Abstract
Tyrosine kinase inhibitors (TKIs) and trastuzumab deruxtecan (T-DXd) have shown efficacy in HER2-positive patients with brain metastases (BMs). This paper analyzed the efficacy and safety of T-DXd in HER2-positive breast cancer patients with BMs who progressed after pyrotinib treatment. We conducted a single-center, retrospective cohort study. HER2-positive patients with BMs who received T-DXd treatment after disease progression following pyrotinib therapy were identified from electronic medical records. The primary endpoint of this study was central nervous system progression-free survival (CNS-PFS). From April 2021 to July 2023, 15 patients were included in the study. The median CNS-PFS was 7.4 months [95% confidence interval (CI) 6.1–8.8 months], the median PFS for patients with extracranial/total lesions was 6.4 months (95% CI 4.4–8.3 months), and the median OS was 9.8 months (95% CI 5.9–13.8 months). The ORRs for intracranial, extracranial, and overall lesions were 33.3%, 71.4%, and 73.3%, respectively. Adverse events of grade 3 or higher with an incidence rate ≥ 5% included leukopenia (20.0%), neutropenia (13.3%), thrombocytopenia (6.7%), and nausea (6.7%). Adverse events of specific interest, interstitial lung disease or pneumonitis, occurred in 2 patents (13.3%), and both were grade 1. The preliminary data in this study suggest that in clinical practice in China, T-DXd is an optional treatment for patients with active/stable BMs who have progressed on pyrotinib. However, further studies are needed to determine its efficacy and the best treatment sequence for these patients.
Similar content being viewed by others
Introduction
Human epidermal growth factor receptor 2 (HER2) is overexpressed in approximately 20–30% of breast cancers, and its increased expression indicates aggressive tumor biology and poor prognosis. The development of anti-HER2 targeted drugs has significantly improved the prognosis of HER2-positive breast cancer patients. Up to 50% of patients with advanced HER2-positive breast cancer develop brain metastases (BMs), which significantly shorten the survival time1,2. Local therapy, such as surgery and radiotherapy, is an important strategy for patients with BMs, but systemic anti-HER2 regimens also show promising therapeutic potential3.
Anti-HER2 drugs are divided into three categories: monoclonal antibodies, tyrosine kinase inhibitors (TKIs), and antibody‒drug conjugates (ADCs). TKIs have the advantages of a small molecular size and a strong ability to penetrate the blood‒brain barrier. To date, more evidence of the effectiveness of TKIs in the treatment of BMs has been reported. Phase 2 LANDSCAPE and TBCRC-022 demonstrated the efficacy of lapatinib and neratinib combined with capecitabine, respectively, in treating patients with BMs4,5. The first phase 3 randomized clinical trial, HER2CLIMB, confirmed the efficacy of tucatinib in treating active/stable BMs. Therefore, tucatinib combined with trastuzumab and capecitabine has become the preferred treatment for HER2-positive breast cancer patients with active BMs6,7. Tucatinib has not yet been approved for use in China. Pyrotinib, which was independently developed in China, has shown clinical effectiveness in treating active/stable BMs and is widely used in clinical practice in China8.
Trastuzumab deruxtecan (T-DXd), a new-generation anti-HER2 ADC drug, has shown promising anticancer efficacy9,10,11 and has been approved for patients with unresectable or metastatic HER2-positive breast cancer who have received one or more prior anti-HER2-based regimens. The DESTINY-Breast01-03 clinical studies included a small number of patients with stable BMs and showed a good objective response rate and duration of response10,11,12,13. Currently, retrospective studies and small-scale clinical studies are exploring the efficacy of T-DXd in HER2-positive patients with active BMs14,15. Five HER2-positive patients with BMs treated with T-DXd after tucatinib progression showed satisfactory therapeutic effects16. In Chinese clinical practice, the efficacy and safety of T-DXd in patients with BMs after failure of pyrotinib-based treatment are unknown. This paper analyzed the efficacy and safety of T-DXd in HER2-positive breast cancer patients with BMs who progressed after pyrotinib-based treatment.
Materials and methods
This was a single-center, retrospective cohort study. Metastatic breast cancer (MBC) patients who received T-DXd treatment were identified from electronic medical records. All patients were then screened for eligibility based on the following criteria: (1) age ≥ 18 years; (2) pathologically diagnosed with HER2-positive breast cancer, defined as positive by immunohistochemistry (IHC) staining (+ + +) or fluorescence in situ hybridization (FISH) positivity; (3) radiologically confirmed BMs; (4) progression of BMs during prior pyrotinib-based therapy, defined as the development of new BMs during pyrotinib-based therapy or progression of preexisting BMs; (5) performance-status score on the Eastern Cooperative Oncology Group (ECOG) scale ≤ 3; (6) at least one efficacy assessment after T-DXd treatment; (7) adequate bone marrow and organ function; and (8) complete medical records. The study was performed in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the Fifth Medical Center of Chinese People’s Liberation Army General Hospital, and all patients provided written informed consent. Study treatment.
Patients received T-DXd at the recommended dose of 5.4 mg/kg by intravenous infusion every 21 days. Dose reduction, interruption, delay, or treatment discontinuation were considered in the case of adverse reactions. The adverse events were promptly managed according to the guidelines. Treatment lasted until disease progression or unacceptable toxicity occurred or for any other reason.
Outcomes
The primary endpoint of this study was central nervous system progression-free survival (CNS-PFS), which indicates the time interval from treatment to disease progression in intracranial lesions or to death from any cause. The secondary endpoints included the objective response rate (ORR) for intracranial, extracranial and overall lesions; the clinical benefit rate (CBR) for intracranial, extracranial and overall lesions; overall survival (OS); and safety. The ORR is defined as the percentage of patients who achieve the best overall response (CR) and the percentage of patients who achieve a partial response (PR) based on investigator assessment. The CBR is defined as the sum of the CR rate, PR rate, and stable disease (SD) rate for more than 6 months. OS was defined as the time interval from treatment to death from any cause. Tumor imaging assessment including brain MRIs was performed every two cycles or when disease progression was suspected clinically based on symptoms and signs until disease progression or death. Efficacy in intracranial lesions was evaluated based on the Response Assessment in Neuro-Oncology-Brain Metastases (RANO-BM) criteria, while that of extracranial/overall lesions was based on the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, both performed by experienced radiologists. Safety assessments included vital sign and clinical laboratory evaluations, and adverse events were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. There is no adjudication committee to evaluate the status of interstitial pneumonia of patients. When interstitial lung disease or pneumonia was suspected, early diagnosis was made through high-resolution CT, laboratory tests, and consultation with a radiologist and a respiratory specialist. In moderate to severe cases, T-DXd was discontinued, and steroids were administered.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software. Qualitative data were presented as sample rates or frequencies, and intergroup differences were compared by using chi-square or Fisher’s exact tests. Kaplan‒Meier (K‒M) curves were used to analyze and calculate PFS and OS, and log-rank tests were used for comparison.
Results
Patient characteristics
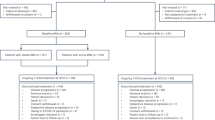
From April 2021 to July 2023, 48 patients with metastatic cancer were selected for treatment with T-DXd. Among them, 20 patients had BMs, and 15 patients previously received pyrotinib therapy and experienced disease progression (Fig. 1). The median age of the patients was 51 years (32–66), and 5 patients were positive for hormone receptors. A total of 14 patients had extracranial metastases and intracranial metastases. The median number of metastatic sites was 5 (1–6). The median number of prior lines of therapy before T-DXd for metastatic disease was 6 (range, 2–11). All patients in the study previously received trastuzumab and pyrotinib, 13 patients (86.7%) previously received pertuzumab, and 9 patients (60%) previously received T-DM1. After being diagnosed with BMs, 13 patients (86.7%) received trastuzumab plus pertuzumab, 8 patients (53.3%) received pyrotinib, and 5 patients (33.3%) received T-DM1. The median number of anti-HER2 therapy lines before T-DXd was 2 (0–5) after the diagnosis of BMs. New BMs developed in 8 patients during pyrotinib-based therapy,while preexisting BMs progressed in 7 patinets. Thirteen patients (86.7%) previously received local therapy for BMs, including whole-brain radiotherapy, stereotactic radiotherapy, surgery, or combinations, while 2 patients did not receive local therapy for BMs. The median time between the initiation of T-DXd treatment and the last local therapy for BMs was 8.1 months (1.1–36.8 months). Nine patients (60.0%) had active BMs (new BMs or progressive BMs), 6 patients (40.0%) had stable BMs (received prior local treatment and remained stable), and 10 (66.7%) of the patients had multiple BMs. Six patients (40.0%) experienced symptoms such as dizziness, headache, nausea, or others related to BMs (Table 1).
Efficacy
As of September 2023, the median follow-up time was 8.6 months (2.1–23.3 months). Five patients continued T-DXd treatment, 10 patients discontinued treatment due to disease progression, 6 of whom experienced simultaneous intracranial and extracranial progression, and 4 of whom experienced only extracranial progression. Eight patients (57.1%) died due to progression of breast cancer. The median CNS-PFS was 7.4 months [95% confidence interval (CI), 6.1–8.8 months] (Fig. 2A), the median PFS for patients with extracranial and all lesions were both 6.4 months (95% CI 4.4–8.3 months) (Fig. 2B), and the median OS was 9.8 months (95% CI 5.9–13.8 months) (Fig. 2C).
Of the 15 patients included in the study, 9 had evaluable intracranial lesions, and 14 had evaluable extracranial lesions. The ORRs for intracranial, extracranial, and overall lesions were 33.3%(3/9), 71.4%(10/14), and 73.3%(11/15), respectively, while the CBRs for intracranial, extracranial, and overall lesions were 53.3%, 66.7%, and 73.3%, respectively. The median time to response was 1.5 months for intracranial lesions, 1.4 months for extracranial lesions, and 1.6 months for overall lesions, while the median duration of response was 4.9 months for intracranial lesions, 5.7 months for extracranial lesions, and 6.0 months for overall lesions (Fig. 3).
Tumor response. (A) Waterfall plot of optimal changes from baseline in intracranial and extracranial target lesions in 15 patients. *, 6 patients had no intracranial target lesions; #, 1 patient had no extracranial target lesion. (B) Swimmer plot of PFS for 15 patients. SD, stable disease; PR, partial response; PD, progressive disease; CNS-PFS, central nervous system progression-free survival.
Nine patients had active BMs, of whom 8 had target lesions in the brain, and 7 progressed after previous local treatment. Three patients achieved PR in their intracranial lesions, all of whom had progressed after previous local BM treatment (Fig. 4). BMs-related symptoms, including dizziness and nausea, were observed in 6 patients. Among these, BMs-related symptoms began at the initiation of T-DXd in 3 cases, were already present in 3 cases prior to T-DXd, and corticosteroids were administered to 3 patients at the start of T-DXd to alleviate BMs-related symptoms. Among them, 4 patients experienced symptom alleviation following T-DXd, with all events being reduced from CTCAE version 5.0 Grade 2 to Grade 1. Six patients had stable BMs, of whom 1 had a target lesion in the brain and none presented with symptoms related to BMs. The intracranial ORR, CBR, CNS-PFS, and OS in patients with active BMs were 33.3%, 44.4%, 7.4 months, and 8.3 months, respectively, while those in patients with stable BMs were 0%, 71.4%, 7.0 months, and 20.3 months, respectively (Fig. 5).
Safety
Treatment-emergent adverse events in 5% or more of the patients are shown in Table 2, most of which were grade 1/2 and thus controllable. Adverse events of grade 3 or higher with an incidence rate ≥ 5% included leukopenia (20.0%), neutropenia (13.3%), thrombocytopenia (6.7%), and nausea (6.7%). Adverse events of specific interest, interstitial lung disease or pneumonitis, occurred in 2 patents (13.3%), and both were grade 1. Two patients had a dose reduction of T-DXd due to nausea and vomiting of Grade 2.
Discussion
In recent years, the incidence of BMs has increased due to prolonged survival caused by novel systemic therapy regimens and improvements in screening and diagnosis for asymptomatic BMs. Patients with BMs usually have a poor prognosis and a poor quality of life, which is a significant challenge in clinical practice. In addition to local therapy for BMs, systemic anti-HER2 treatment also benefits patients with HER2-positive breast cancer. International guidelines recommend that tucatinib plus trastuzumab and capecitabine be considered the preferred therapy for patients with active BMs, while patients with stable BMs should receive T-DXd first17 Tucatinib is not yet approved for use in China, and T-DXd is not listed on the national reimbursement drug list, thus limiting its availability. The PERMEATE study showed that pyrotinib combined with capecitabine had an ORR of 74.6% and 42.1% for untreated patients and previously locally treated patients with active BMs, respectively. The PFS times were 11.3 and 5.6 months, respectively. Hence, pyrotinib is widely used in clinical practice in China8. This study is the first to analyze the efficacy and safety of T-DXd in HER2-positive breast cancer BM patients who were previously treated with pyrotinib and who progressed. T-DXd showed good intracranial and extracranial antitumor activity as well as manageable adverse reactions.
In this study, six patients with stable BMs after failure of prior pyrotinib-based therapy were treated with T-DXd. The extracranial ORR was 100%, the CBR was 100%, the median PFS was 7.0 months, and the median OS was 20.3 months. One patient’s intracranial lesions were assessable, with a response of SD; the intracranial CBR was 66.7%, and the median CNS-PFS was 7.0 months. The efficacy was inferior to the data reported in the DESTINY-Breast01-03 studies, possibly because the patients enrolled in this study had a greater tumor burden and greater refractoriness. All patients had visceral metastases and failed prior anti-HER2 therapies, including trastuzumab, T-DM1, and pyrotinib, with a median of 6 prior systemic treatment lines. In the DESTINY-Breast01-03 studies, the proportion of patients previously treated with TKIs was only 11.5%10,12,13,18. Retrospective analysis of data from the KAMILA study showed that PFS and OS were 5.5 and 18.9 months, respectively, and the intracranial CBR was 42.9% in patients with stable BMS treated with T-DM1 with fewer prior treatment lines, visceral metastases, and lapatinib treatment than that of this study19. This suggested that T-DXd could be used as a treatment regimen for patients with stable BMs after failure of pyrotinib-based therapy.
Active BMs, especially in patients who have failed after previous local treatment, have always been a clinical challenge. This study demonstrated the efficacy of T-DXd in 9 patients with active BMs after failure of pyrotinib-based therapy. The extracranial ORR was 44.4%, the median PFS was 6.4 months, the median OS was 8.3 months, 8 patients with measurable intracranial lesions were evaluated, the intracranial ORR was 33.3%, the CBR was 44.4%, the median intracranial PFS was 7.4 months, and 4 of the 6 patients with baseline BM symptoms experienced symptom relief after T-DXd treatment. The efficacy of T-DXd in this study was inferior to that reported in studies by TUXEDO and DEBBRAH, possibly due to the greater tumor burden and refractoriness of the patients enrolled in this study, limited palliative care after T-DXd progression, and previous pyrotinib-based failure14,15. The median time from the last local brain treatment to enrollment in this study was 8.1 months, which was significantly shorter than the 13 months reported in TUXEDO, suggesting that the patients in this study had faster disease progression and greater malignancy. Isabelle Desmoulins reported that 5 patients with active BMs who received T-DXd after progression of tucatinib had an intracranial ORR of 100%. The possible reason for the different results observed in this study is that pyrotinib and tucatinib have different mechanisms of action. Pyrotinib is an irreversible inhibitor of HER1, HER2, and HER4, while tucatinib is a highly selective inhibitor of HER216.
To date, DESTINY-Breast12 is, to our knowledge, the largest prospective study to report on intracranial activity of T-DXd in patients with HER2 + MBC and baseline BMs. In patients with baseline BMs, 12-month CNS-PFS rate was 58.9% (95% CI 51.9–65.3), with 57.8% (95% CI 48.2–66.1) and 60.1% (95% CI 49.2–69.4) in patients with stable and active BMs, respectively. The confirmed CNS-ORR for these patients was 71.7% (95% CI 64.2–79.3) and 79.2% (95% CI 70.2–88.3) and 62.3% (95% CI 50.1–74.5) in patients with stable and active BMs, respectively20. Compared to DESTINY-Breast12, the efficacy of T-DXd in our study was lower. Several factors may account for the differing outcomes, including the later lines of T-DXd treatment (with a median prior 1 line in DESTINY-Breast12 vs. 6 in our study), the prior use of TKIs (6.5% in DESTINY-Breast12 vs. 100% in our study), a greater tumor burden, and more rapid disease progression.
The adverse reaction profile of T-DXd patients was consistent with that in previous reports, and the incidence of grade 3 or higher neutropenia was similar to that in previous reports. The use of prophylactic triple antiemetics resulted in no grade 3 nausea/vomiting, and the incidence of ILD/pneumonia was relatively low, with no grade 2–5 events.
This study has several limitations. This was a retrospective and single-center study. T-DXd was only approved in China in 2023 and has not yet been included in the national reimbursement list during the period of our study, thus limiting its availability. Furthermore, patients with leptomeningeal metastasis were excluded. As a result, the sample size was relatively small, patient selection bias and significant differences in patients’ prior treatments existed, and the follow-up time was short. However, this study is the first to provide preliminary data on the efficacy and safety of T-DXd in treating patients with active/stable BMs who have progressed on pyrotinib, offering insights into the inevitable challenges faced in clinical practice. T-DXd has now become a standard second-line treatment around the world including China. However, pyrotinib-based treatment is currently the standard first-line or second-line therapy in China. Therefore, evaluating the efficacy and safety of T-DXd in patients with BMs following pyrotinib progression continues to hold important clinical significance.
Conclusions
The preliminary data in this study suggest that in clinical practice in China, T-DXd is an optional treatment for patients with active/stable BMs who have progressed on pyrotinib. However, further studies are needed to determine its efficacy and the best treatment sequence for these patients.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Choong, G. M., Cullen, G. D. & O’Sullivan, C. C. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA Cancer J. Clin. 70(5), 355–374 (2020).
Müller, V. et al. Epidemiology, clinical outcomes, and unmet needs of patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases: A systematic literature review. Cancer Treat Rev. 115, 102527 (2023).
Gennari, A. et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 32(12), 1475–1495 (2021).
Bachelot, T. et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 14(1), 64–71 (2013).
Freedman, R. A. et al. TBCRC 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 37(13), 1081–1089 (2019).
Lin, N. U. et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J. Clin. Oncol. 38(23), 2610–2619 (2020).
Lin, N. U. et al. Tucatinib vs Placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: Updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. 9(2), 197–205 (2023).
Yan, M. et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): A multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 23(3), 353–361 (2022).
Modi, S. et al. Trastuzumab Deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382(7), 610–621 (2020).
André, F. et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 401(10390), 1773–1785 (2023).
Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401(10371), 105–117 (2023).
Jerusalem, G. et al. Trastuzumab Deruxtecan in HER2-positive metastatic breast cancer patients with brain metastases: A DESTINY-Breast01 subgroup analysis. Cancer Discov. 12(12), 2754–2762 (2022).
Hurvitz, S. A. et al. 377O A pooled analysis of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (mBC) with brain metastases (BMs) from DESTINY-Breast (DB) -01, -02, and -03. Ann. Oncol. 34, S335–S336. https://doi.org/10.1016/j.annonc.2023.09.554 (2023).
Bartsch, R. et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: A single-arm, phase 2 trial. Nat. Med. 28(9), 1840–1847 (2022).
Pérez-García, J. M. et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro Oncol. 25(1), 157–166 (2023).
Desmoulins, I., Bellio, H., Méjean, N., Truntzer, C. & Ladoire, S. Intracranial response of brain metastases in patients with HER2-amplified breast cancer treated with trastuzumab-deruxtecan after failure of tucatinib-based therapy. Eur. J. Cancer 187, 161–163 (2023).
Im, S. A. et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, staging and treatment of patients with metastatic breast cancer. ESMO Open 8(3), 101541 (2023).
Jacobson, A. Trastuzumab Deruxtecan improves progression-free survival and intracranial response in patients with HER2-positive metastatic breast cancer and brain metastases. Oncologist. 27(Suppl 1), S3–S4 (2022).
Montemurro, F. et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial(☆). Ann. Oncol. 31(10), 1350–1358 (2020).
Harbeck, N. et al. Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: A phase 3b/4 trial [published correction appears in Nat Med. 30(12):3780 (2024).
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception/design: Zefei Jiang, Tao Wang. Provision of study material or patients: Jinmei Zhou, Jinyi Xiao, Xuexue Wu, Xiaobo Wang, Li Bian, Shaohua Zhang. Data analysis and interpretation: Jinmei Zhou, Jinyi Xiao, and Tao Wang. Manuscript writing: Jinmei Zhou, Jinyi Xiao, and Xuexue Wu. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This single-center, retrospective cohort study has been approved by the Ethics Committee of the Fifth Medical Center of Chinese People’s Liberation Army General Hospital (approval number: 2021-6-11). All patients provided written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, J., Xiao, J., Wu, X. et al. Efficacy and safety of trastuzumab deruxtecan in HER2-positive breast cancer patients with brain metastases after failure of pyrotinib-based therapy. Sci Rep 15, 17731 (2025). https://doi.org/10.1038/s41598-025-02550-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02550-6