Abstract
Clustered protocadherins (cPcdh) are cell adhesion molecules with 58 isoforms, essential for neural circuit formation and higher cognitive functions. This study investigated the impact of reduced cPcdh-α diversity on cognitive function using mutant mice. Behavioral tests revealed that cPcdh-α1-12 mice exhibited specific memory impairments ranging from a few seconds to 2 h short-term memory, while memory from 24 h to 2 weeks long-term memory remained intact. Notably, no abnormalities in appearance or spontaneous behavior were observed in cPcdh-α1-12 mice, suggesting that the deficits were specific to short-term memory. Furthermore, a comprehensive analysis of neural activity during memory recall in 2 h short-term memory and 24 h long-term memory following showed significant reductions in the hippocampus, amygdala, and retrosplenial cortex during short-term memory tasks. No such reductions were observed during long-term memory recall. These results suggest that short-term and long-term memory are supported by partially distinct neural circuits and underscore the critical role of cPcdh-α diversity in establishing the neural pathways necessary for short-term memory retrieval.
Similar content being viewed by others
Introduction
The brain is an extremely complex system that processes vast amounts of information, relying on the specific connections and coordinated interactions of billions of neurons for functions such as sensory integration, memory formation, and cognition. The precise formation of these neural circuits is essential for proper brain function, and disruptions in this process can lead to significant cognitive and behavioral impairments1.
Clustered protocadherins (cPcdh) are cell adhesion molecules that mediate connections between neurons and play an important role in determining the specificity of synaptic connections, making them crucial for neural circuit formation2,3. cPcdh are a family of cell adhesion molecules expressed in neurons, comprising 58 isoforms that are classified into three subgroups: cPcdh-α, cPcdh-β, and cPcdh-γ3,4,5. Each neuron expresses these isoforms in diverse combinations, forming specific synaptic connections through interactions between identical isoforms6,7,8,9,10,11,12. This molecular diversity is thought to be critical for the formation of neural circuits, which are essential for higher brain functions such as memory and cognition13,14. Thus, unraveling the complexity of neural circuit formation requires clarifying the role of cPcdh in synapse formation, an important topic in neuroscience. Although it is known that cPcdh contribute to synaptic specificity, the functional impact of reduced cPcdh isoform diversity on neural circuits and behavior has not been fully elucidated.
To investigate the functional significance of cPcdh diversity, a genetically modified mouse model called cPcdh-α1-12 mice has been developed. In this model, only cPcdh-α1 and α12 of the 12 variable isoforms are expressed, while two αC1 and αC2 constant isoforms are also retained12,15,16. The cPcdh-α1-12 mice exhibit a phenotype similar to that of wild-type mice in appearance and general behavior, but they display deficits in certain higher brain functions, particularly in sensory integration, short-term visual memory, and audiovisual associative memory17,18. These impairments are evident in poorer task performance, emphasizing the critical role of cPcdh-α isoform diversity in neural circuit formation and higher brain functions. Despite these observations, the neural mechanisms responsible for these deficits remain to be elucidated, highlighting the need for further investigation into the molecular and circuit-level changes in cPcdh-α1-12 mice.
The aim of this study is to investigate the effects of reduced cPcdh-α diversity on the behavior and cognitive function of cPcdh-α1-12 mice by first conducting multiple behavioral experiments to comprehensively evaluate their cognitive functions. In addition, changes in neural activity will be observed simultaneously using neuronal activity markers to examine the relationship between cognitive impairments and alterations in neural activity. We hypothesize that reduced cPcdh-α diversity causes behavioral deficits and changes in neural activity, providing new insights into the molecular mechanisms related to neural circuit formation and cognitive functions.
Result
Assessment of behavioral traits in cPcdh α1-12 mice lacking protocadherin diversity
In this study, we assessed the memory and cognitive functions of cPcdh-α1-12 mice, in which α2 to α11 of the 12 clustered protocadherin α isoforms are deleted (Fig. 1a). An open-field test was conducted to measure the total distance traveled and the time spent in the center area, assessing spontaneous locomotor activity and anxiety-like behavior. The results showed no significant differences between cPcdh-α1-12 mice and wild-type mice in either total distance traveled, or time spent in the center area (Fig. 1b, c) (Fig. S1). Next, a fear conditioning test conducted to evaluate long-term memory. The results showed that both cPcdh-α1-12 mice and wild-type mice exhibited significantly high levels of freezing responses, indicating no abnormalities in long-term memory formation (Fig. 1d, Table S1).
Activity and long-term memory assessment in cPcdh-α1-12 mice. (a) Schematic of cPcdh-α1-12 mice, showing the deletion of cPcdh α2 to α11. (b, c) Open field test results: (b) total distance traveled, (c) total time in the central area. No significant differences between wild-type (n = 6, male 3, female 3) and cPcdh-α1-12 (n = 5, male 3, female 2). (d) Fear conditioning test. Both wild-type (n = 10, male 5, female 5) and cPcdh-α1-12 (n = 10, male 5, female 5) mice showed significant freezing at 24 h and 2 weeks, with no significant differences between genotypes. Three mice (male 2, female 1) were tested only at the 24 h time point. Data are mean ± SEM. Statistical analysis used an independent t-test for the open field test and mixed two-way ANOVA with genotype as a between-groups factor and interval as a within-groups factor, followed by Bonferroni’s multiple comparisons test for post-hoc analysis for the fear conditioning test.
Short-term memory deficits in cPcdh α1-12 mice
Previous studies have suggested that cPcdh-α1-12 mice may have specific deficits in short-term memory compared to wild-type mice, as assessed by the T-maze task, which consists of visually guided and memory-guided tasks18. However, in previous studies, mice took over a minute to reach their destination, leading to significant variability between trials and making it difficult to accurately measure memory retention time. Therefore, I followed the previously described protocol while minimizing stress on the mice by using a transfer cylinder to move them into the arena instead of handling them directly, and re-tested the experiment under improved conditions18. First, the mice were trained on a visually guided task. Wild-type mice learned to select the correct arm marked by a visual cue to receive a reward, and both cPcdh-α1-12 and wild-type mice showed similar performance, reaching stable scores within 15 days. Once mice achieved a success rate of 80% or more higher on three separate days, they transitioned to the memory-guided task, where visual cues were replaced with memory-based cues, requiring them to rely on short-term memory to select the correct arm. Following the transition to the memory-guided task, which required short-term memory, the performance of wild-type mice was initially suppressed to chance levels immediately after a task switch and gradually recovered to the performance level before the switch (Fig. 2a). In contrast, cPcdh-α1-12 mice showed a similar initial suppression after the task switch, but no subsequent recovery was observed. As a result, the performance of cPcdh-α1-12 mice was significantly lower than that of wild-type mice (Fig. 2b, Table S2). In addition, the time required for choice selection was significantly reduced compared to previous studies, confirming that cPcdh-α1-12 mice exhibit abnormalities in the formation of short-term memory lasting only a few seconds compared to wild-type mice (Fig. 2c). These findings suggest that while long-term memory capacity remains intact, cPcdh-α1-12 mice exhibit deficits in short-term memory.
Short-term memory assessment in cPcdh-α1-12 mice using T-maze test. (a) After task switching (x = 0), wild-type mice initially showed a decline in performance in both visually-guided and memory-guided tasks but subsequently recovered. In contrast, cPcdhα1-12 mice showed a decline specifically in the memory-guided task without recovery (wild-type n = 4, male 2, female 2; cPcdh-α1-12 n = 4, male 2, female 2). (b) Mean performance in the last five sessions (100 trials). There was no significant difference between groups in the visually-guided task, but cPcdh-α1-12 mice showed significantly lower performance in the memory-guided task (**p < 0.01). (c) Time taken to reach the correct goal. Both groups reached the goals within a few seconds in both tasks, with no significant difference. Data are presented as mean ± SEM. Statistical significance was determined using mixed two-way ANOVA, with genotype as a between-groups factor and task as a within-groups factor, followed by Bonferroni’s multiple comparisons test for post-hoc analysis.
Assessment of short-term and long-term associative memory in cPcdh-α1-12 mice
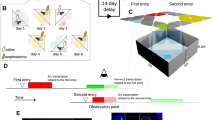
The standard Context Pre-exposure Facilitation Effect (CPFE) paradigm typically uses a fixed 24 h-interval in which the interval between context exploration (Pre-exposure) and electric shock (Immediate Shock, IS)19. In order to investigate memory formation across the different phases between short-term and long-term, we designed a modified CPFE test in which the interval between Pre-exposure and IS was fixed 2 and 24 h. Twenty-four hours after IS, the memory recall tests were conducted once in the shock-administered context (context A) and once in another context (context B) (test 1, test 2). Freezing rates during Pre-exposure, test 1, and test 2 were quantified to assess the ability to discriminate between the two contexts (Fig. 3a). Wild-type mice exhibited significantly higher freezing rates in Test 1 compared to Test 2, regardless of the interval between Pre-exposure and IS (Fig. 3b, c). Similarly, cPcdh-α1-12 mice showed significantly increased freezing in test 1 compared to test 2 at the 24 h interval. In contrast, at the 2 h interval, cPcdh-α1-12 mice did not exhibit any freezing behavior during pre-exposure, test 1, and test 2, indicating a failure to form an association between the context and the shock in cPcdh-α1-12 mice (Fig. 3b). This suggests that they did not recall the context associated with the shock, reflecting impaired short-term associative memory (Table S3, S4). These results indicate that cPcdh-α1-12 mice retain long-term associative memory but have deficits in short-term associative memory formation.
Evaluation of short-term memory deficits and long-term memory retention in cPcdh-α1-12 mice using CPFE tests (a) Schematic of the CPFE test protocol. The interval between Pre-exposure and Immediate Shock (IS) was set at 24 or 2 h. Memory recall Test1 was conducted 24 h after IS, followed by Test2 in a different context 1.5 h later. (b-c) Freezing response results in the CPFE test (wild-type: n = 8, male 4, female 4; cPcdh-α1-12: n = 8, male 4, female 4). (b) shows the test results at the 2 h interval, and (c) shows the results at the 24 h interval. At the 24 h interval, both wild-type and cPcdh-α1-12 mice exhibited significantly higher freezing in Test 1 than in Test 2. However, at the 2 h interval, cPcdh-α1-12 mice showed no significant difference in freezing between Test 1 and Test 2. Data are presented as mean ± SEM. Statistical significance was determined using mixed two-way ANOVA, with genotype as a between-groups factor and phase (Pre-exposure, Test 1, Test 2) as a within-groups factor, followed by Bonferroni’s multiple comparisons test for post-hoc analysis.
c-Fos analysis of neuronal activity during memory recall in cPcdh-α1-12
Previous behavioral experiments have shown that cPcdh-α1-12 mice exhibit specific deficits in short-term memory, while their long-term memory retention remains intact. These results suggest that different neural circuits and molecular mechanisms may be involved in short-term and long-term memory. To further investigate, I next analyzed neuronal activity during memory recall using c-Fos staining, a marker for neuronal activation, to examine which brain regions are associated with these memory impairments at the cellular level. Mice were sacrificed 1.5 h after being placed in context A, and brain tissues were processed for c-Fos staining. For detailed analysis of neuronal activity, coronal sections were prepared along the anterior–posterior axis of the brain, and quantitative analyses were performed on each section. c-Fos-positive cells were automatically detected, and the obtained brain sections were registered with the Allen Brain Atlas using Serial Section Registration20,21. For each mouse, 96 coronal sections spanning AP 2.3 to AP −4.0 were analyzed to assess neuronal activity across the anterior–posterior axis (Fig. 4a). In tests conducted at a 2 h interval, c-Fos-positive neurons were observed in wild-type mice across multiple cortical and subcortical regions, including the hippocampus, prefrontal cortex, and basolateral amygdala, indicating that these areas are strongly involved in memory recall. These results were consistent with the active regions observed in conventional CPFE tests conducted previously22,23. In contrast, cPcdh-α1-12 mice showed significantly reduced neuronal activity during recall in regions such as the hippocampus (CA1, CA3), retrosplenial area (RSP), amygdala (MEA, BLA), and endopiriform nucleus (EP), compared to wild-type mice (Fig. 4b, d (i-v), e) (Fig. S2b). These findings are consistent with the behavioral experiments and suggest that the specific impairments in short-term memory recall observed in cPcdh-α1-12 mice may be caused at the neuronal level. Furthermore, when tested at a 24 h interval, no significant differences in c-Fos-positive neuronal activity were observed between cPcdh-α1-12 mice and wild-type mice in the hippocampus, prefrontal cortex, amygdala, and other mentioned brain regions (Fig. 4c, d (i’-v’), e) (Fig. S2a). These findings indicate that while cPcdh-α1-12 mice exhibit abnormal neuronal activity related to short-term memory, their neuronal activity during long-term memory recall appears to be normal.
Comparison of neuronal activity in brain regions during short-term and long-term memory recall in cPcdhα1-12 mice (a) Coronal brain Sects. (50 μm thick, AP 2.3 to AP −4.0) were prepared and aligned with the Allen Brain Atlas for c-Fos quantification. 2 h interval : cPcdh-α1-12 (n = 5, male 3, female 2), wild-type (n = 5, male 3, female 2), 24 h interval : cPcdh-α1-12 (n = 4, male 2, female 2), wild-type (n = 4, male 2, female 2). Approximately 98 slices were obtained per mouse. (b) Quantification of c-Fos-positive neurons in brain regions of cPcdhα1–12 mice and wild-type mice tested at a 24 h interval. The top 16 regions with the highest number of cells in wild-type mice are shown. No significant differences were observed between wild-type and cPcdh-α1-12 mice across any of the brain regions. (c) Quantification of c-Fos-positive neurons in brain regions of cPcdh-α1-12 and wild-type mice tested at a 2 h interval. In cPcdh-α1-12 mice, significantly fewer c-Fos-positive neurons were observed in the hippocampus (CA1, CA3), retrosplenial area (RSP), amygdala (MEA, BLA), and endopiriform nucleus (EP) compared to wild-type mice. Statistical significance is indicated by *p < 0.05. (d) Representative images of c-Fos staining observed in cPcdh-α1-12 (i’–vi’) and wild-type mice (i–vi) 2 h after memory recall. (i), (i’) CA3 (ii), (ii’) CA1 (iii), (iii’) RSP (iv), (iv’) MEA (v), (v’) BLA, EP. Scale bar = 100 μm. (e) 3D reconstruction of c-Fos-positive neurons. Activated neurons (green dots) are visualized in regions where cPcdh-α1-12 mice showed significantly reduced activity, highlighting the differences in neuronal activity patterns between the 4 groups. Data are presented as mean ± SEM. Statistical significance was determined using unpaired t-test.
Neuronal activation changes during memory retrieval in mice with reduced cPcdh diversity
In the previous section, we showed that cPcdh-α1-12 mice exhibit reduced neuronal activity during short-term memory recall, as indicated by decreased numbers of activated cells across several brain regions. In this section, we examined whether the density of activated neurons during short-term and long-term memory tasks is affected by the reduction in cPcdh diversity. To address this, we performed statistical comparisons using genotype and interval as independent factors. In the CA3 and CA1 regions, a significant interaction between genotype and interval was observed (Table S5). At the 2 h interval, cPcdh-α1-12 mice showed significantly lower c-Fos expression than wild-type mice (Fig. 5a, b). This difference was not observed at the 24 h interval, suggesting that the reduction in neuronal activity is specific to short-term memory recall. In addition, c-Fos expression in cPcdh-α1-12 mice was higher at 24 h than at 2 h. In the BLA and EP regions, a significant interaction between genotype and interval was also observed. At the 2 h interval, cPcdh-α1-12 mice showed significantly lower c-Fos expression than wild-type mice, whereas no such difference was observed at the 24 h interval (Fig. 5c, d). In the RSP and MEA regions, a similar trend of decreased c-Fos expression in cPcdh-α1-12 mice at the 2 h interval was observed, although neither the genotype nor interval effect reached statistical significance (Fig. 5e, f). These results indicate that reduced cPcdh diversity selectively impairs neuronal activation during short-term memory recall. Notably, decreases in neuronal activity were found in multiple brain regions at the 2 h interval but not at the 24 h interval, suggesting that different neural circuits may be recruited for short-term and long-term memory.
Comparison of neural activity density at 2 h and 24 h in wild-type and cPcdh-α1-12 mice c-Fos expression in six brain regions in wild-type and cPcdh-α1-12 mice. (a) CA3, (b) CA1, (c) BLA, (d) EP, (e) RSP, and (f) MEA. 2 h interval: cPcdh-α1-12 (n = 5, male 3, female 2), wild-type (n = 5, male 3, female 2). 24 h interval: cPcdh-α1-12 (n = 4, male 2, female 2), wild-type (n = 4, male 2, female 2). Data are presented as mean ± SEM. Statistical significance was determined using a mixed 2-way ANOVA, with genotype as a between-groups factor and interval as a within-groups factor, followed by Bonferroni’s multiple comparisons test for post-hoc analysis.
Discussion
cPcdh-α1-12 mice exhibit short-term memory deficits but retain normal long-term memory
In this study, we conducted a detailed analysis of how the diversity of cPcdh-α affects short-term and long-term memory. The results indicated that while cPcdh-α1-12 mice exhibited deficits in short-term memory, long-term memory was not significantly affected. Specifically, in the T-maze task and CPFE test, cPcdh-α1-12 mice performed significantly worse than wild-type mice in tasks requiring short-term memory, demonstrating difficulties in retaining information for short periods and distinguishing contexts. While there is some concern that genetic background differences may have influenced these phenotypes, cPcdh-α1-12 mice were generated on a C57BL/6 background15, and maintained as a separate colony from control C57BL/6 J mice in the same room under identical environmental conditions. These findings suggest that the molecular diversity of cPcdh α plays a crucial role in the formation and function of neural circuits.
Short-term memory deficits and neural activity patterns
Short-term memory deficits were further corroborated by analyses of neuronal activity using c-Fos staining. In conditions requiring short-term memory, cPcdh-α1-12 mice showed significantly reduced neuronal activity in brain regions such as the hippocampus, amygdala, and retrosplenial cortex. In contrast, in tests conducted 24 h later, no significant differences in neuronal activity were observed between cPcdh-α1-12 mice and wild-type mice, indicating that normal neural activity was maintained in long-term memory. This reduction in neuronal activity during short-term memory tasks aligns with the observed behavioral deficits and provides further evidence of direct impairments in cellular activity in cPcdh-α1-12 mice. The hippocampus, amygdala, and retrosplenial cortex each play distinct but interconnected roles in memory processing: the hippocampus is involved in encoding and retrieving episodic memory24,25,26,27,28,29, the amygdala contributes to emotional and associative memory30,31,32, and the retrosplenial cortex integrates spatial and contextual information33. These regions are interconnected through neural circuits, functioning in a coordinated manner to support memory retention26,34,35. These findings strongly support the idea that the reduced neuronal activity observed in cPcdh-α1-12 mice reflects not just localized dysfunction but a disruption in circuit-wide coordination. The short-term memory deficits are likely driven by direct impairments in cellular function.
Molecular mechanisms underlying short-term memory deficits in cPcdh-α1-12 mice
The observation that cPcdh-α1-12 mice exhibit impairments in short-term memory while retaining normal long-term memory suggests that short-term and long-term memories are not merely sequential stages of a unified process but instead rely on partially independent molecular pathways. This distinction between short-term and long-term memories is further supported by findings from multiple levels of analysis, as knockout mice lacking PKC-gamma, which regulates critical aspects of synaptic plasticity and memory, or brevican, a major component of perineuronal nets that stabilizes synaptic connections and modulates neural plasticity, also exhibit selective impairments in short-term memory36,37. Additionally, pharmacological studies have demonstrated that inhibition of specific hippocampal receptors selectively disrupts short-term memory while sparing long-term memory38, reinforcing the notion that distinct molecular mechanisms govern these processes. Together, these findings consistently support the functional separation of short-term and long-term memories across engram, synaptic, and pharmacological levels. In this study, neuronal activity in the hippocampal CA3, CA1, BLA, and EP of cPcdh-α1-12 mice significantly decreased during memory recall at a 2 h interval, suggesting that these regions play a key role in short-term memory retention. These results also support the idea that, while short-term and long-term memory share neural circuits, they depend on distinct molecular mechanisms.
Clustered protocadherins, including those in the cPcdh-α family, have been identified as cell adhesion molecules localized at synaptic junctions, where they contribute to synapse formation and stabilization3. Synaptic modifications that support short-term memory heavily depend on the regulation of cell adhesion molecules, which mediate synapse formation and structural remodeling in response to neuronal activity. Notably, adhesion molecules such as NCAM and FasII have been shown to be associated with synaptic plasticity and transient synaptic modifications and are specifically involved in processes related to short-term memory38,39,40,41,42.. A potential explanation for the short-term memory deficits in cPcdh-α1-12 mice is that cPcdhs, as cell adhesion molecules, play a key role in regulating synaptic remodeling. On the other hand, the molecular diversity of cPcdhs has been shown to contribute to self-avoidance during dendritic synapse formation43,44 and is also considered essential for the formation of specific neural circuits between different neurons45,46. These findings suggest that the reduced molecular diversity of cPcdh-α disrupts the specificity of cPcdh-dependent synaptic formation, potentially impairing the formation of specialized neural circuits required for short-term memory in cPcdh-α1-12 mice.
Role of cPcdh-α diversity in neural circuit formation
Previous research has shown that the molecular diversity of cPcdh contributes to the formation of neural circuits and the specificity of intercellular adhesion. This study newly demonstrates that this diversity is critical for the function of neural circuits, particularly in short-term memory formation. Because cPcdh-α1-12 mice lack cPcdh-α diversity, specific interactions within neural circuit connectivity may be limited, resulting in short-term memory impairments. This finding suggests that specific neural circuits are vital for short-term information retention and that the diversity of cPcdh-α is essential for their formation.
Additionally, given that cPcdhs contribute to neural circuit formation, their dysfunction has been implicated in neurodevelopmental and neuropsychiatric disorders47,48,49,50,51. Mutations in cPcdh genes have been linked to autism spectrum disorder52 and schizophrenia53,54,55, both of which involve cognitive and memory deficits. The findings of this study suggest that the loss of cPcdh-α diversity impairs synaptic plasticity and memory retention, potentially providing insights into how protocadherin dysfunction contributes to cognitive impairments in these disorders. Investigating the role of cPcdh-α diversity in synaptic plasticity across different brain regions may further elucidate its contributions to neurodevelopmental conditions.
Methods
Animal experiments
All the experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the Science Council of Japan and were approved by the Animal Experiment Committee of Osaka University. After the experiments, mice were anaesthetized with isoflurane to ensure minimal distress. Euthanasia was performed by cervical dislocation, in compliance with institutional animal care and use guidelines. All procedures adhered to the ARRIVE guidelines.
Animal model preparation
All experiments used C57BL/6 mice and cPcdh-α1-12 mice (BRC No. RBRC02798), which were generated and characterized according to the method described previous research56. All mice were maintained and bred in the laboratory of Prof. Takeshi Yagi at the University Osaka. They were from our own breeding stock and housed in groups of 5–8 mice per cage. The mice used in the experiments were 2 to 4 months old, including both male and female animals. The animals were housed at a temperature of 23 ± 1 °C with 50% ± 10% relative humidity under a 12 h light/dark cycle (lights on at 8:00 a.m., lights off at 8:00 p.m.). Prior to the experiments, all mice were handled for 5 min per day and transitioned to individual housing starting three days before the experiments to acclimate them to the experimental environment. Mice were transitioned to individual housing starting three days before the experiments to minimize the influence of social interactions with other mice on c-Fos expression and to reduce variability among individuals caused by such interactions.
Behavioral experiments
The open field test was conducted to evaluate spontaneous locomotor activity and anxiety-like behavior in mice. Testing was carried out in a white acrylic open field box (50 × 50 × 40 cm) cleaned with 70% ethanol between trials to minimize odor cues. Each mouse was placed in the center of the box and allowed to explore freely for 60 min. Mouse behavior was recorded by an overhead video camera at 30 frames per second, and the time spent in the central area and total distance traveled were used as assessment metrics. An increase in time spent in the center was used as an indicator of reduced anxiety, while the total distance traveled was used as an indicator of spontaneous locomotor activity. Mouse movements were analyzed using DeepLabCut, a deep-learning-based markerless tracking tool57. The nose tip, right ear, left ear, and tail base were tracked to calculate movement trajectories. The central area was defined as the inner 25 × 25 cm square, and the time spent in this area was calculated as a percentage of the total exploration time. The total distance traveled was computed by summing the displacement of the tracked points frame by frame. All analyses were conducted using MATLAB software.
The contextual fear conditioning test was conducted to evaluate fear memory in mice. Testing was carried out in a conditioning chamber (30 × 24 × 21 cm), equipped with a stainless-steel grid floor for administering electric shocks. The chamber was cleaned with 70% ethanol between trials to minimize odor cues. Additionally, the chamber was placed inside a soundproof box (51 × 51 × 34 cm) with dim lighting. In this between-subjects design, each mouse was placed in the chamber on the test day and allowed to explore freely for 4 min, followed by three 2 s electric shocks of 0.5 mA, administered at 1 min intervals. After 24 h or two weeks, each mouse was reintroduced to the same chamber for 5 min without electric shocks, and the percentage of freezing responses was recorded as an index of fear memory. Freezing was defined as the state in which each of the four tracked points remained within a 0.8 cm radius for a minimum of 2 s. The freezing percentage was calculated using MATLAB. Automated measurements were validated by comparison with manual visual scoring, confirming no significant differences between the two methods. Statistical analysis used an independent t-test for the open field test and mixed two-way ANOVA with genotype as a between-groups factor and interval as a within-groups factor, followed by Bonferroni’s multiple comparisons test for post-hoc analysis for the fear conditioning test.
The CPFE test was conducted to assess the formation of contextual memory. Testing was conducted in a conditioning chamber (30 × 24 × 21 cm), with two distinct contexts, context A and context B. In context A, a square tube (19.8 × 19.8 × 21 cm) covered with paper was used, while in context B, an acrylic cylindrical tube (22 cm diameter) was set up. The chamber was cleaned with 70% ethanol between trials to minimize odor cues. On the day before the test, each mouse was pre-exposed to context A for 5 min. Two or twenty-four h after pre-exposure, each mouse was reintroduced to the chamber, and a 0.5 mA electric shock (2 s) was administered 5 s after placement. After 1 min, each mouse was removed from the chamber. Twenty-four h after conditioning, each mouse was placed in context A for 5 min and allowed to move freely, followed by a 5 min placement in context B 1.5 h later. Freezing responses were recorded as an index of contextual memory. Statistical analysis used an independent t-test for the open field test and mixed two-way ANOVA with genotype as a between-groups factor and interval as a within-groups factor, followed by Bonferroni’s multiple comparisons test for post-hoc analysis for the CPFE test.
The T-maze test was conducted to assess short-term memory, following a previously described protocol18. T-maze was constructed from black plastic with arms measuring 30 cm wide, 100 cm long, 10 cm high, and with a passage width of 5 cm. White plastic cues (5 × 5 × 10 cm) were placed either at the choice point or 10 cm from the starting point. Training and testing began when mice were 2 months old. Each correct trial was rewarded with 50 μl of a 5% glucose solution, while in incorrect trials, the mice were reintroduced to the correct arm. Trials were terminated if a mouse failed to choose an arm within 4 min of placement in the maze. Each mouse performed 20 trials per day and consumed 1 mL of the 5% glucose solution per session. To maintain motivation for the reward, mice were housed under intermittent water restriction. The first session began two days after the start of water deprivation, and mice were trained for a maximum of five consecutive days before water deprivation was discontinued. The training duration varied among mice to ensure that their body weight was maintained at approximately 85% of their pre-deprivation weight while allowing for normal body growth throughout the intermittent water deprivation period. Adjustments in training duration were made as needed to balance effective task learning with maintaining the health and well-being of the animals. Statistical significance was determined using mixed two-way ANOVA, with genotype as a between-groups factor and task as a within-groups factor, followed by Bonferroni’s multiple comparisons test for post-hoc analysis.
Tissue preparation and acquisition of serial sections
Mice were anaesthetized with isoflurane, perfused transcardially first with 25 mm PBS and then with 4% paraformaldehyde (PFA) in PB (pH 7.3). The brains, removed from the skull, were stored in PFA at 4 °C overnight, then transferred to 25 mm PBS with 30% sucrose and kept at 4 °C overnight. Brains were embedded in a cryomold (Tissue-Tek) with the cortex facing upwards, using a mixture of OCT compound (Tissue-Tek) and 30% sucrose at a 2:1 ratio. The samples were frozen in isopentane cooled with liquid nitrogen and sectioned coronally at 50 µm thickness using a cryostat (CM3050S, Leica).
Immunohistochemistry
Sections were washed with 25 mM PBS, and a blocking solution (500 μl per well) was applied for 1 h at room temperature to prevent non-specific binding. Sections were then incubated overnight at 4 °C with a 1:1000 dilution of the primary antibody (rabbit anti-c-Fos, Cell Signaling Technology) in blocking solution. After washing with 25 mM PBS, sections were incubated with a 1:1000 dilution of the secondary antibody (AlexaFluor488, Invitrogen) in PBS with 0.1% Triton X-100 for 2 h at room temperature. Following the secondary antibody incubation, the plate was shielded from light with aluminum foil. Sections were then washed in 25 mM PBS, mounted on glass slides (MAS-01, MATSUNAMI), covered with antifade mounting medium (Immunoselect Antifading Mounting Medium, dianovaTM), and sealed with nail polish.
Cell counting
The localization and number of cells were quantified semi-automatically using MATLAB. c-Fos-positive cells were defined as bright spots with a radius of 3–4 pixels and a brightness above a threshold that matched visual counting in randomly selected images with an agreement of over 95% or more per mouse. Images were deformed to align 40–60 landmark points on each section to account for shifts due to section distortion or damage, thereby ensuring precise cell localization based on the Allen Mouse Brain Common Coordinate Framework (CCFv3)20,21.
Data analysis
GraphPad Prism was used for data analysis. All graphs present data as mean ± SEM, and statistical significance was set at *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Data availability
The experimental data and the simulation results that support the findings of this study are available in Figshare with the identifier https://doi.org/https://doi.org/10.6084/m9.figshare.28904420.
References
Bassett, D. S. & Sporns, O. Network neuroscience. Nat. Neurosci. 20, 353–364 (2017).
Sano, K. et al. Protocadherins: A large family of cadherin-related molecules in central nervous system. EMBO J. 12, 2249–2256 (1993).
Kohmura, N. et al. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron 20, 1137–1151 (1998).
Wu, Q. & Maniatis, T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 97, 779–790 (1999).
Yagi, T. & Takeichi, M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 14, 1169–1180 (2000).
Brasch, J. et al. Visualization of clustered protocadherin neuronal self-recognition complexes. Nature 569, 280 (2019).
Goodman, K. M. et al. Protocadherin cis-dimer architecture and recognition unit diversity. Proc. Natl. Acad. Sci. 114, E9829–E9837 (2017).
Goodman, K. M. et al. γ-Protocadherin structural diversity and functional implications. Elife 5, e20930 (2016).
Goodman, K. M. et al. Structural basis of diverse homophilic recognition by clustered α- and β-Protocadherins. Neuron 90, 709–723 (2016).
Tasic, B. et al. Promoter choice determines splice site selection in protocadherin α and γ Pre-mRNA splicing. Mol. Cell 10, 21–33 (2002).
Wang, X., Su, H. & Bradley, A. Molecular mechanisms governing Pcdh-gamma gene expression: evidence for a multiple promoter and cis-alternative splicing model. Genes Dev 16, 1890–1905 (2002).
Kaneko, R. et al. Allelic gene regulation of Pcdh-alpha and Pcdh-gamma clusters involving both monoallelic and biallelic expression in single Purkinje cells. J. Biol. Chem. 281, 30551–30560 (2006).
Fukuda, E. et al. Down-regulation of protocadherin-alpha A isoforms in mice changes contextual fear conditioning and spatial working memory. Eur. J. Neurosci. 28, 1362–1376 (2008).
Asai, H. et al. Pcdhβ deficiency affects hippocampal CA1 ensemble activity and contextual fear discrimination. Mol. Brain 13, 7 (2020).
Noguchi, Y. et al. Total expression and dual gene-regulatory mechanisms maintained in deletions and duplications of the Pcdha cluster. J. Biol. Chem. 284, 32002–32014 (2009).
Esumi, S. et al. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat. Genet. 37, 171–176 (2005).
Yoshitake, K. et al. Visual map shifts based on whisker-guided cues in the young mouse visual cortex. Cell Rep. 5, 1365–1374 (2013).
Yamagishi, T. et al. Molecular diversity of clustered protocadherin-α required for sensory integration and short-term memory in mice. Sci. Rep. 8, 9616 (2018).
Rudy, J. W., Huff, N. C. & Matus-Amat, P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci. Biobehav. Rev. 28, 675–685 (2004).
Chen, S. et al. High-throughput strategy for profiling sequential section with multiplex staining of mouse brain. Front. Neuroanat. 15, 771229 (2021).
Wang, Q. et al. The allen mouse brain common coordinate framework: A 3D reference atlas. Cell 181, 936-953.e20 (2020).
Rudy, J. W. Context representations, context functions, and the parahippocampal–hippocampal system. Learn. Mem. 16, 573–585 (2009).
Schiffino, F. L., Murawski, N. J., Rosen, J. B. & Stanton, M. E. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol. Learn. Mem. 95, 190–198 (2011).
Ramirez, S. et al. Creating a false memory in the hippocampus. Science 341, 387–391 (2013).
Ramirez, S. et al. Activating positive memory engrams suppresses depression-like behaviour. Nature 522, 335–339 (2015).
Ryan, T. J., Roy, D. S., Pignatelli, M., Arons, A. & Tonegawa, S. Memory Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015).
Ohkawa, N. et al. Artificial association of pre-stored information to generate a qualitatively new memory. Cell Rep. 11, 261–269 (2015).
Redondo, R. L. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Reijmers, L. G., Perkins, B. L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Gore, F. et al. Neural representations of unconditioned stimuli in basolateral amygdala mediate innate and learned responses. Cell 162, 134–145 (2015).
Zhou, Y. et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 12, 1438–1443 (2009).
Cowansage, K. K. et al. Direct reactivation of a coherent neocortical memory of context. Neuron 84, 432–441 (2014).
Tonegawa, S., Morrissey, M. D. & Kitamura, T. The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci. 19, 485–498 (2018).
Sun, W. et al. Spatial transcriptomics reveal neuron–astrocyte synergy in long-term memory. Nature 627, 374–381 (2024).
Gomis-González, M. et al. Protein Kinase C-Gamma knockout mice show impaired hippocampal short-term memory while preserved long-term memory. Mol. Neurobiol. 58, 617–630 (2021).
Favuzzi, E. et al. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 95, 639-655.e10 (2017).
Izquierdo, I. et al. Mechanisms for memory types differ. Nature 393, 635–636 (1998).
Martin, K. C. & Kandel, E. R. Cell adhesion molecules, CREB, and the formation of new synaptic connections. Neuron 17, 567–570 (1996).
Sutton, M. A., Masters, S. E., Bagnall, M. W. & Carew, T. J. Molecular mechanisms underlying a unique intermediate phase of memory in aplysia. Neuron 31, 143–154 (2001).
Trannoy, S., Redt-Clouet, C., Dura, J.-M. & Preat, T. Parallel processing of appetitive short- and long-term memories in drosophila. Curr. Biol. 21, 1647–1653 (2011).
Izquierdo, L. A. et al. Molecular pharmacological dissection of short- and long-term memory. Cell Mol. Neurobiol. 22, 269–287 (2002).
Rubinstein, R. et al. Molecular logic of neuronal self-recognition through protocadherin domain interactions. Cell 163, 629–642 (2015).
Lefebvre, J. L., Kostadinov, D., Chen, W. V., Maniatis, T. & Sanes, J. R. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature 488, 517–521 (2012).
Yagi, T. Genetic basis of neuronal individuality in the mammalian brain. J. Neurogenet. 27, 97–105 (2013).
Tarusawa, E. et al. Establishment of high reciprocal connectivity between clonal cortical neurons is regulated by the Dnmt3b DNA methyltransferase and clustered protocadherins. BMC Biol. 14, 103 (2016).
Iacono, G. et al. Increased H3K9 methylation and impaired expression of Protocadherins are associated with the cognitive dysfunctions of the Kleefstra syndrome. Nucleic Acids Res. 46, 4950–4965 (2018).
Naskar, T. et al. Ancestral variations of the PCDHG Gene Cluster Predispose to Dyslexia in a Multiplex family. EBioMedicine 28, 168–179 (2018).
Li, Y. et al. Synaptic adhesion molecule Pcdh-γC5 mediates synaptic dysfunction in Alzheimer’s Disease. J. Neurosci. 37, 9259–9268 (2017).
El Hajj, N., Dittrich, M. & Haaf, T. Epigenetic dysregulation of protocadherins in human disease. Semin. Cell Dev. Biol. 69, 172–182 (2017).
El Hajj, N. et al. Epigenetic dysregulation in the developing Down syndrome cortex. Epigenetics 11, 563–578 (2016).
Anitha, A. et al. Protocadherin α (PCDHA) as a novel susceptibility gene for autism. J. Psychiatry Neurosci. 38, 192–198 (2013).
Shao, Z. et al. Dysregulated protocadherin-pathway activity as an intrinsic defect in induced pluripotent stem cell-derived cortical interneurons from subjects with schizophrenia. Nat. Neurosci. 22, 229–242 (2019).
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Pedrosa, E. et al. Analysis of protocadherin alpha gene enhancer polymorphism in bipolar disorder and schizophrenia. Schizophr. Res. 102, 210–219 (2008).
Noguchi, Y. et al. Total Expression and Dual Gene-regulatory Mechanisms Maintained in Deletions and Duplications of the Pcdha Cluster*. J. Biol. Chem. 284, 32002–32014 (2009).
Mathis, A. et al. DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Acknowledgements
We thank the members of the KOKORO Biology Laboratory for assistance and discussions.
Funding
JSPS KAKENHI,JP22 J20803,a MEXT Grant-in-Aid for Scientific Research (A) from JSPS,No. 18H04016,a Grant-in-Aid for Scientific Research on Innovative Areas “Integrated analysis and regulation of cellular diversity”,No. 20H05035,cientific Research on Transformative Research Areas (A) Adaptive Circuit Census,No. 22H05498.
Author information
Authors and Affiliations
Contributions
T.O. and T.Y. planned the experiments and wrote the manuscript. T.O. and H.Y. performed the experiments. T.Y. supervised the experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Osuka, T., Yamamoto, H. & Yagi, T. Diversity of clustered protocadherin-α genes in neuronal identity and its role in short-term specific associative memory formation. Sci Rep 15, 19334 (2025). https://doi.org/10.1038/s41598-025-02546-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02546-2