50 kg; however, weight data were unavailable for 55.4% of cases. Age stratification indicated that 45.6% of patients were aged 18–65 years, 23.9% were over 65 years, and 0.1% were under 18 years, with 30.5% of reports lacking age information.
The majority of ADR reports (72.5%) originated from drug consumers, with healthcare professionals accounting for the remainder. The most frequently reported clinical outcomes included initial hospitalization or prolonged hospitalization (13.6%) and other serious medical events (12.3%). Geographically, the United States contributed the majority of reports (85.0%), followed by France (2.6%) and the United Kingdom (2.3%).
Temporal analysis, as illustrated in Fig. 2, demonstrated a progressive increase in ADR reporting beginning in 2005, reaching peak levels in 2007, and subsequently stabilizing after 2015. This temporal pattern suggests potential changes in reporting practices or drug utilization trends during the study period.
Signal detection based on SOC levels
The pharmacovigilance analysis revealed that adverse drug reactions (ADRs) associated with concomitant administration of GLP-1 receptor agonists and metformin spanned 27 distinct System Organ Classes (SOCs), as depicted in Fig. 3. However, only five SOCs demonstrated statistically significant associations based on the predefined Reporting Odds Ratio (ROR) criteria, excluding product-related issues. The significant SOCs with their respective ROR values were as follows:
-
General disorders and administration site conditions (n = 32,110; ROR = 1.1, 95% CI 1.09–1.11)
-
Gastrointestinal disorders (n = 33,685; ROR = 2.64, 95% CI 2.61–2.67)
-
Metabolism and nutrition disorders (n = 10,775; ROR = 3.09, 95% CI 3.03–3.15)
-
Investigations (n = 30,802; ROR = 3.35, 95% CI 3.31–3.39)
As illustrated in Fig. 4, the three most frequently reported SOC categories were "Gastrointestinal disorders," "General disorders and administration site conditions," and "Investigations." Notably, while "Injury, poisoning, and procedural complications" and "Nervous system disorders" demonstrated higher reporting frequencies compared to other SOCs, these associations did not reach statistical significance in our analysis, suggesting potential reporting bias or confounding factors that warrant further investigation.
Signal detection based on PT levels
In the Preferred Term (PT) level analysis, we implemented a rigorous screening protocol to exclude conditions that might represent therapeutic indications for GLP-1 receptor agonists and metformin combination therapy, as well as events clearly unrelated to pharmacologically-induced adverse reactions. Following this exclusion process, we conducted a quantitative analysis of the remaining adverse events (AEs), ranking them by reporting frequency and selecting the top 50 PTs for detailed evaluation (Fig. 5). The complete list of analyzed PTs is provided in Supplementary Table 2.
The pharmacovigilance analysis revealed a positive correlation between reporting frequency and signal strength. Notably, several well-documented AEs demonstrated significant reporting patterns, including nausea (n = 9651; ROR = 4.61, 95% CI 4.51–4.7), vomiting (n = 4383; ROR = 3.44, 95% CI 3.34–3.55), weight loss (n = 7815; ROR = 10.62, 95% CI 10.37–10.86), and pancreatitis (n = 1389; ROR = 9.59, 95% CI 9.09–10.12), all of which are consistent with established safety profiles documented in the prescribing information.
Furthermore, our analysis identified several potentially novel safety signals that warrant further investigation:
-
Acute kidney injury (n = 675; ROR = 1.62, 95% CI 1.5–1.75)
-
Pancreatic cancer (n = 507; ROR = 5.96, 95% CI 5.46–6.5)
-
Visual impairment (n = 379; ROR = 1.13, 95% CI 1.02–1.25)
-
Lactic acidosis (n = 567; ROR = 6.71, 95% CI 6.18–7.3)
-
Diabetic ketoacidosis (n = 383; ROR = 5.72, 95% CI 5.17–6.33)
These findings not only validate known safety concerns but also highlight potential areas for further pharmacovigilance monitoring and clinical investigation.
Time-to-onset analysis
The time-to-onset analysis encompassed 46,336 reports containing complete temporal data for adverse event (AE) occurrence. Following rigorous quality control measures, which included the exclusion of duplicate entries, reports with incomplete demographic data (age or gender), and cases with missing temporal information, we retained 13,047 AE reports associated with concomitant GLP-1 receptor agonist and metformin therapy for final analysis. The temporal distribution of AE onset demonstrated a median time of 59 days (interquartile range: 16–212 days), indicating substantial variability in the latency period of adverse events.
Gender-stratified analysis revealed distinct temporal patterns in AE manifestation, as illustrated in Fig. 6. Male patients predominantly experienced AEs within the initial 30-day window following treatment initiation, whereas female patients exhibited a delayed onset pattern, with the majority of AEs emerging after 360 days of continuous therapy. Notably, both gender groups demonstrated the potential for AE development beyond the one-year treatment threshold, underscoring the importance of sustained pharmacovigilance throughout the treatment course.
These findings emphasize the necessity for continuous, long-term monitoring of patients receiving combination therapy with GLP-1 receptor agonists and metformin, regardless of treatment duration, to ensure timely identification and management of potential adverse events.
Group design and comparative analysis
Comparative safety profile: GLP-1 receptor agonist monotherapy versus combination therapy with metformin
The pharmacovigilance analysis encompassed 262,355 adverse event (AE) reports associated with GLP-1 receptor agonist therapy. Through systematic evaluation based on report frequency and reporting odds ratio (ROR) calculations, the top 50 preferred terms (PTs) were identified for comprehensive visualization (Supplementary Fig. 1), with the complete dataset available in Supplementary Table 3.
Comparative safety assessment demonstrated a statistically significant reduction in adverse reaction incidence with combination therapy (GLP-1 receptor agonists plus metformin) compared to monotherapy. The most pronounced differences were observed in gastrointestinal and metabolic events: nausea (combination: n = 9651 vs monotherapy: n = 38,030), vomiting (4383 vs 17,062), pancreatitis (1389 vs 6655), and hypoglycemia (773 vs 1778). These findings suggest that the therapeutic combination may offer an improved safety profile while maintaining therapeutic efficacy.
Comparative safety evaluation: metformin monotherapy versus combination therapy with GLP-1 receptor agonists
The safety analysis incorporated 82,244 adverse event (AE) reports associated with metformin-based therapies. Through systematic evaluation utilizing report frequency and reporting odds ratio (ROR) metrics, the top 50 preferred terms (PTs) were identified for detailed visualization (Supplementary Fig. 2), with the complete dataset cataloged in Supplementary Table 4.
The pharmacovigilance data revealed a significantly elevated incidence of adverse reactions in the metformin monotherapy cohort compared to the combination therapy group. Particularly noteworthy were the marked differences in serious metabolic and renal complications: lactic acidosis (monotherapy: n = 15,789 vs combination: n = 567), acute kidney injury (7694 vs 675), diarrhea (6922 vs 3890), and metabolic acidosis (4713 vs 203). These findings suggest that the addition of GLP-1 receptor agonists to metformin therapy may mitigate certain serious adverse effects associated with metformin monotherapy.
Subgroup analysis methodology
Subgroup analysis represents a critical methodological approach in pharmacovigilance studies, involving the stratification of study populations based on predefined demographic and clinical characteristics, including age, gender, body mass index, and comorbid conditions. This analytical framework facilitates the calculation of effect size estimates within each stratified subgroup, thereby enhancing the precision of adverse drug reaction (ADR) signal detection. Furthermore, this approach enables the identification of previously unrecognized or population-specific adverse events through comparative analysis across subgroups, contributing to more comprehensive drug safety profiling.
Gender-specific adverse event profiling
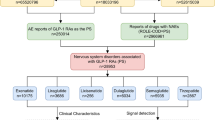
A comprehensive gender-based subgroup analysis was conducted to evaluate differential adverse reaction patterns associated with GLP-1 receptor agonist and metformin combination therapy. The analysis incorporated 47,226 adverse event reports, stratified by gender (male: n = 19,518; female: n = 27,708). Through systematic evaluation based on report frequency, the top 50 preferred terms (PTs) for each gender subgroup were identified and visualized (Supplementary Figs. 3–4), with complete datasets available in Supplementary Tables 5–6.
The analysis revealed four common adverse events across both genders: nausea, weight loss, vomiting, and diarrhea. Subsequent stratification by report frequency and reporting odds ratio (ROR) identified the top 25 gender-specific PTs, which were subsequently visualized through volcano plot analysis (Fig. 7). The visualization employed a dual-color coding system (red: female-specific PTs; blue: male-specific PTs), revealing distinct gender-based patterns. Notably, injection site reactions demonstrated significantly higher prevalence in female patients (p < 0.05). Of particular clinical importance was the identification of metastatic pancreatic cancer reports exclusively within the male subgroup among the top 50 PTs, warranting further investigation into potential gender-specific pharmacological responses.
Age-stratified adverse event analysis
A comprehensive age-based subgroup analysis was conducted to evaluate adverse reaction profiles associated with GLP-1 receptor agonist and metformin combination therapy across three distinct age cohorts: pediatric (< 18 years; n = 54), adult (18–65 years; n = 21,975), and geriatric (> 65 years; n = 11,497) populations. Through systematic evaluation utilizing report frequency and reporting odds ratio (ROR) metrics, the top 50 preferred terms (PTs) for each age group were identified and visualized (Supplementary Figs. 5–7), with complete datasets cataloged in Supplementary Tables 7–8.
The analysis revealed distinct age-related patterns in adverse event profiles. Gastrointestinal-related events, including nausea, vomiting, decreased appetite, and weight loss, were prevalent across both adult and geriatric populations. Notably, injection site reactions demonstrated significantly higher incidence in the adult cohort compared to geriatric patients (p < 0.05), while renal complications, particularly acute kidney injury, showed increased prevalence in the geriatric population. The pediatric cohort, though limited in sample size (n = 10), demonstrated unique adverse event patterns, with acute pancreatitis emerging as a relatively common occurrence, alongside notable reports of somnolence, warranting further investigation into age-specific pharmacological responses.
Weight-stratified adverse event profiling
Given the well-established positive correlation between body weight and type 2 diabetes risk, a weight-based subgroup analysis was conducted to evaluate differential adverse reaction patterns associated with GLP-1 receptor agonist and metformin combination therapy. Patients were stratified into three weight categories: low-weight (< 50 kg; n = 142), intermediate-weight (50–100 kg; n = 12,737), and high-weight (> 100 kg; n = 8613) groups. Through systematic evaluation based on report frequency and reporting odds ratio (ROR) metrics, the top 50 preferred terms (PTs) for each weight category were identified and visualized (Supplementary Figs. 8–10), with complete datasets available in Supplementary Tables 9–10.
The analysis revealed consistent gastrointestinal-related adverse events across all weight groups, including nausea, vomiting, decreased appetite, and weight loss. Notably, weight-specific patterns emerged in serious adverse events: papillary thyroid carcinoma demonstrated significantly higher incidence in the high-weight cohort compared to other groups (p < 0.05), while acute kidney injury showed increased prevalence in the intermediate-weight group. These findings suggest that body weight may serve as a significant modifier of adverse event profiles in patients receiving GLP-1 receptor agonist and metformin combination therapy, warranting further investigation into weight-dependent pharmacological responses.
Sensitivity analysis
We performed sensitivity analysis using the Bayesian Confidence Propagation Neural Network (BCPNN) to validate signals initially screened by the Reporting Odds Ratio (ROR). The reliability of signals was assessed by evaluating consistency between the two methodologies.
Based on the combined results of ROR and BCPNN analyses, we compared drug safety signals between monotherapy and combination therapy. The findings demonstrated that the reporting frequency of gastrointestinal adverse reactions (such as nausea and vomiting) in the combination therapy group was lower than in the monotherapy group, although the signal strength of these risks was comparable between the two groups. This suggests a relatively lower risk of gastrointestinal adverse reactions in combination therapy, yet a persistent strong association. Additionally, metabolic adverse reactions like weight loss and hypoglycemia showed similar profiles between combination therapy and monotherapy, supported by BCPNN results, indicating no significant differences in metabolic safety. However, combination therapy exhibited advantages over monotherapy in reducing the incidence of severe adverse reactions, particularly renal impairment and lactic acidosis. For example, the reporting frequency of lactic acidosis was significantly higher in the metformin monotherapy group compared to the combination therapy group (ROR = 293.53, 95% CI 286.44–300.8 vs. ROR = 6.71, 95% CI 6.18–7.3). This result was further corroborated by BCPNN analysis (IC[IC025] = 6.85 [6.83] vs. IC[IC025] = 2.72 [2.59]), highlighting the superior safety profile of combination therapy in mitigating severe metabolic abnormalities such as lactic acidosis.
To further explore safety differences among different subgroups, we performed sensitivity analyses stratified by gender, age, and body weight. The results revealed that gender, age, and body weight significantly influenced both the incidence and types of adverse drug reactions. In the gender subgroup analysis, we found notable differences in the timing of adverse reactions between male and female patients. Male patients showed higher reporting rates of kidney stones and early-onset adverse reactions (≤ 30 days), while female patients were more prone to delayed adverse reactions (> 360 days). The BCPNN analysis further validated these findings, demonstrating that female patients had higher risks of long-term treatment-related adverse reactions, whereas male patients experienced more acute adverse events in the short term.
In the age-stratified analysis, elderly patients (> 65 years) receiving combination therapy demonstrated significantly elevated risks of acute kidney injury. Both disproportionality analyses confirmed this heightened risk: ROR analysis showed a statistically significant association (ROR = 1.81, 95% CI 1.62–2.03), while BCPNN yielded an information component of IC = 0.85 (IC025 = 0.69), both indicating substantially greater renal impairment risks in this older population treated with the combination therapy. This observed risk profile likely stems from two pharmacological factors: (1) the contraindication of metformin in patients with renal insufficiency, and (2) the potential dehydrating effects associated with GLP-1 receptor agonists. In marked contrast, younger patients (18–64 years) exhibited no statistically significant renal adverse reactions when receiving the same combination treatment regimen.
In the body weight subgroup analysis, obese patients (> 100 kg) demonstrated significantly higher incidence rates of papillary thyroid carcinoma, as evidenced by both disproportionality analyses: ROR analysis revealed a strong association (ROR = 36.87, 95% CI 25.65–53.02) and BCPNN showed a substantial information component (IC = 4.48, IC025 = 4.00), consistently validating this safety signal. Furthermore, this patient subgroup reported increased adverse events related to weight loss (ROR = 10.85, 95% CI 9.12–12.91) and metabolic disturbances. In contrast, underweight patients (< 50 kg) did not exhibit any statistically significant metabolic-related adverse reaction signals in our analyses.
In summary, the sensitivity analyses conducted across all subgroups demonstrated consistent results between both BCPNN and ROR methodologies, thereby providing robust evidence to support the identification of these drug safety signals. All preferred terms (PTs) meeting the BCPNN algorithm criteria are comprehensively presented in Supplementary Tables 11–21.
Discussion
This study, based on data from the U.S. FDA Adverse Event Reporting System (FAERS), systematically extracted and analyzed adverse drug reactions associated with the combination therapy of GLP-1 receptor agonists and metformin for the first time. The results demonstrated that combination therapy is superior to monotherapy in reducing severe risks such as lactic acidosis and acute kidney injury. However, gastrointestinal reactions (e.g., nausea, vomiting) and metabolism-related events (e.g., weight loss) remain primary concerns. While previous studies have well-established the safety profiles of GLP-1RA and metformin monotherapies18,19, there remains a critical knowledge gap regarding combination therapy safety and demographic heterogeneity. Our investigation conducted a comprehensive pharmacovigilance analysis across sex-, age-, and body weight-stratified subgroups, revealing clinically significant disparities in adverse reaction patterns. These findings provide actionable insights for personalized safety assessment of antidiabetic combination regimens.
Existing studies suggest that combination therapy generally does not increase the risk of gastrointestinal adverse reactions20,21. GLP-1RA activates GLP-1 receptors on the gastric vagal afferent fibers, inhibiting the release of NO from nitrergic neurons, reducing the amplitude of gastric antrum smooth muscle contraction, and thus delaying gastric emptying22. Metformin inhibits mitochondrial respiratory chain complex I, leading to an increase in the intracellular AMP/ATP ratio, which activates AMPK. AMPK upregulates the activity of sodium-glucose co-transporter 1 (SGLT1), enhancing the active absorption of glucose in the intestine23. The combination of these two may prevent excessive delay in gastric emptying, thus reducing the occurrence of gastrointestinal adverse reactions24,25. However, a meta-analysis indicated a potential elevation in risk26.
The safety advantages of combination therapy may stem from the synergistic mechanisms of the drugs: Metformin activates hepatic AMP-activated protein kinase (AMPK), and AMPK phosphorylates the transcription factor CREB, reducing its binding to the promoters of PEPCK and G6Pase, thereby inhibiting the expression of key enzymes in gluconeogenesis and suppressing hepatic glucose production. Metformin also enhances GLUT4 translocation in muscle cells via AMPK activation, promoting glucose uptake and improving insulin sensitivity27,28. Meanwhile, GLP-1 receptor agonists activate GLP-1 receptors on gastric vagal afferent fibers, inhibiting the release of NO from nitrergic neurons, reducing the amplitude of gastric antrum smooth muscle contraction, and thus delaying gastric emptying. GLP-1RA also activates GLP-1 receptors on β-cells, increasing intracellular cAMP levels, activating protein kinase A (PKA), promoting insulin gene transcription, synthesis, and glucose-dependent secretion, thereby stimulating insulin secretion and exerting a synergistic effect29,30. In this study, the reporting rates of severe metabolic acidosis (n = 567) and acute kidney injury (n = 675) in combination therapy were significantly lower than those in metformin monotherapy (15,789 cases vs. 7694 cases), consistent with guideline recommendations that combination regimens can reduce metformin dependence31. GLP-1RA improves blood sugar control, reduces insulin resistance, and promotes weight loss, indirectly reducing glomerular hyperfiltration and delaying the progression of diabetic nephropathy32. This, in turn, allows metformin to be used within a safer range of kidney function, thereby indirectly reducing nephrotoxicity33,34. However, although the reporting rates of nausea (n = 9651) and vomiting (n = 4383) in combination therapy were lower than those in GLP-1RA monotherapy (38,030 cases vs. 17,062 cases), they remained significantly higher than those in the background population. This observation aligns with findings from multicenter trials—Marso et al.35 noted that gastrointestinal reactions in combination therapy were predominantly concentrated in the initial phase and could be alleviated through gradual dose adjustments35.
The study detected potential signals such as pancreatic cancer and papillary thyroid carcinoma (in the > 100 kg group), although the number of cases was limited (507 cases), necessitating cautious interpretation. The carcinogenic risk of GLP-1RAs remains controversial: large-scale cohort studies have not identified an association with pancreatic cancer36, but animal experiments suggest that long-term high-dose use may induce ductal hyperplasia37,38. Notably, obesity (> 100 kg) as an independent risk factor for various cancers39 may confound causality determination. The signal for acute kidney injury (675 cases) was more prominent in elderly and overweight groups, potentially linked to metformin’s contraindication in renal impairment34 and the dehydration effects of GLP-1RAs40, highlighting the need for strict baseline renal function screening and monitoring of blood volume status.
There are significant demographic differences in adverse reactions: 57.5% of adverse events (AEs) in females occur after one year of treatment, whereas 40.5% in males are concentrated within 30 days. This may be related to gender-related pharmacokinetic differences (lower clearance of GLP-1RA in females)41 and postmenopausal changes in gastrointestinal motility42. The elderly group (> 65 years) has an increased risk of acute kidney injury and hypoglycemia, which is associated with declining renal function and polypharmacy43. The overweight group (> 100 kg) shows a higher reporting rate of papillary thyroid carcinoma, potentially linked to the proliferative mechanisms of GLP-1RA37 and the synergistic effects of obesity-related chronic inflammation44. Regular thyroid screening is recommended for such patients.
The study has the following limitations: (1) underreporting and selection bias in FAERS data,as it lacks a comprehensive list of concomitant medications, which may lead to biased analysis results. Further in-depth research will be conducted in future studies; (2) incomplete control of confounding factors (comorbidities, concomitant medications); (3) insufficient sample size in some subgroups (e.g., 54 cases in the < 18 years group); (4) the ROR method can only suggest associations. Recommendations for clinical practice include: (1) monitoring gastrointestinal reactions during the initial phase and adjusting doses incrementally; (2) regular assessment of renal function and thyroid morphology in elderly and overweight patients; (3) attention to rare events during long-term treatment (e.g., visual impairment). Future research should focus on: (1) drug metabolic interactions; (2) dose–response relationships in obesity-cancer; (3) the development of AI-based models for predicting adverse reactions45.
Conclusion
This first comprehensive pharmacovigilance analysis of GLP-1 receptor agonist (GLP-1RA) and metformin combination therapy, utilizing FDA Adverse Event Reporting System (FAERS) data (2004–2024), demonstrates superior safety profiles compared to monotherapy, with significant reductions in severe adverse events (AEs) such as lactic acidosis (ROR = 6.71, 95% CI 6.18–7.3 vs. 15,789 monotherapy cases) and acute kidney injury (ROR = 1.62, 95% CI 1.5–1.75 vs. 7694 cases). Synergistic mechanisms—metformin’s insulin sensitization and GLP-1RA’s renal protection—underpin these benefits. However, gastrointestinal reactions (nausea, vomiting) and metabolic disturbances (weight loss) persist as predominant concerns, albeit at lower rates than GLP-1RA monotherapy. Novel signals, including pancreatic cancer (ROR = 5.96, 95% CI 5.46–6.5) and visual impairment (ROR = 1.13, 95% CI 1.02–1.25), emerged alongside demographic disparities: females exhibited delayed AE onset (> 360 days; 57.5%), while males experienced early-phase events (≤ 30 days; 40.5%). High-risk subgroups (elderly > 65 years, obese > 100 kg) showed elevated risks of acute kidney injury and papillary thyroid carcinoma. Despite FAERS limitations (underreporting bias, confounding factors), these findings advocate for tailored monitoring protocols—gradual dose titration, renal/thyroid surveillance in high-risk groups, and long-term vigilance for rare AEs. Future research should prioritize prospective validation of signals, pharmacokinetic interactions, and obesity-cancer dose–response relationships to optimize diabetes management strategies.
Data availability
All original data can be accessed in the The FDA Adverse Event Reporting System (FAERS) database (https://open.fda.gov/data/downloads/).
References
Khan, M. A. B. et al. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 10(1), 107–111 (2020).
Zhang, S. et al. Research hotspots and trends in diabetes and insulin resistance: A bibliometric analysis. Front. Nutr. 11, 1480491 (2024).
Baur, J. A. & Birnbaum, M. J. Control of gluconeogenesis by metformin: Does redox trump energy charge?. Cell. Metab. 20(2), 197–199 (2014).
Viollet, B. et al. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 122(6), 253–270 (2012).
Rena, G., Hardie, D. G. & Pearson, E. R. The mechanisms of action of metformin. Diabetologia 60(9), 1577–1585 (2017).
Wang Y, Liu Q, Kang SG, Huang K, Tong T. Dietary Bioactive Ingredients Modulating the cAMP Signaling in Diabetes Treatment. Nutrients. 2021;13(9).
Nauck, M. A. & Meier, J. J. Incretin hormones: Their role in health and disease. Diabetes Obes Metab. 20(Suppl 1), 5–21 (2018).
Prada-Oliveira, J. A. et al. GLP-1 mediated improvement of the glucose tolerance in the T2DM GK rat model after massive jejunal resection. Ann. Anat. 223, 1–7 (2019).
Inoue, H. et al. Efficacy of DPP-4 inhibitors, GLP-1 analogues, and SGLT2 inhibitors as add-ons to metformin monotherapy in T2DM patients: A model-based meta-analysis. Br. J. Clin. Pharmacol. 85(2), 393–402 (2019).
Suissa, K., Cromer, S. J. & Patorno, E. GLP-1 receptor agonists and gastrointestinal adverse events. JAMA 331(10), 884–885 (2024).
He, L. et al. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: A systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med. 182(5), 513–519 (2022).
Nabrdalik, K. et al. Gastrointestinal adverse events of metformin treatment in patients with type 2 diabetes mellitus: A systematic review, meta-analysis and meta-regression of randomized controlled trials. Front. Endocrinol. 13, 975912 (2022).
Salpeter, S. R., Greyber, E., Pasternak, G. A. & Salpeter, E. E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2010(4), Cd002967 (2010).
Klein, K. R. et al. Occurrence of gastrointestinal adverse events upon GLP-1 receptor agonist initiation with concomitant metformin use: A post hoc analysis of LEADER, STEP 2, SUSTAIN-6, and PIONEER 6. Diabetes Care 47(2), 280–284 (2024).
Xie, Z., Hu, J., Gu, H., Li, M. & Chen, J. Comparison of the efficacy and safety of 10 glucagon-like peptide-1 receptor agonists as add-on to metformin in patients with type 2 diabetes: A systematic review. Front. Endocrinol. 14, 1244432 (2023).
Gorgojo-Martínez, J. J. et al. Clinical recommendations to manage gastrointestinal adverse events in patients treated with Glp-1 receptor agonists: A multidisciplinary expert consensus. J. Clin. Med. 12(1), 145 (2022).
Giunchi, V., Fusaroli, M., Hauben, M., Raschi, E. & Poluzzi, E. Challenges and opportunities in accessing and analysing FAERS data: A call towards a collaborative approach. Drug Saf. 46(10), 921–926 (2023).
Wu, T. et al. Safety of glucagon-like peptide-1 receptor agonists: A real-world study based on the US FDA adverse event reporting system database. Clin. Drug Investig. 42(11), 965–975 (2022).
Du, Y. et al. Metformin adverse event profile: A pharmacovigilance study based on the FDA adverse event reporting system (FAERS) from 2004 to 2022. Expert. Rev. Clin. Pharmacol. 17(2), 189–201 (2024).
Nathan, D. M. et al. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy—A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32(1), 193–203 (2009).
Ren, G., Ma, X. & Jiao, P. Effect of liraglutide combined with metformin or acarbose on glucose control in type 2 diabetes mellitus and risk factors of gastrointestinal adverse reactions. Am. J. Transl. Res. 14(5), 3207–3215 (2022).
Imeryüz, N. et al. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am. J. Physiol. 273(4), G920–G927 (1997).
Foretz, M., Guigas, B. & Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 19(8), 460–476 (2023).
Tran, K. L. et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am. Health Drug Benefits 10(4), 178–188 (2017).
Deacon, C. F., Mannucci, E. & Ahrén, B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes—A review and meta analysis. Diabetes Obes. Metab. 14(8), 762–767 (2012).
Gu, J. et al. The efficacy and safety of liraglutide added to metformin in patients with diabetes: A meta-analysis of randomized controlled trials. Sci. Rep. 6, 32714 (2016).
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the study of diabetes (EASD). Diabetes Care 45(11), 2753–2786 (2022).
Inzucchi, S. E. et al. Management of hyperglycaemia in type 2 diabetes: A patient-centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 55(6), 1577–1596 (2012).
Nauck, M. A. & Meier, J. J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 4(6), 525–536 (2016).
Zhao, X. et al. GLP-1 receptor agonists: Beyond their pancreatic effects. Front. Endocrinol. 12, 721135 (2021).
Buse, J. B. et al. 2019 Update to: management of hyperglycemia in type 2 Diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 43(2), 487–493 (2020).
Granata, A. et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: State of the art. Clin. Kidney J. 15(9), 1657–1665 (2022).
Mann, J. F. E., Ørsted, D. D. & Buse, J. B. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377(22), 2197–2198 (2017).
Inzucchi, S. E., Lipska, K. J., Mayo, H., Bailey, C. J. & McGuire, D. K. Metformin in patients with type 2 diabetes and kidney disease: A systematic review. JAMA 312(24), 2668–2675 (2014).
Marso, S. P., Holst, A. G. & Vilsbøll, T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 376(9), 891–892 (2017).
Cao, C., Yang, S. & Zhou, Z. GLP-1 receptor agonists and risk of cancer in type 2 diabetes: An updated meta-analysis of randomized controlled trials. Endocrine 66(2), 157–165 (2019).
Gier, B. et al. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model. Diabetes 61(5), 1250–1262 (2012).
Nachnani, J. S. et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia 53(1), 153–159 (2010).
Lauby-Secretan, B. et al. Body fatness and cancer-viewpoint of the IARC working group. N. Engl. J. Med. 375(8), 794–798 (2016).
Tuttle, K. R. et al. Diabetic kidney disease: A report from an ADA consensus conference. Diabetes Care 37(10), 2864–2883 (2014).
Soldin, O. P. & Mattison, D. R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 48(3), 143–157 (2009).
Gandhi, M., Aweeka, F., Greenblatt, R. M. & Blaschke, T. F. Sex differences in pharmacokinetics and pharmacodynamics. Annu. Rev. Pharmacol. Toxicol. 44, 499–523 (2004).
ElSayed, N. A. et al. 13. Older adults: Standards of care in diabetes-2023. Diabetes Care 46(Suppl 1), S216–S229 (2023).
Brown, K. A. & Simpson, E. R. Obesity and breast cancer: mechanisms and therapeutic implications. Front. Biosci. 4(7), 2515–2524 (2012).
Härmark, L. & van Grootheest, A. C. Pharmacovigilance: Methods, recent developments and future perspectives. Eur. J. Clin. Pharmacol. 64(8), 743–752 (2008).
Funding
The authors declare that the research, writing, and publication of this article received funding. This study was jointly supported by the following grants: Hunan Provincial Department of Education Fund Project (23A0667); Changsha Outstanding Youth Innovation Talent Development Program (kq2106074); National Undergraduate Innovation and Entrepreneurship Training Program 2024 (Letter No. 13 [2024] from the Department of Higher Education—S202410823011); The "14th Five-Year Plan" Applied Characteristic Discipline (Clinical Medicine) of Hunan Province.
Author information
Authors and Affiliations
Contributions
Conceptualization: LBY, CDN. Data curation: LBY, ZWC. Formal analysis: LBY, HRZ, ZWC, TJ. Funding acquisition: CDN. Investigation: LBY. Methodology: LBY, HRZ. Software: LBY, ZWC, YX. Validation: LBY. Visualization: LBY. Writing—original draft: LBY, HRZ, CDN. Writing—review & editing: LBY, HRZ, CDN, YX. All the authors read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, B., Huang, R., Zhang, W. et al. Disproportionality analysis of GLP-1 receptor agonists combined with metformin based on the FAERS database. Sci Rep 15, 34673 (2025). https://doi.org/10.1038/s41598-025-02394-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02394-0
Keywords
This article is cited by
-
AEs with GLP-1RA and metformin combination therapy
Reactions Weekly (2025)