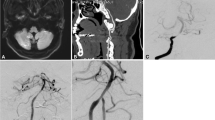
Abstract
Paclitaxel-coated balloon (PCB) treatment has shown promise for intracranial atherosclerotic disease (ICAD). However, studies on restenosis and its associated factors after PCB use are lacking. This study aimed to elucidate risk factors of restenosis after PCB treatment for ICAD. We retrospectively enrolled consecutive patients who had been successfully treated with PCBs for symptomatic ICAD between January 2016 and December 2022. Eligible patients were classified into “no-restenosis” and “restenosis” groups by follow-up DSA. Demographic, clinical, laboratory test, and angiographic data were analyzed. Multivariate logistic regression analysis was used to explore the risk factors of restenosis after PCB treatment. A total of 261 eligible patients were enrolled in this study. There was no death and there were 6.51% (17/261) patients had ipsilateral stroke within 30 days. During 6 months clinical follow up, there was no death and there were 3.07% (8/261) patients had ipsilateral stroke after 30 days, and 13.41% (35/261) patients were identified with restenosis. The restenosis group had significantly higher follow-up hs-CRP (P = 0.040), higher stenosis degree (P = 0.011), higher total occlusion rate (P = 0.009), longer lesion length (P < 0.001), higher residual stenosis after intervention (P < 0.001) and lower stenting rate (P = 0.017).Multivariate logistic regression analysis showed that baseline neutrophil count (OR 1.356, 95% CI 1.015–1.812; P = 0.039), lesion length (OR 1.113, 95% CI 1.032–1.199; P = 0.005) and residual stenosis after intervention (OR 1.066, 95% CI 1.036–1.097; P < 0.001) were risk factors of restenosis.
Similar content being viewed by others
Introduction
Intracranial atherosclerotic disease (ICAD) is one of the most common causes of ischemic stroke and is associated with a high risk of recurrent stroke despite aggressive medical treatment, especially in Asian populations1,2. Angioplasty and stenting have been attempted for patients who are refractory to medication3. However, the safety and long-term efficacy of conventional angioplasty and stenting were challenged by the high incidence of periprocedural complications and high restenosis rate, which accounted for most subsequent recurrence of ischemic events. The in-stent restenosis (ISR) rate has been reported to be 23–30% for bare metal stents4,5,6, and ISR affects the long-term outcome in these patients by increasing the risk of recurrent stroke7.
Decreasing the risk of restenosis is an important way to reduce stroke recurrence. Paclitaxel-coated balloon (PCB) is coated with paclitaxel, it can effectively inhibit intimal hyperplasia and reduce the risk of restenosis8,9.With the growth in endovascular devices, PCB has been gradually applied in the treatment of ICAD to reduce restenosis recently. Preliminary studies have showed the safety and feasibility of PCB for ICAD with a lower incidence of restenosis10,11,12,13,14,15,16. However, large-scale clinical studies on restenosis and its associated factors after PCB use are lacking.
This study aimed to elucidate vessel patency and associated factors of restenosis from the perspectives of demographic profile, blood parameters and features of the lesion in patients with ICAD undergoing PCB treatment.
Methods
Study population
We retrospectively reviewed our prospective stroke database to identify consecutive patients who had been successfully treated with PCBs for symptomatic ICAD between January 2016 and December 2022. Informed consent was obtained from patients or their authorized family members before surgery. The procedure was complied with the Declaration of Helsinki. The Institutional Review Board of the First Affiliated Hospital of Shandong First Medical University approved the study.
Study inclusion criteria were as follows: (1) intracranial atherosclerosis was the primary etiology; (2) transient ischemic attacks (TIA) or ischemic stroke related to a stenotic or occlusive intracranial artery despite optimal medical treatment, which was defined as treatment that included dual-antiplatelet treatment (DAPT), statin, blood pressure and glucose control, smoking cessation, and an emphasis on a healthy lifestyle; (3) stenosis degree ≥ 70% or total occlusion in the intracranial internal carotid artery (Intra ICA), middle cerebral artery (MCA), intracranial vertebral artery (Intra VA), or basilar artery (BA); The stenosis degree was determined according to the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study on digital subtraction angiography (DSA); (4) vascular imaging follow-up using DSA was performed after treatment. And study exclusion criteria were: (1) nonatherosclerotic diseases, such as suspected cerebral vasculitis, arterial dissection, and potential source of cardiac embolism; (2) the target artery with tandem multiple stenotic lesions.
Procedures
The details of the interventional procedure have been described previously10,12. In brief, all patients were given individual standard medical treatment, risk factor management, and lifestyle interventions. DAPT with aspirin 100 mg and clopidogrel 75 mg daily was given at least for 5 days before procedure. The endovascular procedures were performed under general anesthesia. Adequate predilation using conventional balloons was mandatory before the use of PCB (SeQuent Please; B. Braun, Berlin, Germany). PCB angioplasty was applied after conventional balloons angioplasty. If patients suffered dissection after conventional balloons angioplasty, the treatment was the same, we still applied PCB angioplasty in the next step. The diameter of the PCB corresponded to approximately 80 to 100% of the normal vessel and was 0.5 to 1 mm larger than the conventional balloon. The PCB covered the entire lesion and was slowly inflated at a nominal pressure for 60 s to transit the paclitaxel into the vessel wall. After withdrawal of the PCB, an angiogram was reperformed to evaluate the lumen and exclude vessel dissection, perforation, and distal embolization. Remedial stenting implantation was performed if the residual stenosis was > 50% or there was flow limiting dissection after PCB dilation. Post-procedural antegrade flow was graded using the TICI grading system, and technical success was determined by recanalization with a TICI grade 2b/3 on post-procedural angiography. DAPT was maintained for 3 months for patients with only PCB dilation and 6 months for patients with remedial stenting implantation. Aspirin or clopidogrel monotherapy was maintained thereafter.
Data collection and Follow-up outcomes
Demographic, clinical, laboratory test, angiographic data were collected. All patients were followed up clinically at 1 month, 3 months, 6 months, 1 year, and yearly thereafter. They were scheduled to return for DSA examination at 6 mo (± 1mo) after the index procedure. The primary follow-up outcomes were angiographic restenosis, recurrent ischemic events, and symptomatic restenosis at 6 months. Ipsilateral stroke and death within 30 days, ipsilateral stroke and death after 30 days, and restenosis rate were analyzed. Restenosis was defined as > 50% stenosis within or immediately adjacent (within 5 mm) of the treated segment and > 20% absolute luminal loss. Recurrent ischemic events were defined as any focal neurological symptoms related to the corresponding vascular territory. Symptomatic restenosis was defined as restenosis associated with ischemic symptoms of the offending vessel territory. The imaging and clinical outcomes were reviewed by 2 investigators. Disagreements were resolved by consensus.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation (SD) or as the median with interquartile range, and compared using the Student t test or the Mann-Whitney U test. Categorical variables are expressed as numbers and percentages, and compared using the chi-square or Fisher exact test. The Kolmogorov-Smirnov test was used to test distribution normality. Multivariate logistic regression analyses were conducted to test the relationship between restenosis and the variables. The results of regression analysis are presented as odds ratios (ORs) and 95% CIs. A two-tailed P value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 27.0 for Windows (IBM, Armonk, New York).
Results
Study population and follow-up outcomes
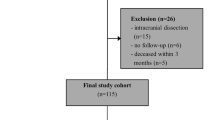
A total of 261 eligible patients treated with PCBs for symptomatic ICAD were enrolled between January 2016 to December 2022 (Fig. 1). Among them, 226 patients were identified without restenosis, 35 patients were identified with restenosis. The restenosis rate is 13.41% in these patients. The median DSA follow-up period was 6.00 months (IQR 4.00–7.00 months). The restenosis incidence in the PCB group were significantly lower than those in the plain balloon group (13.41% [35/261] vs. 52.63% [10/19], P < 0.001) and the conventional stenting group (13.41% [35/261] vs. 22.58% [28/124], P = 0.023).
There was no death and there were 17 patients (17/261, 6.51%) had ipsilateral stroke within 30 days. Ischemic stroke occurred in 8 patients, intracranial hemorrhage occurred in 8 patients, subarachnoid hemorrhage occurred in 1 patient. There were 2 major and 15 minor complications in these patients. The most frequently encountered complication was hyperperfusion or reperfusion intracerebral hemorrhage. There was 1 major instance of symptomatic intracerebral hemorrhage, 1 minor instance of symptomatic intracerebral hemorrhage and 6 instances of asymptomatic intracerebral hemorrhage. The patient who suffered major intracerebral hemorrhage presented restlessness and gaze immediately after the procedure, and was transferred to the intensive care unit, the symptoms relieved after aggressive pressure control and medicine treatment. The patient who suffered minor symptomatic intracerebral hemorrhage presented dizziness and headache immediately after the procedure, and the symptoms relieved after pressure control and medicine treatment. The second most commonly encountered complication was perforator or branch artery stroke. There were 7 instances of minor symptomatic perforator or branch artery stroke. The symptoms including dizziness, headache, blurred vision, limb weakness, limb numbness and speech disorder were relieved after medicine treatment. There was 1 major ischemic stroke due to acute thrombosis formation in stent 1 day after the procedure. The patient presented restlessness, speech disorder and limb weakness. Tirofiban was applied for this patient. The symptoms improved and relieved after aggressive medicine and rehabilitation treatment. The patient who suffered minor subarachnoid hemorrhage was asymptomatic.
During 6 months clinical follow up, there was no death and there were 3.07% (8/261) patients had ipsilateral stroke after 30 days. 7 patients in restenosis group and 1 patient in no-restenosis group had recurrent minor ischemic events of the offending vessel territory respectively. The symptoms including limb numbness, limb weakness, speech disorder and the symptoms relieved after medicine treatment. The recurrent ischemic events rate is 3.07% (8/261) and symptomatic restenosis rate is 2.68% (7/261) in these patients. The recurrent ischemic events rate of restenosis group was significantly higher than no-restenosis group (20.00% [7/35] vs. 0.44% [1/226], P < 0.001).
Baseline demographic parameters of the patients
The baseline demographic parameters of the patients are shown in Table 1. There was no significant difference between the two groups concerning the age, sexes, medical history including smoking, hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease and ischemic stroke history. In the restenosis group, 94.29% were admitted to the hospital because of stroke and 5.71% due to TIA; these values were 95.13% and 4.87% in the no-restenosis group. Differences were also not statistically significant in qualifying ischemic events (P = 1.000), baseline NIHSS score (P = 0.104) and mRS score (P = 0.316).
Baseline and follow-up blood parameters of the patients
In terms of baseline blood parameters (Table 2), differences were not statistically significant in leukocyte count, neutrophil count, monocyte count, lymphocyte count, platelet count, neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), fibrinogen, fasting plasma glucose, glycated hemoglobin (HbA1c), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride, total cholesterol, homocysteine, uric acid, ESR (erythrocyte sedimentation rate), and high-sensitivity C-reactive protein(hs-CRP) between the two groups.
The follow-up blood parameters were tested when the patients underwent DSA follow-up (Table 2). Patients in the restenosis group had a significantly higher level of follow-up hs-CRP (median 0.74 vs. 1.16 mg/L, P = 0.040). But differences were not statistically significant in other follow-up blood parameters including leukocyte count, neutrophil count, monocyte count, lymphocyte count, platelet count, NLR, PLR, fibrinogen, fasting plasma glucose, HbA1c, LDL, HDL, triglyceride, total cholesterol, homocysteine, uric acid, and ESR between the two groups.
Lesion and procedure characteristics
The lesion and procedure characteristics of the patients are shown in Table 3. The restenosis group had significantly higher stenosis degree before intervention (median 89.50 [77.58,100.00]% VS 100.00 [83.96,100.00]%, P = 0.011), higher total occlusion rate (36.73% [83/226] VS 60.00% [21/35], P = 0.009), longer lesion length (median 5.58 [3.57,8.46]mm VS 10.92 [5.70,13.49]mm, P < 0.001), higher residual stenosis after intervention (21.35 [0.00,32.39]% VS 38.11 [26.45,48.39]%, P < 0.001) and lower stenting rate ( 47.35% [107/226] VS 25.71% [9/35], P = 0.017). There was no significant difference for target artery distribution (P = 0.880). There were 2 patients suffered dissection after conventional balloons angioplasty. 15 patients suffered dissection after PCB dilation, and dissection rate after PCB dilation (5.75% [13/226] VS 5.71% [2/35], P = 1.000) between the two groups was not significantly different.
Risk factors associated with restenosis
The univariate logistic regression analysis was initially performed to identify risk factors with P < 0.05 (Table 4). Univariate logistic regression analysis results revealed that baseline neutrophil count (OR 1.291, 95% CI 1.017–1.637; P = 0.036); stenosis degree before intervention (OR 1.039, 95%CI 1.004–1.075; P = 0.027); total occlusion (OR 2.584, 95% CI 1.247–5.354; P = 0.011), lesion length (OR 1.133, 95%CI 1.068–1.203; P < 0.001); residual stenosis after intervention (OR 1.066, 95%CI 1.039–1.093; P < 0.001); stenting (OR 0.385, 95%CI 0.173–0.858; P = 0.020) were associated with restenosis.
The factors with significance levels of P < 0.05 in the univariate logistic regression analysis were further analyzed in a multivariate logistic regression analysis for the risk factors of restenosis. Multivariate logistic regression analysis showed that baseline neutrophil count (OR 1.356, 95% CI 1.015–1.812; P = 0.039); lesion length (OR 1.113, 95% CI 1.032–1.199; P = 0.005); residual stenosis after intervention (OR1.066, 95%CI 1.036–1.097; P < 0.001) were risk factors of restenosis. The results of the multivariate logistic regression analysis are shown in Table 5.
Discussion
Over the years, managing ICAD has remained challenging. Previous studies reported restenosis rate after PCB angioplasty varied from 0 to 15% 10–16. The restenosis rate at 6-mo DSA follow-up in this study (13.41%, 35/261) was consistent with them. The recurrent ischemic events rate is 3.07% (8/261) and symptomatic restenosis rate is 2.68% (7/261) in these patients. The recurrent ischemic events rate of restenosis group was significantly higher than no-restenosis group (20.00% [7/35] vs. 0.44% [1/226], P < 0.001). In aspect of target artery distribution, there was no significant difference in restenosis rate.
Compared with plain balloon angioplasty, PCB angioplasty can effectively reduce the incidence of restenosis. Tang et al.11 reported that the incidence of restenosis in the PCB (SeQuent Please drug-coated balloon, the same as we used in our study) group was significantly lower than that in the plain balloon group (6.3% vs. 31.3%, P = 0.01) without increasing the risk of periprocedural stroke or death. In our previous study12, we compared the efficacy and safety of PCB dilation (with or without stenting) with conventional stenting angioplasty for symptomatic ICAD in routine clinical practice. The median stenosis degree (0 [0-20.0%] vs. 15.0 [0-62.5%], P = 0.005) and total restenosis incidence (5.3% vs. 34.2%, P = 0.003) in the PCB group were significantly lower than those in the conventional stenting group. For periprocedural complications, although there was no statistical difference, the absolute incidence of periprocedural complications was lower in the PCB group than that in the conventional stenting group. Although there were no statistical differences, the absolute incidence of recurrent ischemic events and symptomatic restenosis were lower in the PCB group than those in the non-PCB group in the 2 studies. This study also indicated that the restenosis incidence in the PCB group were significantly lower than those in the plain balloon group (13.41% vs. 52.63%, P < 0.001) and the conventional stenting group (13.41% vs. 22.58%, P = 0.023). The safety and efficacy comparation among PCB angioplasty, conventional balloon angioplasty and stenting still need further study.
PCB treatment reducing the risk of restenosis decreases the risk of recurrent stroke. Identifying which patients with ICAD will respond optimally to PCB treatment is of paramount importance for improving outcomes and using resources efficiently. We analyzed the vessel patency and associated factors of restenosis in patients with symptomatic ICAD undergoing PCB treatment.
The restenosis group had significantly higher level of follow-up hs-CRP (P = 0.040), higher stenosis degree before intervention (P = 0.011), higher total occlusion rate (P = 0.009), longer lesion length (P < 0.001), higher residual stenosis after intervention (P < 0.001) and lower stenting rate (P = 0.017). Multivariate logistic regression analysis showed that baseline neutrophil count (OR 1.356, 95% CI 1.015–1.812; P = 0.039); lesion length (OR 1.113, 95% CI 1.032–1.199; P = 0.005); residual stenosis after intervention (OR 1.066, 95%CI 1.036–1.097; P < 0.001) were risk factors of restenosis.
The pathophysiological mechanisms of restenosis showed that intimal hyperplasia and inflammatory responses contribute to in-stent neointimal growth following arterial injury17, and the number of inflammatory cells around stent struts was significantly higher in restenosis compared with no restenosis. These data suggest the use of strategies that reduce intimal hyperplasia and inflammation to reduce the incidence of restenosis. Paclitaxel coated on the balloon can inhibit neointimal proliferation, arterial smooth muscle cell proliferation and migration, reduce inflammation, and suppress the occurrence of neo-atherosclerosis8,9,18,19. We have evaluated dynamic changes in the vessel wall features after PCB angioplasty for intracranial atherosclerotic lesions by vessel wall MRI previously20. This study showed that vascular healing with plaque modification and stabilization occurred following PCB treatment of intracranial atherosclerotic lesions. These results strongly highlight the potential of PCB angioplasty to treat ICAD.
Dissection after dilatation can provoke inflammation of the vessel wall and it may be related to restenosis. But there was no significant difference for dissection after PCB dilation (P = 1.000) between the two groups in this study. We speculated this result may related to the anti-neointimal hyperplasia and anti-inflammatory function of paclitaxel coated on the balloon. And the bailout stenting was also helpful to heal the dissection.
Baseline neutrophil count was a risk factor for restenosis in our study. Increased neutrophil count may indicate elevated inflammatory reaction of the body, leading to excessive reaction of the arterial wall and final restenosis. Preprocedural inflammation factors hs-CRP, NLR were found to be risk factors of intracranial ISR in previous studies21,22. But we did not find the inflammation makers such as hs-CRP, NLR, PLR were risk factors of the restenosis after PCB treatment for ICAD. This inconsistency may because that the effects of systemic hyperinflammation on the local vascular wall were partly offset by the antiinflammation and antiproliferation effect of paclitaxel eluted by PCB.
In our study, the lesion length was found to be a risk factor of restenosis after multivariate logistic regression analysis. Longer lesions are prone to be more difficult to treat. A longer lesion means greater plaque burden, more serious atherosclerotic damage and more inflammatory cells which may be one of the underlying mechanisms leading to the intracranial restenosis. As paclitaxel treatment demonstrated a dose-dependently inhibition of inflammation and intimal hyperplasia8, appropriately increasing paclitaxel eluted to these lesions may help to reduce restenosis occurrence.
Residual stenosis was found to be another risk factor of restenosis after PCB treatment in this study. However medial injury and increased medial fracture length cause increased neointimal growth17, the potential benefit of “bigger is better” to reduce restenosis is limited by increased arterial injury and inflammation. Plaque disruption and arterial injury of varying degrees are unavoidable if lumen dilatation is to be achieved. Arterial injury is difficult to quantify clinically in present interventional practice, and the operator cannot tightly control the degree of arterial injury (plaque disruption, medial stretch, medial dissection, or medial rupture) produced during procedures. We suppose that proper restoration of normal physiological blood flow and avoidance of excessive medial injury are desirable goals in the ICAD treatment rather than only maximizing lumen area. The optimal immediate residual stenosis rate needs further investigation in the future.
In this study, there were 6.51% patients (17/261) had ipsilateral stroke within 30 days. Ischemic stroke occurred in 3.07% (8/261) of patients, intracranial hemorrhage occurred in 3.07% (8/261) of patients, and subarachnoid hemorrhage occurred in 0.38% (1/261) of patient. After treatment with PCB angioplasty, complications were not different from those after conventional balloon angioplasty or stenting in previous studies. Periprocedural stroke rate within 30 days in this study seemed to be high (6.51%). One of the most important things is to reduce periprocedural stroke rate as much as possible to justify this therapy. Within the periprocedural time window of 72 h after the procedure, WEAVE Trial23 demonstrated 2.6% (4/152 patients) periprocedural complication rate, which was lower than this study. Comparing with WEAVE Trial, there are some differences between the two studies, such as study inclusion criteria and follow-up windows. WEAVE trial enrolled patients with symptomatic ICAD lesion of 70–99% in an artery 2 mm or larger. While patients with symptomatic ICAD lesion of stenosis degree ≥ 70% or total occlusion were enrolled in this study. The enrolled patients in this study remained hemodynamically unstable and presented with recurrent ischemic events despite aggressive medical therapy. These patients were in danger of high risk of stroke recurrence. The treatment of occlusion lesions is much more difficult with higher risk than stenotic lesions. Besides, the follow-up time window for periprocedural complication rate was 72 h after the procedure in WEAVE Trial, which was shorter than 30 days follow-up time window in this study. It seems to be reasonable for the higher periprocedural complication rate in this study because of these differences between the two studies. WEAVE trial recommended to choose the balloon size with nominal diameter at 6 atmospheres to be 80% of the true luminal diameter or ≈ 60% in lesions directly adjacent to angiographically visible perforators. Underdilation is recommended to avoid arterial dissection, vessel rupture, and snow plow effect of compressed plaque into perforator arteries. Maybe this method is helpful to reduce the periprocedural complication rate and further studies are necessary to assess the efficacy and feasibility of this method for reducing periprocedural complication rate of PCB treatment in symptomatic ICAD.
Our study has several limitations. First, this study is a retrospective study and may lead to selection bias. Second, this study is a single-center study, so it may not be generalized in a wide range. Third, the independent predictors of restenosis identified in the multivariable analysis appear to be consistent with those reported in previous studies on conventional balloon angioplasty and stenting. Exploring the specific predictors within the context of PCB treatment is needed in further research.
In conclusion, for patients with symptomatic ICAD, the use of PCB treatment can effectively lower restenosis risk. The baseline neutrophil count, lesion length and residual stenosis after intervention were risk factors of restenosis after PCB treatment for ICAD.
Data availability
The datasets are available from the corresponding author on reasonable request.
References
Gorelick, P. B., Wong, K. S., Bae, H. J. & Pandey, D. K. Large artery intracranial occlusive disease: A large worldwide burden but a relatively neglected frontier. Stroke 39, 2396–2399. https://doi.org/10.1161/strokeaha.107.505776 (2008).
Wang, Y. et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: The Chinese intracranial atherosclerosis (CICAS) study. Stroke 45, 663–669. https://doi.org/10.1161/strokeaha.113.003508 (2014).
Leung, T. W., Wabnitz, A. M., Miao, Z. & Chimowitz, M. I. Angioplasty and stenting. Front. Neurol. Neurosci. 40, 152–163. https://doi.org/10.1159/000448311 (2016).
Ma, N. et al. Stenting for symptomatic intracranial arterial stenosis in China: 1-year outcome of a multicentre registry study. Stroke Vasc. Neurol. 3, 176–184. https://doi.org/10.1136/svn-2017-000137 (2018).
Levy, E. I. et al. Wingspan in-stent restenosis and thrombosis: Incidence, clinical presentation, and management. Neurosurgery 61, 644–650. https://doi.org/10.1227/01.Neu.0000290914.24976.83 (2007). discussion 650 – 641.
Kang, K. et al. Balloon-mounted stenting for ICAS in a multicenter registry study in China: A comparison with the WEAVE/WOVEN trial. J. Neurointerv. Surg. 13, 894–899. https://doi.org/10.1136/neurintsurg-2020-016658 (2021).
Derdeyn, C. P. et al. Nonprocedural symptomatic infarction and In-Stent restenosis after intracranial angioplasty and stenting in the SAMMPRIS trial (Stenting and aggressive medical management for the prevention of recurrent stroke in intracranial Stenosis). Stroke 48, 1501–1506. https://doi.org/10.1161/strokeaha.116.014537 (2017).
Chowdhury, M. M. et al. Paclitaxel Drug-Coated balloon angioplasty suppresses progression and inflammation of experimental atherosclerosis in rabbits. JACC Basic Transl. Sci. 5, 685–695. https://doi.org/10.1016/j.jacbts.2020.04.007 (2020).
Scheller, B. et al. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 110, 810–814. https://doi.org/10.1161/01.Cir.0000138929.71660.E0 (2004).
Han, J. et al. Drug-coated balloons for the treatment of symptomatic intracranial atherosclerosis: initial experience and follow-up outcome. J. Neurointerv. Surg. 11, 569–573. https://doi.org/10.1136/neurintsurg-2018-014237 (2019).
Tang, Y. et al. Comparison of drug-coated balloon with conventional balloon for angioplasty in symptomatic intracranial atherosclerotic stenosis. J. Neurointerv. Surg. https://doi.org/10.1136/jnis-2022-019685 (2023).
Zhang, J. et al. Drug-Coated balloon dilation compared with conventional stenting angioplasty for intracranial atherosclerotic disease. Neurosurgery 87, 992–998. https://doi.org/10.1093/neuros/nyaa191 (2020).
Qiao, H. et al. Safety and efficacy of drug coated balloon angioplasty for intracranial atherosclerotic disease. J. Neurointerv. Surg. https://doi.org/10.1136/jnis-2022-019122 (2022).
Gruber, P. et al. Percutaneous transluminal angioplasty using the novel drug-coated balloon catheter sequent please NEO for the treatment of symptomatic intracranial severe stenosis: Feasibility and safety study. J. Neurointerv Surg. 11, 719–722. https://doi.org/10.1136/neurintsurg-2018-014378 (2019).
Gruber, P. et al. Neuro elutax SV drug-eluting balloon versus wingspan stent system in symptomatic intracranial high-grade stenosis: A single-center experience. J. Neurointerv. Surg. 10, e32. https://doi.org/10.1136/neurintsurg-2017-013699 (2018).
Zhao, W. et al. Drug-Coated balloon treatment for delayed recanalization of symptomatic intracranial artery occlusion. Transl. Stroke Res. 14, 193–199. https://doi.org/10.1007/s12975-022-01024-5 (2023).
Farb, A., Weber, D. K., Kolodgie, F. D., Burke, A. P. & Virmani, R. Morphological predictors of restenosis after coronary stenting in humans. Circulation 105, 2974–2980. https://doi.org/10.1161/01.cir.0000019071.72887.bd (2002).
Hou, D., Rogers, P. I., Toleikis, P. M., Hunter, W. & March, K. L. Intrapericardial Paclitaxel delivery inhibits neointimal proliferation and promotes arterial enlargement after Porcine coronary overstretch. Circulation 102, 1575–1581. https://doi.org/10.1161/01.cir.102.13.1575 (2000).
Axel, D. I. et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 96, 636–645. https://doi.org/10.1161/01.cir.96.2.636 (1997).
Meng, Y. et al. Plaque modification and stabilization after drug-coated balloon angioplasty for intracranial atherosclerotic lesions. Eur. Radiol. 33, 1112–1120. https://doi.org/10.1007/s00330-022-09129-z (2023).
Yu, Y. et al. Multiple predictors of in-stent restenosis after stent implantation in symptomatic intracranial atherosclerotic stenosis. J. Neurosurg. 136, 1716–1725. https://doi.org/10.3171/2021.6.Jns211201 (2022).
Guo, X. et al. Long-Term risk factors for intracranial In-Stent restenosis from a multicenter trial of stenting for symptomatic intracranial artery stenosis registry in China. Front. Neurol. 11, 601199. https://doi.org/10.3389/fneur.2020.601199 (2020).
Alexander, M. J. et al. WEAVE trial: Final results in 152 On-Label patients. Stroke 50, 889–894. https://doi.org/10.1161/strokeaha.118.023996 (2019).
Author information
Authors and Affiliations
Contributions
Y.K.C. and K.Y.D. contributed to the acquisition, analysis of the data; the revision of content and the final approval of the article. Y.S., L.L.S., and M.M.Z. contributed to the analysis of the data and the final approval of the article. H.Y., J.Z., W.W., Y.M., W.L.L. and X.H. contributed to the statistical analysis and the final approval of the article. W.Z. contributed to the design of the study; acquisition, analysis and interpretation of the data; drafting the article and revising the content; and final approval of the paper. J.H. contributed to the conception and design of the study, acquisition and analysis of the data, revision of the content, and final approval of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Dong, K., Song, Y. et al. Risk factors of restenosis after paclitaxel-coated balloon treatment in symptomatic intracranial atherosclerotic disease. Sci Rep 15, 18061 (2025). https://doi.org/10.1038/s41598-025-02538-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02538-2