Abstract
Supercapacitors exhibit limitations such as low energy density, high self-discharge rates, and degradation of electrochemical performance over extended cycling. This study presents the development of a high-performance asymmetric supercapacitor by synthesizing novel ZnO-VSe2 nanocomposites through wet-chemical methods, aiming to enhance capacity, energy density, and durability. The capacitive performance of these materials was systematically evaluated in an aqueous alkaline electrolyte (KOH) at a concentration of 2 M. ZnO-VSe2 composite demonstrates superior electrochemical energy storage capabilities, achieving a specific capacitance of 898 F/g and reducing overall resistance, enabling rapid electrolyte diffusion. These optimized electrochemical characteristics underscore the potential of ZnO-VSe2 for energy storage applications. Specifically, the ZnO-VSe2||AC asymmetric supercapacitor achieved an impressive capacitance of 260 F/g, an energy density of 71 Wh/kg, and a maximum power output of 6948 W/kg, along with a remarkable stability of 89.1% at a current density of 10 A/g over 5000 cycles. The proposed methodology offers a cost-effective and promising approach for evolving high-energy hybrid supercapacitors for energy storage applications.
Similar content being viewed by others
Introduction
The rapid increase in population and industrialization has significantly increased global energy consumption. The depletion of fossil fuels and the attending energy crisis have increased the pursuit of renewable and efficient energy storage systems1,2,3. Storing energy generated from renewable sources demands advanced and highly efficient storage technologies4,5. Among these, batteries and supercapacitors are the most extensively researched6,7. Batteries offer the advantage of exceptionally high specific energy compared to other storage devices, while supercapacitors provide superior specific power, a characteristic in which batteries are deficient3,4,6. Furthermore, the extended cycle life, broad operating temperature range, and enhanced safety of supercapacitors make them particularly attractive compared to batteries8,9,10. Supercapacitors are classified into two types, based on their charge storage mechanisms: electric double-layer capacitors (EDLCs) and pseudocapacitors. EDLCs store charge by developing an electric double-layer, which results from the adsorption of electrolyte ions at the surface of the electrode and electrolyte. This mechanism is purely electrostatic and does not involve faradaic reactions. In contrast, pseudocapacitors store charge through fast redox reactions at the electrode-electrolyte interface, enabling them to store more energy than EDLCs. Pseudocapacitance can arise from three distinct mechanisms: under potential deposition, redox reactions, and ion intercalation. Intercalation pseudocapacitance involves both capacitive and diffusion-controlled contributions. The capacitive contribution includes the electric double-layer formation and rapid redox processes at the interface5. Research efforts in developing supercapacitor electrode materials focus on identifying candidates with high specific energy and power, alongside long cycle life11,12,13,14.
Recently, transition metal oxides such as zinc, iron, cobalt, and manganese oxides have emerged as prominent materials for supercapacitor electrodes, with their performance primarily driven by fast faradaic redox reactions15. Among these, zinc oxide (ZnO) has been widely studied and is known for its potential as a functional material because of its superior energy density16. ZnO stands out as a suitable material for pseudocapacitor applications because of its enhanced electrochemical activity, high stability, affordability, and environmentally friendly properties17,18,19,20. Despite its advantages, the practical use of ZnO is limited by challenges such as poor rate capability, and inadequate electrical conductivity18,21,22. Integrating ZnO with materials exhibiting high electrical conductivity can significantly enhance its electrochemical performance, particularly in rate capability and cycle stability. Notably, transition metal selenides demonstrate superior electrochemical properties compared to their oxide and sulfide counterparts, primarily due to the higher electrical conductivity of selenium ions23. In the past few years, transition metal selenides also have garnered considerable interest across the sector of renewable energy, particularly for enhancing their electrochemical properties as electrode materials24,25,26. Owing to selenium’s superior electrical conductivity, transition metal selenides are considered to possess enhanced electrochemical characteristics. Numerous electrode materials have been extensively studied, including SnSe nanosheets27, hierarchical NiSe2 nanostructures28, CoSe nanosheets29, CuSe nanosheet arrays30, and MoSe2 nanosheets31 all of which have demonstrated promising potential for advanced supercapacitor applications7. Despite their promising progress as active electrodes, there remain significant challenges for transition metal selenides, such as low ion/electron conduction rates, volume expansion during charge-discharge cycles, limited surface area, reactivity issues, and unstable cycling performance. Addressing these gaps is crucial for further advancing their performance. Among the transition metal selenides Vanadium diselenide (VSe2), a prominent material within the family of two-dimensional transition metal dichalcogenides (TMDs), has demonstrated significant potential as a supercapacitor electrode material for energy storage applications. Compared to other TMDs, VSe2 exhibits metallic behavior in its stable state32,33. Structurally, it consists of monolayers of VSe2 separated by van der Waals gaps. A vanadium atom is positioned between two selenium atoms in each unit cell. This unique layered structure, combined with the electronic properties of VSe2, contributes to its exceptional intercalation capabilities within the van der Waals gaps, enhancing its energy storage performance34,35,36. VSe2 boasts high electrical conductivity, approximately 1000 S/m at 300 K, which is advantageous for electrochemical applications. However, its relatively high Gibbs free energy poses a challenge to its overall electrochemical efficiency. In most TMD materials, electrochemical activity primarily arises from edge sites, while the basal plane remains largely inactive in such reactions. This characteristic also applies to VSe2, influencing its electrochemical behavior37,38. VSe2 has already found applications in energy storage devices. For instance, thin VSe2 nanosheets have been created using a chemical vapor deposition (CVD) method and employed in in-plane solid-state supercapacitors33. These devices demonstrated a capacitance of 4.167 mFcm-2 at 1 mAcm-2. Similarly, 3D cuboidal VSe2 and graphene hybrids were produced using a hydrothermal technique, exhibiting a capacitance of 680 F/g at a current density of 1 A/g39. The hybrid electrode material, composed of pseudocapacitive VSe2, electric double-layer capacitance (EDLC) type reduced graphene oxide (rGO), and carbon nanotubes (CNTs), improves both the power density and cyclic stability of the electrode. VSe2/SWCNTs/rGO composite electrode for supercapacitors, showing nearly a six-fold increase in specific capacitance compared to pure VSe2, noting enhanced cyclic stability in the material32,40. Assemble composite with metal selenides can modify the electronic conductivity and charge density of ZnO, potentially altering their charge storage mechanism. Additionally, selenide materials can improve the rate capability and long-term cycling performance of oxide materials.
The ZnO-VSe2 nanocomposite offers a novel approach to supercapacitor electrode design by integrating the pseudocapacitive behavior of ZnO with the high electrical conductivity and layered structure of VSe2. These composite addresses key challenges highlighted in the literature, such as low conductivity, poor cycling stability, and scalability limitations, making it a significant advancement over previously reported materials. Furthermore, its potential to facilitate hybrid energy storage mechanisms underscores its novelty and importance in advancing supercapacitor technology.
Experimental
Materials
Sigma-Aldrich provided zinc nitrate (Zn(NO3)2.6H2O), sodium hydroxide (NaOH), vanadium pentoxide (V₂O₅), selenium dioxide (SeO2), and oxalic acid dihydrate (C2H2O4·2H2O). Since all the materials used were of analytical grade, no supplementary purification was required before their use.
Synthesis of ZnO
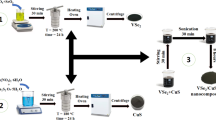
ZnO nanorods were prepared using the hydrothermal method. First, a 0.3 M solution of Zn(NO3)2·6 H₂O was prepared by dissolving it in deionized water. A 0.5 M NaOH solution was then added dropwise until the pH reached 10, while continuously stirring the mixture for 30 min at room temperature. Once a homogeneous solution was achieved, then the mixture was placed in a Teflon-lined stainless-steel autoclave and kept at 120 °C for 10 h. After allowing the sample to cool down to room temperature naturally, the resulting precipitate was washed and centrifuged multiple times with deionized water and absolute ethanol. The sample was then dried at 80 °C for 24 h and annealed in air at 600 °C for 2 h to enhance crystallinity, reduce defects, and improve the structural stability41.
Synthesis of VSe2
To prepare VSe2, 3.00 mmol of SeO₂, 0.75 mmol of V₂O₅, and 14.63 mmol of oxalic acid dihydrate (C₂H₂O₄·2 H₂O) were placed in a 50 mL Teflon-lined autoclave. Following the addition of the precursors, 40 mL of DI water was introduced into the autoclave. The autoclave was sealed and placed in an oven, where it was heated at 190 °C for 24 h to carry out the hydrothermal reaction. After the reaction was complete, the precipitate was separated by centrifugation and washed several times with hot deionized water and ethanol to remove impurities. The sample was then dried at 80 °C for 8 h to remove remaining moisture.
During the synthesis of VSe2 oxalic acid acts as a reducing and complexing agent in VSe₂ synthesis, promoting vanadium precursor reduction and controlling nucleation for uniform and highly purity particles.
The following reaction takes place during the synthesis of VSe2. First reduction of Vanadium pentoxide (V₂O₅) by oxalic acid (C2H2O4) to vanadyl ions (VO2+).
V₂O₅ + C2H2O4 + 2 H+ → 2VO2+ + 2CO2 + H2O.
2VO2+ + C2H2O4 + 2 H+ → 2VO2+ + 2CO2 + H2O.
Secondly, Selenium dioxide (SeO2) decomposes under thermal conditions to release selenium ion.
SeO2 + 2 H+ + 2e− → Se + H2O.
Than,
VO2+ + 2Se + 2 H+ → VSe2 + H2O.
Preparation of ZnO–VSe2 composite
ZnO-VSe2 nanocomposites were created using wet chemical methods. The ZnO and VSe2 components were combined in a 50:50 weight ratio, respectively. To each formulation, 40 mL of methanol was added, followed by sonication for 40 min. The solution was subsequently stirred magnetically for an additional 40 min to ensure complete dissolution. The solvent was removed by heating the mixture at 80 °C for 20 h.
Preparation of electrodes
To study the electrochemical performance of the prepared samples, a uniform slurry of the active material was prepared by mixing 80% of the active material, 10% of adhesive polyvinylidene fluoride (PVDF), and 10% carbon black in 10 µl of N-methyl-2-pyrrolidone (NMP). This mixture was stirred for 12 h. The resulting slurry was then applied evenly to a 1 × 1 cm² area of a Nickle foam (Cleaned in an ultrasonic bath sonicator for 40 min in ethanol, followed by rinsing with deionized water, and then dried overnight in an oven at 90 °C). Drying of the coated electrodes was carried out in a vacuum oven at 80 °C for 10 h, yielding electrodes with an active material weight of approximately 2 mg with ± 0.002 mg.
Electrochemical measurements
The electrochemical analysis of individual working electrodes was performed using cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS) within a standard three-electrode setup. This configuration included a working electrode made of the active material, an Ag/AgCl reference electrode, and a platinum wire as the counter electrode. CV and GCD measurements were conducted over a potential range of 0 V to 0.6 V (vs. Ag/AgCl) to analyze the redox behavior of the ZnO–VSe2 nanocomposite in the KOH electrolyte with varying scan rates and specific currents from low to high. This window was chosen based on the material’s electrochemical stability without degradation. For EIS, we measured in a frequency range from 100 kHz to 0.01 Hz, maintaining a small AC amplitude of 10 mV to evaluate charge transfer resistance and ion diffusion properties. The results from these electrochemical tests enabled the calculation of several key parameters, including specific capacitance, power density, current density, and electrochemical series resistance (ESR). Specific capacitance was determined using GCD data through the following Eq. 1 42.
Cs denotes the specific capacitance (F/g), I represents the current density (mA), t indicates the discharge time (s), m refers to the total mass of the active material (mg), and V signifies the potential window (V). Energy density E(D) (Wh/kg) and power density P(D) (W/kg) of the composite material can be calculated by using Eqs. 2 and 3 43.
Here, Δt is the discharging time and ΔV is a potential window.
Characterization
The synthesized samples were characterized using different analytical instruments; to evaluate the structural properties X-ray diffractometer (XRD, Bruker D8 Advance) with Cu Kα radiation (λ = 0.154056 nm) is used, a scanning electron microscope (TESCAN MAIA3) is used to analyze the surface morphology. To study the vibrational modes Raman spectroscopy (Model, Dongwoo Optron Co. Ltd) is used. Electrochemical properties of the samples were assessed with Gamry 101 (Potentiostatic/Galvanostatic/ZRA), conducting tests such as galvanostatic charge-discharge (GCD), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS).
Result and discussion
Structural analysis
The X-ray diffraction patterns of ZnO, VSe2, and ZnO-VSe2 nanocomposite are displayed in Fig. 1a. The main diffraction peaks for ZnO appear at 2θ values of 32.40°, 35.26°, 37.10°, 48.52°, 57.62°, 63.92°, 67.37°, 69.03°, 70.33°, 73.74°, and 78.2°, corresponding to the (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), and (202) planes. These peaks confirm the wurtzite hexagonal structure of the synthesized ZnO, as indicated by JCPDS card No. 36-1451 with space group p63/mc44. For the synthesized VSe2, the XRD pattern shows peaks at 2θ values of 14.58°, 28.50°, 34.31°, 43.25°, 54.82°, 55.41°, 61.70°, 63.98°, and 66.90°, which align with the (001), (002), (011), (102), (110), (103), (004), (112) and (201) reflections, respectively. This pattern matches JCPDS card No. 89-1641 and is associated with the hexagonal VSe2, space group P63/mmc45. The XRD spectra for ZnO-VSe2 nanocomposites confirm the presence of both ZnO and VSe2, though the peaks in the composite samples are notably weaker than those of the individual components. This may result from the interaction between ZnO and VSe2 nanoparticles. The peaks associated with VSe2 are less pronounced in the composites, likely due to ZnO nanoparticles covering the VSe2. The lattice mismatch between ZnO and VSe2 can introduce strain and defects at the interface of the ZnO-VSe2 composite46. Changes in surface morphology, such as increased roughness or porosity due to composite formation, scatter X-rays non-coherently, reducing the diffracted peak intensity captured by the detector. The broad and relatively weak diffraction peak in the nanocomposite indicates lower face-to-face stacking due to hybridization47. Additionally, the broad peaks in the XRD patterns suggest small crystallite sizes, supporting the formation of nanocomposites. The crystallite sizes (D) for ZnO, VSe2, and ZnO-VSe2, calculated using Debye-Scherrer’s formula19,48, are approximately 28.9, 24.7, and 22.1 nm, respectively.
Here, D represents the crystallite size in nm, λ is the X-ray wavelength used for CuKα radiation, which is 0.154 nm, while β (in radians) represents the full width at half maximum, and θ denotes Bragg’s angle.
Raman spectroscopy was performed on ZnO, VSe2, and ZnO–VSe2 nanocomposites to support and verify the XRD findings. Figure 1b presents the Raman spectra of ZnO, VSe2, and their composite. The Raman spectrum of ZnO displays three distinct peaks at 331 cm⁻¹, 385 cm⁻¹, and 437 cm⁻¹, corresponding to the 2LA, A1(TO), and E1 (high) modes of ZnO. The peak at 330 cm⁻¹ results from second-order Raman scattering49. For VSe2, three prominent vibrational peaks are observed at 217 cm⁻¹, 314 cm⁻¹, and 447 cm⁻¹, which are attributed to the A1g, A2g, and 2LA modes, respectively50. These peaks correspond to the characteristic vibrational modes of VSe2, confirming its structural integrity. In the ZnO-VSe2 nanocomposites, the Raman spectrum exhibits vibrational modes from both ZnO and VSe2, aligning with the XRD data and verifying the efficacious synthesis of the nanocomposite. A slight shift in peak positions and changes in intensity in the composite compared to the individual ZnO and VSe2 samples is observed. This suggests strong interactions at the interface between the two materials. These changes likely indicate charge transfer between ZnO and VSe2, which can improve electronic conductivity and enhance electrochemical performance51. Furthermore, peak broadening in the composite may be attributed to lattice distortions or strain effects, further confirming the formation of a well-integrated heterostructure. These Raman analysis findings highlight the synergistic interaction between ZnO and VSe2, which plays a crucial role in improving the electrochemical properties of the ZnO-VSe2 nanocomposite.
Morphological and elemental analysis
The surface morphology was further examined using scanning electron microscopy (SEM), as illustrated in Fig. 2. The pristine ZnO sample exhibits nanorods of several micrometers in length, with a smooth surface (Fig. 2a), revealing that the ZnO nanorods are interconnected, a feature that enhances rapid and favorable redox reactions while maintaining structural stability. The VSe2 sample as depicted in Fig. 2b, reveals a spherical structure with extensive pores. This interconnected structure significantly shortens diffusion paths by expanding the contact area between the electrode and electrolyte, thus offering more active sites for faradaic reactions during electrochemical processes. Additionally, the high clarity of the VSe2 sample is demonstrated by the non-existence of significant stacking or aggregation, in agreement with XRD results. The SEM image in Fig. 2c displays the combined morphology of ZnO and VSe2, showing nanorods and spheres with negligible agglomeration. Moreover, the ZnO-VSe2 nanocomposite retains its original morphology after integration, with no substantial changes observed, suggesting that the nanocomposite structure may offer superior electrochemical performance compared to the bulk materials. This morphology introduces additional voids and pores, promoting rapid and reversible faradaic reactions. The high pores reduce ion diffusion paths during charge/discharge processes while preserving the structural integrity of the core. The combined nanorods and spheres morphology enhances ion and electron transport efficiency and accommodates volume changes more effectively.
The elemental analysis confirmed the high purity of all prepared samples, as illustrated in Fig. 3a–c. The EDAX spectrum for pure ZnO consists of signals corresponding to Zn and O, while the spectrum for VSe2 verifies the presence of V and Se. Similarly, the EDAX spectrum of ZnS–VSe2 nanocomposites contains signals for Zn, O, V, and Se. These results indicate that the prepared samples possess high purity, consistent with the findings from XRD and Raman spectroscopy. The value of atomic and weight% of ZnO, VSe2 and ZnO-VSe2 are shown in Table 1.
Electrochemical performance of ZnO, VSe2, and ZnO-VSe2
The electrochemical characteristics of active materials can be assessed using a three-electrode system to confirm their capacitive performance and potential use as electrodes in supercapacitor applications in 2 M KOH as an electrolyte. The KOH is used as electrolyte which plays a vital role in the electrochemical performance of the ZnO–VSe2 nanocomposite. As a strong alkaline electrolyte, it enables the smooth movement of OH- ions, which enhances redox reactions at the electrode/electrolyte interface. This improves charge transfer kinetics, leading to better electrochemical performance, including higher capacitance and improved cycling stability. The electrochemically active surface area of the electrodes is 1 × 1 cm, which provides insight into the amount of surface available for electrochemical reactions, directly influencing the material’s capacity and efficiency. A higher Electrochemically active surface area (ECSA) typically leads to enhanced charge/discharge rates and improved overall performance in energy storage devices52. To check the electrochemical characteristics of synthesized electrodes cyclic voltammetry (CV) analysis, charge/discharge (CD) measurements, and electrochemical impedance spectroscopy (EIS) were used. CV curves of all samples are performed under similar conditions from 5 to 50 mV/s. Specifically, the CV profiles of ZnO, VSe2, and ZnO-VSe2 nanocomposite electrodes are illustrated in Fig. 4a-c. For a comparative analysis, a constant scan rate was applied to the CV spectra of these electrodes, as depicted in Fig. 4d. Conspicuously, the ZnO-VSe2 nanocomposite electrode exhibited a greater area beneath the CV curve within the potential range of 0.0 to 0.6 V at a fixed scan rate, indicating superior capacitance compared to the individual ZnO and VSe2 electrodes. This enhanced capacitance, along with distinct redox peaks, can be attributed to faradaic reactions. Furthermore, VSe2 displayed a larger enclosed area than ZnO, indicating its higher capacitance. As previously mentioned, the ZnO electrode exhibits a smaller enclosed loop area, indicating lower capacitance and inferior electrochemical properties compared to VSe2 under similar electrochemical conditions. The CV curves of the ZnO-VSe2 nanocomposites display distinct redox peaks, suggesting that the predominant capacitance arises from a Faradaic mechanism. The prominent redox peaks are attributed to the Zn²⁺ and V⁴⁺ oxidation states of ZnO and VSe2, which provide multiple sites for charge storage during the charge-discharge process, particularly when interrelating with the KOH electrolyte53,54. The ZnO-VSe2 nanocomposites electrode exhibits a significantly enhanced current response and a larger area beneath the voltammogram compared to its bulk counterparts, leading to the highest capacitance Fig. 4c. The key distinction between Fig. 4a and b, and 4c is that the ZnO-VSe2 nanocomposites electrode shows the largest current, suggesting the superior electrode performance. All electrodes displayed a similar behavior across different scan rates, indicating that minor potential shifts as a consequence of polarization and heightened resistance at the electrode/electrolyte interface55.
The layered structure of VSe₂ allows it to exhibit multiple oxidation states, such as V3+ and V4+, which play a key role in the charge storage process. During this process, VSe₂ undergoes various redox reactions, such as V4+/V3 + 56. Similarly, selenium (Se) also participates in redox changes, often shifting between Se²⁻ and Se⁴⁺ states. These reactions contribute to a higher charge storage capacity, ultimately improving the overall energy storage performance. During the charging process, K⁺ ions from the electrolyte and OH⁻ diffusion gradually occupy the available space at lower scan rates and currents, leading to increased capacity. The weak bonding of Se in the V–Se system, whether chemical or electronic, can change with different oxidation states, making the material more active during electrochemical reactions57. From the Figure, it is clear that the CV curves of all the synthesized samples reveal pseudocapacitor behavior. The redox peaks observed in the CV curve of ZnO-VSe2 are due to the intercalation and deintercalation of K+ ions into the surface of electrodes and may be due to the variable oxidation states of vanadium. The two redox peaks are associated with the conversion between V³⁺/V⁴⁺ and Zn⁺/Zn²⁺ redox transition demonstrating that ZnO and VSe₂ have good electrochemical activity in an alkaline electrolyte. During the charging and discharging process following reactions are associated with the ZnO-VSe2 electrode56,58.
ZnO + OH− ↔ ZnO(OH) + e−.
ZnO + 2OH− ↔ ZnO2 + 2 + H2O.
VSe2 + OH− ↔ VSe2OH + e−.
VSe2 + K+ + e− ↔ VSe + SeK.
(VSe2)surface + K+ + e− ↔ (VSe− + K+)surface.
Increased contact between the active electrodes and the electrolyte enhances the utilization of active materials, thereby improving the supercapacitor’s performance. During discharge, the host ions migrate back into the electrolyte, while external electrons maintain charge balance and ensure charge neutrality59,60.
Generally, the specific current response (i) at a particular potential (V) is proportional to the scan rate (v) due to the capacitive effect, whereas the diffusive effect results in i being proportional to the square root of the scan rate (v1/2). The square root of the scan rate vs. peak current of all the synthesized samples at different scan rates is shown in Fig. 5(a-c).
The charge storage mechanism of the synthesized electrodes’ CV curve was analyzed using the power law described in Eq. (5) 43.
where a and b are variable parameters, and the b-value lies between 0.5 and 1. This relationship allows for the determination of the capacitive and/or diffusive contributions. When the b-value is closer to 0.5, the capacity response is more likely to be diffusion-dominated (battery-type behavior). Conversely, when the b-value approaches 1, the response is more likely to be dominated by the capacitive effects (surface reactions) of the electrode42. The b-values derived from the slopes at different scan rates of the linear fits for ZnO, VSe2 and ZnO-VSe2 electrodes are 0.64, 0.86, and 0.81, respectively. The b-values for the ZnO-VSe2 electrode fall between 0.5 and 1, with values closer to 1, suggesting that the charge storage mechanism involves both capacitive and diffusive responses, with a greater contribution from the capacitive response. On the other hand, the total contribution from both capacitive and diffusive responses can be determined using the following equation
Here, k1v represents the capacitive contribution, while k2v1/2 corresponds to the diffusive contribution61. Figure 6a-f illustrates the contribution ratios of the two electrochemical charge storage mechanisms at different scan rates for the ZnO, VSe2 and ZnO-VSe2 electrodes. Lower scan rates show mostly diffusion-based charge storage; however, capacitive contributions grow more prominent at higher scan rates. This occurs because, at higher scan rates, there is less time for ions to diffuse into the nanospheres. Additionally, this suggests a high-rate capability or fast ion insertion/extraction, indicating that the intercalation mechanism takes place both within the material and on its surface. At higher scan rates, surface-level interactions become more prominent at the interface62,63.
Furthermore, ECSA of all the synthesized samples were quantitatively estimated from the electrochemical-double-layer-capacitance (Cdl) of the catalytic surface. The capacitive current (ΔJ) at a fixed potential (~ 0.05 V) was calculated using the relation:
where Ja and Jc are the anodic and cathodic current densities, respectively, at the same potential. A linear plot of ΔJ versus scan rate yields the Cdl from the slope. The ECSA is proportional to the Cdl and can be estimated from Eq. 10 64.
where Cs is the specific capacitance of a smooth surface, assumed to be 40 µF cm− 2. As shown in Fig. 7 (a, b), the ZnO-VSe2 composite electrode exhibited the highest slope, indicating a significantly larger Cdl and hence a greater ECSA (7.45 cm2, compared to ZnO (0.934 cm2 and VSe2 (3.63 cm2. This enhancement is attributed to the synergistic effects between ZnO and VSe2, which increase the number of accessible electroactive sites and facilitate charge transfer processes. The analysis offers a thorough knowledge of the charge storage mechanism of the ZnO-VSe2 nanocomposite electrodes, further supporting their appropriateness for high-performance supercapacitor systems. The results have been used in the text to fortify the treatment of the electrochemical properties of the fabricated device. The exceptional performance can be investigated in depth through the analysis of CD platforms, as described below.
The charge-discharge (CD) profiles of the ZnO, VSe2 and ZnO-VSe2 composite electrodes were evaluated at different current densities under comparable potentials, as illustrated in Fig. 8 (a-c). A comparative plot for all electrodes is presented in Fig. 8d, covering the potential range from 0.0 to 0.6 V. Based on CV analysis, the ZnO-VSe2 nanocomposite electrode exhibits a higher discharge time than the individual ZnO and VSe2 electrodes, specifying superior capacitance. The performance of the ZnO, VSe2, and ZnO-VSe2 electrodes is further detailed in Figs. 8a-c. The CD profiles remain consistent across various currents, with no significant deviations observed, reflecting excellent rate capability. The uniformity in CD curves suggests high coulombic efficiency for all electrodes. These CD results align with the findings from the CV measurements discussed earlier.
Specific capacitance is computed based on Eq. 1, with the corresponding values exhibited in Table 2 and graphically depicted in Fig. 9a. The ZnO-VSe2 nanocomposite electrode demonstrates a significantly higher capacitance compared to the individual ZnO and VSe2 electrodes at all experienced current rates. The ZnO–VSe2 nanocomposite electrode exhibited the highest capacitance values, reaching 898 Fg− 1 and 562 Fg− 1 at current densities of 1 Ag− 1 and 5 Ag− 1 respectively with an excellent retention of 62%. This indicates a synergistic effect between the ZnO and VSe2 nanomaterials. It is noteworthy that an exponential decline in capacitance is observed with increasing current rates, suggesting reduced ion diffusion interaction within the host electrode, likely due to time constraints66,67.
The retention of all the synthesized was calculated using the following formula and values are given in Table 2.
The electrochemical properties of ZnO, VSe2, and ZnO-VSe2 nanocomposite electrodes are further elucidated through impedance spectroscopy, as presented in Fig. 9b. The Nyquist plot indicates that all electrodes ZnO, VSe2, and the ZnO-VSe2 nanocomposite exhibit a negligible semicircular feature, indicating minimal charge transfer resistance (Rct) and solution resistance (Rs) with the sharp vertical line corresponds to the near-ideal capacitive behavior of these materials. The relatively higher resistance of the ZnO electrode points to its suboptimal electrochemical performance, corroborated by GCD and CV analyses. In contrast, VSe2 displays lower resistance than ZnO, signifying enhanced electrochemical behavior. Specifically, the ZnO-VSe2 nanocomposite demonstrates the smallest values for Rs and Rct, suggesting superior ion diffusion and mobility during the charge-discharge process. The greater diffusion resistance observed in the individual ZnO and VSe2 electrodes, as compared to their composite, supplementary validates the superior electrochemical performance of the ZnO-VSe2 nanocomposite electrode. The outstanding energy storage performance can be attributed to the following factors: (a) The composite structure provides a larger effective surface area as compare to bulk counterparts, offering more active sites for electrochemical reactions. This increase in active sites directly contributes to higher current responses and improved energy storage performance. (b) The incorporation of VSe2 into the ZnO material reduces the electrode resistance, enhancing its performance for energy storage applications. (c) The combination of ZnO and VSe₂ forms a close-contact heterojunction, enhancing electron transfer efficiency. This improved conductivity promotes faster charge movement during redox reactions25. An enlarged view of the Nyquist plot is provided in Fig. 9b inside, with specific resistance values detailed in Table 3.
Electrochemical performance evaluation of ZnO-VSe2||AC ASC
The exceptional performance of the ZnO-VSe2 composite electrodes in a three-electrode configuration has shifted our focus towards assembling an asymmetric supercapacitor (ASC) in a 2 M KOH aqueous solution as electrolyte. Before conducting two-electrode measurements, CV and GCD tests were performed on the activated carbon (AC) electrode in a three-electrode configuration. The corresponding CV and GCD graphs are presented in Fig. 10a and b, respectively. The CV curve of the AC electrode was obtained at the potential window of -0.4 V to 0.4 V. The typical rectangular-shaped CV curves, characteristic of EDLC is observed over a scan rate range of 10–50 mV/s. The GCD curves of the AC electrode exhibit an isosceles triangular shape within the current density range of 1–5 A/g. The good linear response of the AC electrode over time indicates excellent reversibility of the electrochemical charge-discharge process68.
The electrochemical behavior of the ZnO-VSe₂ || AC asymmetric supercapacitor was investigated using CV at scan rates ranging from 10 to 50 mV/s as illustrated in Fig. 11a. The ZnO-VSe2 composite electrode works as a positive while activated carbon is a negative electrode. The voltage window was successfully achieved up to 1.4 V indicates a stable operating range, reflecting the effective combination of the faradaic ZnO-VSe2 positive electrode and the electrical double-layer capacitive (EDLC) AC negative electrode. The CV curves display distinct redox peaks, confirming a pseudocapacitive charge storage mechanism driven by reversible redox reactions in the ZnO-VSe2 composite. As the scan rate increases, both anodic and cathodic peak currents rise proportionally, with a slight peak shift attributed to internal resistance and ion diffusion limitations. Even at higher scan rates, suggests excellent electrochemical reversibility and efficient charge transport. Both the higher and lower scan rates demonstrate remarkable electrochemical performance69. There was no distortion in the ZnO-VSe2||AC voltammogram, indicating excellent energy storage capacity and reversibility throughout the successive electrochemical processes. The charge-discharge (CD) behavior of the ZnO-VSe2||AC ASC was investigated at various current rates, and the corresponding data is illustrated in Fig. 11b. The nonlinear CD curves at different current rates highlight the combined energy storage capacity of the ZnO-VSe2||AC ASC. Consistent with the CV results, CD curves at current rates of 1 to 10 Ag− 1 were used to calculate the capacitance of the ZnO-VSe2||AC ASC, as illustrated in Fig. 10c. A high capacitance of 260 F/g was observed at 1 A/g, which decreased to 71 F/g as the current rate increased ten times (Fig. 11c). This indicates the outstanding rate performance of the ZnO-VSe2||AC ASC, as shown in Table 4. A steady decrease in capacitance was noted as the current rates increased, suggesting that the rapid charge-discharge process limited the number of ions reaching the electrode surface.
We also determined the specific energy and power using Eqs. 2 and 3, and the findings are illustrated in the Ragone plot (Fig. 11d). Specifically, a relatively high specific energy of 71 Wh/kg was attained at 1 Ag⁻¹, with a specific power of 675 W/kg. As the discharge rate increased to 10 Ag⁻¹, the specific energy decreased to 19.3 Wh/kg, while the specific power increased to a maximum of 6948 W/kg. The corresponding values are provided in Table 4. The energy and power delivery results confirm the interaction between ZnO and VSe2 within a composite, which maintains high conductivity due to the presence of carbon materials70.
Several studies have investigated ZnO and VSe2 based electrode materials for energy storage. ZnO-SnO2||AC shows 133 F/g specific capacitance, 47.2 Wh/kg energy, 10,455 W/kg power, and 92.8% stability after 4,000 cycles71. Mo-doped ZnO||AC delivers capacitance of 125.2 F/g, energy density 39 Wh/kg, power density 7425 W/kg, with 75.6% stability after 8,000 cycles16. Graphene-ZnO composites achieve the specific capacitance of 196 F/g, specific energy 21 Wh/kg, specific power 2,600 W/kg, and 94% stability after 5,000 cycles72. On the other hand, VSe₂-based materials exhibit superior performance. VSe₂-rGO//Fe3O4||C reaches 1,129 F/g, 65 Wh/kg, 1,280 W/kg, with 98.5% stability after 2,000 cycles56. Mn-doped VSe₂ with red phosphorus offers 60 F/g, 31 Wh/kg, 25,600 W/kg, and 95% stability over 5,000 cycles57. VSe₂-rGO composites report 680 F/g, 212 Wh/kg, 3,300 W/kg, but with 81% stability after 10,000 cycles39. The ZnO-VSe₂ composite exhibits superior electrochemical performance compared to previously reported materials, with high specific capacitance, excellent energy and power densities, and outstanding cyclic stability. This is attributed to the synergistic interaction between ZnO and VSe₂, enhancing charge storage and structural integrity. Consequently, it outperforms the electrochemical performance of previously reported literature (Table 5). This exceptional energy storage capability underscores the significance of ZnO-based metal selenides composite as a viable substitute for achieving high-performance, energy-efficient storage solutions.
The impedance plot was also evaluated for the overall functionality of the ZnO-VSe2||AC ASC, as illustrated in Fig. 12a. The equivalent circuit was modelled based on the EIS data using ZSimDemo 3.20 simulation software. As depicted in the inset of Fig. 12a, the circuit consists of solution resistance (Rs), charge-transfer resistance (Rct), and Warburg impedance (W), representing the electrochemical processes occurring within the system. In the figure, the plot exhibits a sharp increase, transitioning into a nearly vertical straight line at approximately 45° to the real axis, indicating a lower Warburg impedance and enhanced ion diffusion. The figure illustrates that the experimental data closely match the simulated curve. The designed equivalent circuit provides detailed insights into the components influencing the charging and discharging behavior of ZnO-VSe2||AC ASC. The small semicircle indicates a negligible charge transfer resistance (Rct = 3.8 Ω), whereas the intercept on the real axis reflects the solution resistance (Rs = 0.93 Ω), signifying the superior conductivity of the ZnO-VSe2||AC ASC66, leading to improved capacitance.
Modern electronics require substantial energy storage solutions, creating a significant demand for durable functional materials. A variety of materials are currently under investigation, with their performance scalable to industrial levels and primed for commercialization to address this need. Cycling durability is a crucial criterion for evaluating the efficacy of active electrode materials, as highlighted in the GCD analysis. In the present study, cycling tests were carried out on the ZnO-VSe2||AC ASC at a current density of 10 Ag⁻¹ for 5000 cycles, with the results presented in Fig. 12b. Furthermore, the ZnO-VSe2||AC ASC demonstrated exceptional cycling durability, retaining 89.1% of its capacitance after extensive cycling. This translates to a mere 10.9% degradation in capacitance, underscoring its remarkable stability. The loss of capacitance is may be due to the active materials like ZnO and VSe₂ may undergo changes in their structure, which can lead to a decrease in capacitance. The KOH electrolyte may also degrade over time, causing a reduction in its conductivity or the formation of by-products that affect the performance. Repeated cycling may lead to the formation of defects or oxidation on the surface of the electrode material, increasing resistance and reducing the number of active sites available for energy storage. The interface between the electrode and electrolyte may degrade with use, leading to higher resistance and less efficient charge transfer, which can contribute to capacitance loss73. The inset of Fig. 12b (blue and red CD curves) shows the initial and last ten cycles, upholding a stable voltage window of 1.4 V even after 5000 cycles. The coulombic efficiency is calculated using the following relation;
The value of the coulombic efficiency of the ZnO-VSe2 electrode is about 98.8% signifying that electrochemical reactions are reasonable during measurement as shown in Fig. 12b. Moreover, the consistent and reliable cycling performance observed indicates the effective integration of ZnO and VSe2 in the composite, along with commendable structural integrity. These compelling findings highlight the significant potential of ZnO, alongside other nanomaterials, in applications related to energy conversion and storage. In addition, a self-discharge analysis evaluated the device’s practical utility, essential for real-world energy storage applications. The positive electrode in this study comprised a ZnO-VSe₂ nanocomposite, while the negative electrode consisted of activated carbon, effectively synthesizing a high-performance ASC. Furthermore, four ASC devices were connected in series and integrated with a red light-emitting diode (LED). The constructed device generated sufficient energy output to power the LED, demonstrating its suitability for energy storage. Figure 13a shows the slurry-coated electrodes while Fig. 13b displays a photograph of a LED illuminated by a package supercapacitor. Charged to 1.4 V, this configuration provided a bright red LED beam for 70 s in a working demonstration. Figure 13c demonstrates multiple LEDs i.e. red, green, and yellow connected in series. This device successfully illuminates all three LEDs, demonstrating its practical energy storage capability. The outstanding performance of the ZnO-VSe₂-based ASC demonstrates great potential for storage applications.
Summary
In conclusion, this study presents the synthesis of a composite material using ZnO and VSe2 as base components using hydrothermal and wet-chemical methods for the optimization of electrochemical performance. The structural analysis indicates that both ZnO and VSe2 exhibit a hexagonal crystal structure characterized by nanorods and spherical morphologies, demonstrating high crystallinity and purity. A comprehensive evaluation of the electrochemical characteristics of the prepared ZnO, VSe2 and ZnO-VSe2 electrodes revealed good charge storage capabilities. Remarkably, the ZnO-VSe2 composite electrode demonstrated superior performance in a three-electrode configuration utilizing an aqueous electrolyte. Furthermore, the ZnO-VSe2 composite electrode was employed as the positive terminal in asymmetric supercapacitor configuration with activated carbon (AC) as the negative terminal, which exhibited remarkable energy density of 71 Wh/kg and power density of 6948 W/kg along with excellent cycling stability of 89.1%. The optimization of voltage and capacitance, in conjunction with enhanced conductivity, significantly elevates the whole energy storage performance of these electrode materials, highlighting their potential for advanced energy storage applications.
Data availability
The data presented in this study are included in the manuscript.
References
Liu, D. D. et al. Physics-informed neural networks for phase-field simulation in designing high energy storage performance polymer nanocomposites. Appl. Phys. Lett. 126 (2025).
Wang, Z., Feng, Z., Hu, C., Li, X. & Zhu, Y. Enhancing battery performance under motor overload drive with a battery–supercapacitor hybrid energy storage system. J. Power Sources. 642, 236680 (2025).
Cai, D. et al. Binder-Free MOF‐Based and MOF‐Derived nanoarrays for flexible electrochemical energy storage: progress and perspectives. Small 20, 2305778 (2024).
Gao, L. et al. Zinc Selenide/cobalt Selenide in nitrogen-doped carbon frameworks as anode materials for high-performance sodium-ion hybrid capacitors. Adv. Compos. Hybrid. Mater. 7, 144 (2024).
Li, X. et al. Effect of microstructure on electrochemical performance of electrode materials for microsupercapacitor. Mater. Lett. 346, 134481 (2023).
Cao, M. et al. Cross-linked K2Ti4O9 nanoribbon arrays with superior rate capability and cyclability for lithium-ion batteries. Mater. Lett. 279, 128495 (2020).
Zhan, Y., Ren, X., Zhao, S. & Guo, Z. Enhancing prediction of electron affinity and ionization energy in liquid organic electrolytes for lithium-ion batteries using machine learning. J. Power Sources. 629, 235992 (2025).
Wang, J. et al. A modified molten-salt method to prepare graphene electrode with high capacitance and low self-discharge rate. Carbon 102, 255–261 (2016).
Dutta, A., Mitra, S., Basak, M. & Banerjee, T. A comprehensive review on batteries and supercapacitors: development and challenges since their inception. Energy Storage. 5, e339 (2023).
Suriyan, K. & Nagarajan, R. A review of renewable energy efficiency technologies. Optim. Techniques Hybrid. Power Systems: Renew. Energy Electr. Veh. Smart Grid. 362, 377 (2024).
Wang, Q., Yan, J. & Fan, Z. Carbon materials for high volumetric performance supercapacitors: design, progress, challenges and opportunities. Energy Environ. Sci. 9, 729–762 (2016).
Augustyn, V., Simon, P. & Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597–1614 (2014).
Simon, P. & Gogotsi, Y. Capacitive energy storage in nanostructured carbon–electrolyte systems. Acc. Chem. Res. 46, 1094–1103 (2013).
Das, T. K. & Prusty, S. Review on conducting polymers and their applications. Polym.-Plast. Technol. Eng. 51, 1487–1500 (2012).
Ramli, N. I. T. et al. Incorporation of zinc oxide into carbon nanotube/graphite nanofiber as high performance supercapacitor electrode. Electrochim. Acta. 228, 259–267 (2017).
Ali, A. et al. Mo-doped ZnO nanoflakes on Ni-foam for asymmetric supercapacitor applications. RSC Adv. 9, 27432–27438 (2019).
Shaheen, I. et al. Sustainable synthesis of organic framework-derived ZnO nanoparticles for fabrication of supercapacitor electrode. Environ. Technol. 43, 605–616 (2022).
Mahmood, M. A. et al. Diluted magnetic semiconductor behavior in Co-and Gd-co-doped ZnO nanotubes for spintronic applications. J. Mater. Sci.: Mater. Electron. 34, 1784 (2023).
Elboughdiri, N. et al. Enhanced electrical and magnetic properties of (Co, Yb) co-doped ZnO memristor for neuromorphic computing. RSC Adv. 13, 35993–36008 (2023).
Khan, R. et al. Carrier-mediated ferromagnetism and dielectric tailoring in dual-doped ZnO semiconductor nanoparticles for spintronics. Mater. Sci. Semiconduct. Process. 193, 109487 (2025).
Hou, L. et al. Monodisperse metallic NiCoSe2 Hollow sub-microspheres: formation process, intrinsic charge‐storage mechanism, and appealing pseudocapacitance as highly conductive electrode for electrochemical supercapacitors. Adv. Funct. Mater. 28, 1705921 (2018).
Rehman, N. U. et al. Dual-doped ZnO-based magnetic semiconductor resistive switching response for memristor-based technologies. J. Mater. Sci.: Mater. Electron. 35, 1557 (2024).
Ali, Z., Asif, M., Huang, X., Tang, T. & Hou, Y. Hierarchically porous Fe2CoSe4 binary-metal Selenide for extraordinary rate performance and durable anode of sodium‐ion batteries. Adv. Mater. 30, 1802745 (2018).
Khan, K. et al. Development of 1.6 V hybrid supercapacitor based on ZnO nanorods/MnO2 nanowires for next-generation electrochemical energy storage. J. Electroanal. Chem. 922, 116753 (2022).
Sajjad, M. et al. A novel high-performance all-solid-state asymmetric supercapacitor based on cuse nanoflakes wrapped on vertically aligned TiO2 nanoplates nanocomposite synthesized via a wet-chemical method. J. Energy Storage. 55, 105304 (2022).
Shah, M. Z. U. et al. A novel TiO2/CuSe based nanocomposite for high-voltage asymmetric supercapacitors. J. Science: Adv. Mater. Devices. 7, 100418 (2022).
Zhang, C. et al. Two-dimensional Tin Selenide nanostructures for flexible all-solid-state supercapacitors. ACS Nano. 8, 3761–3770 (2014).
Li, H. et al. Synthesis of nickel Selenide thin films for high performance all-solid-state asymmetric supercapacitors. Chin. Chem. Lett. 31, 2275–2279 (2020).
Wu, L. et al. Component-controllable bimetallic nickel Cobalt selenides (NixCo1-x) 0.85 se for high performance supercapacitors. J. Alloys Compd. 766, 527–535 (2018).
Li, J. C. et al. Design of 2D self-supported hybrid CuSe@ PANI core/shell nanosheet arrays for high-performance flexible microsupercapacitors. J. Phys. Chem. C. 123, 29133–29143 (2019).
Balasingam, S. K., Lee, J. S. & Jun, Y. Few-layered MoSe 2 nanosheets as an advanced electrode material for supercapacitors. Dalton Trans. 44, 15491–15498 (2015).
Sree Raj, K., Shajahan, A. S., Chakraborty, B. & Rout, C. S. Two-dimensional layered metallic VSe2/SWCNTs/rGO based ternary hybrid materials for high performance energy storage applications. Chemistry–A Eur. J. 26, 6662–6669 (2020).
Wang, C. et al. Metallic few-layered VSe 2 nanosheets: high two-dimensional conductivity for flexible in-plane solid-state supercapacitors. J. Mater. Chem. A. 6, 8299–8306 (2018).
Kumar, K. S., Choudhary, N., Jung, Y. & Thomas, J. Recent advances in two-dimensional nanomaterials for supercapacitor electrode applications. ACS Energy Lett. 3, 482–495 (2018).
Chhowalla, M. et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275 (2013).
Wu, Z. et al. Ultrathin VSe2 nanosheets with fast ion diffusion and robust structural stability for rechargeable zinc-ion battery cathode. Small 16, 2000698 (2020).
Yuan, J. et al. Facile synthesis of single crystal vanadium disulfide nanosheets by chemical vapor deposition for efficient hydrogen evolution reaction. Adv. Mater. 27, 5605–5609 (2015).
Zhao, W. et al. Colloidal synthesis of VSe 2 single-layer nanosheets as novel electrocatalysts for the hydrogen evolution reaction. Chem. Commun. 52, 9228–9231 (2016).
Marri, S. R., Ratha, S., Rout, C. S. & Behera, J. 3D cuboidal vanadium diselenide embedded reduced graphene oxide hybrid structures with enhanced supercapacitor properties. Chem. Commun. 53, 228–231 (2017).
Lee, Y. et al. Pulsed laser-patterned high-entropy single-atomic sites and alloy coordinated graphene oxide for pH-universal water electrolysis. J. Mater. Chem. A (2025).
Goswami, M., Adhikary, N. C. & Bhattacharjee, S. Effect of annealing temperatures on the structural and optical properties of zinc oxide nanoparticles prepared by chemical precipitation method. Optik 158, 1006–1015 (2018).
Kanaujiya, N., Kumar, N., Singh, M., Sharma, Y. & Varma, G. D. CoMn2O4 nanoparticles decorated on 2D MoS2 frame: a synergetic energy storage composite material for practical supercapacitor applications. J. Energy Storage. 35, 102302 (2021).
Shukla, P. S., Agrawal, A., Gaur, A. & Varma, G. Facile synthesis of mesoporous MnCo2O4@ MoS2 nanocomposites for asymmetric supercapacitor application with excellent prolonged cycling stability. J. Energy Storage. 59, 106580 (2023).
Verma, N. & Pathak, D. Rietveld refinement and optical parameters of ZnO nanopowder synthesized by co-precipitation method. Int. J. Mod. Phys. B. 37, 2350078 (2023).
Kuo, F. Y. et al. Synthesis of surfactant-free and morphology-controllable vanadium diselenide for efficient counter electrodes in dye-sensitized solar cells. ACS Appl. Mater. Interfaces. 11, 25090–25099 (2019).
Chen, A., Su, Q., Han, H., Enriquez, E. & Jia, Q. Metal oxide nanocomposites: a perspective from strain, defect, and interface. Adv. Mater. 31, 1803241 (2019).
Hazra, K. et al. Thinning of multilayer graphene to monolayer graphene in a plasma environment. Nanotechnology 22, 025704 (2010).
Shah, W. H. et al. Tuning of the band gap and dielectric loss factor by Mn doping of Zn1-xMnxO nanoparticles. Sci. Rep. 13, 8646 (2023).
Khan, A. Raman spectroscopic study of the ZnO nanostructures. J. Pak Mater. Soc. 4, 5–9 (2010).
Jin, Z. et al. Strong charge-density-wave order of large-area 2D metallic VSe2 nanosheets discovered by temperature-dependent Raman spectra. Appl. Phys. Lett. 116 (2020).
Gayathri, S., Arunkumar, P., Saha, D. & Han, J. H. Composition engineering of ZIF-derived Cobalt phosphide/cobalt monoxide heterostructures for high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 588, 557–570 (2021).
Cho, S. et al. Optimizing nanosheet nickel Cobalt oxide as an anode material for bifunctional electrochemical energy storage and oxygen electrocatalysis. Electrochim. Acta. 274, 279–287 (2018).
Purushothaman, K., Priya, V. S., Nagamuthu, S., Vijayakumar, S. & Muralidharan, G. Synthesising of ZnO nanopetals for supercapacitor applications. Micro Nano Lett. 6, 668–670 (2011).
KA, S. R., Pramoda, K. & Rout, C. S. Assembling a high-performance asymmetric supercapacitor based on pseudocapacitive S-doped VSe 2/CNT hybrid and 2D borocarbonitride nanosheets. J. Mater. Chem. C. 11, 2565–2573 (2023).
Shah, M. Z. U., Sajjad, M., Hou, H., ur Rahman, S. & Shah, A. Copper sulfide nanoparticles on titanium dioxide (TiO2) nanoflakes: a new hybrid asymmetrical Faradaic supercapacitors with high energy density and superior lifespan. J. Energy Storage. 55, 105651 (2022).
Wu, X., Zhai, Z. B., Huang, K. J., Ren, R. R. & Wang, F. Boosting energy and power performance of aqueous energy storage by engineering ultra-fine metallic VSe2 nanoparticles anchored reduced graphene oxide. J. Power Sources. 448, 227399 (2020).
Raj, K. S. & Rout, C. S. Facile synthesis of manganese-doped 2D vanadium diselenide nanosheets for high-performance supercapacitor applications. Emergent Mater. 4, 1037–1046 (2021).
Wang, T. et al. P-N heterojunction NiO/ZnO electrode with high electrochemical performance for supercapacitor applications. Electrochim. Acta. 392, 138976 (2021).
Zhang, L., Wang, H., Zhang, X. & Tang, Y. A review of emerging dual-ion batteries: fundamentals and recent advances. Adv. Funct. Mater. 31, 2010958 (2021).
Khan, I. et al. Superior electrochemical performance of CuS/FeSe2 for advanced asymmetric supercapacitor applications. Electrochem. Commun. 107915 (2025).
Ahmad, S. A. et al. Rational design of a novel MnO2-FeSe2 nanohybrid with nanowires/cubic architecture as promising supercapattery electrode materials. J. Electroanal. Chem. 936, 117318 (2023).
Lu, W. et al. Synergistic effects of Fe and Mn dual-doping in Co3S4 ultrathin nanosheets for high-performance hybrid supercapacitors. J. Colloid Interface Sci. 590, 226–237 (2021).
Giraldo, D., Almodóvar, P., Álvarez-Serrano, I., Chacón, J. & López, M. Electrochemical performance of tunnelled and layered MnO2 electrodes in Aluminium-Ion batteries: A matter of dimensionality. J. Electrochem. Soc. 169, 100538 (2022).
Deb Nath, N. C., Shah, S. S., Qasem, M. A. A., Zahir, M. H. & Aziz, M. A. Defective carbon nanosheets derived from syzygium cumini leaves for electrochemical energy-storage. ChemistrySelect 4, 9079–9083. https://doi.org/10.1002/slct.201900891 (2019).
Aziz, M. A. et al. A simple and direct preparation of a substrate-free interconnected nanostructured carbon electrode from date palm leaflets for detecting hydroquinone. ChemistrySelect 2, 4787–4793 (2017). https://doi.org/10.1002/slct.201700429
Sajjad, M., Khan, Y. & Lu, W. One-pot synthesis of 2D SnS2 nanorods with high energy density and long term stability for high-performance hybrid supercapacitor. J. Energy Storage. 35, 102336 (2021).
Sajjad, M. et al. NiCo2S4 nanosheet grafted SiO2@ C core-shelled spheres as a novel electrode for high performance supercapacitors. Nanotechnology 31, 045403 (2019).
Wei, Q. et al. Preparation and electrochemical performance of orange Peel based-activated carbons activated by different activators. Colloids Surf. A. 574, 221–227 (2019).
Kim, Y., Cho, E., Park, S. J. & Kim, S. One-pot microwave-assisted synthesis of reduced graphene oxide/nickel Cobalt double hydroxide composites and their electrochemical behavior. J. Ind. Eng. Chem. 33, 108–114 (2016).
Zardkhoshoui, A. M., Davarani, S. S. H., Ashtiani, M. M. & Sarparast, M. Designing an asymmetric device based on graphene wrapped yolk–double shell NiGa 2 S 4 Hollow microspheres and graphene wrapped FeS 2–FeSe 2 core–shell cratered spheres with outstanding energy density. J. Mater. Chem. A. 7, 10282–10292 (2019).
Ali, A. et al. A honeycomb-like ZnO/SnO 2 nanocomposite on nickel foam for high-performance asymmetric supercapacitors. New J. Chem. 43, 10583–10589 (2019).
Li, Z. et al. High-performance solid-state supercapacitors based on graphene-ZnO hybrid nanocomposites. Nanoscale Res. Lett. 8, 1–9 (2013).
Kurzweil, P., Schottenbauer, J. & Schell, C. Past, present and future of electrochemical capacitors: pseudocapacitance, aging mechanisms and service life Estimation. J. Energy Storage. 35, 102311 (2021).
Acknowledgements
The authors would like to acknowledge the Higher Education Commission of Pakistan (HEC) via the National Research Program for Universities (NRPU) Project No. 20-16971/NRPU/R&D/HEC/2021 for funding this study. The authors also acknowledge the financial support extended by the Researchers Supporting Project number (RSP2025R242), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
D.A, M.Z.U.S, R.K and A.S; data curation, D.A, A.U.S, K.S, and H.U; formal analysis, A.S, K.M.A, and W.H.S; methodology, M.Z.U.S, D.A, A.S, A.U.S and K.S; project administration, A.A, H.U, and W.M.G; resources, K.M.A; supervision, A.U.S, M.Z.U.S, K.S and D.A; writing-original draft, R.K, A.S, W.M.G, and D.A; writing-review and editing, K.S, K.M.A; all authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Arif, D., Khan, R., Shah, A.U. et al. Design of ZnO–VSe2 nanocomposite for high performance asymmetric supercapacitors. Sci Rep 15, 17592 (2025). https://doi.org/10.1038/s41598-025-02531-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02531-9