Abstract
Polysialic acid (polySia), a glycoepitope critical for neural development and plasticity, remains difficult to quantify owing to its structural complexity. Here, we present a highly sensitive sandwich enzyme-linked immunosorbent assay (ELISA) utilizing novel probes to measure polySia expression. Using this method, we quantified polySia levels in mouse brain samples across various developmental and aging stages. Notable age-related changes were observed, particularly in neuroplastic regions such as the hippocampus and olfactory bulb, where polySia levels increased at 12 months, potentially reflecting resilience mechanisms against brain aging. Elevated polySia levels in blood samples were also detected in both a schizophrenia mouse model and human patients, with a notable male preponderance. In contrast, no significant changes were observed in patients with chronic inflammatory demyelinating polyneuropathy. These findings, enabled by the novel probes, highlight a potential role for polySia in brain aging and neuropsychiatric disorders, offering new insights into developmental and disease mechanisms and supporting its utility as a diagnostic biomarker for brain impairments.
Similar content being viewed by others
Introduction
Glycans are important biomolecules that play pivotal roles in numerous biological processes and are implicated in a wide range of physiological and pathological conditions1. All cells are coated with a glycan layer known as the glycocalyx, and alterations in its composition often distinguish normal cells from abnormal ones, such as cancer cells. Consequently, glycan-specific antibodies or lectins serve as valuable diagnostic tools for various diseases, for example, carbohydrate antigen 19 − 9 (CA19-9)2. This marker is relevant not only for identifying cancer tissues but also for detecting biomarkers in serum. Despite their biological importance and diagnostic utility, precise structural and functional analyses of glycans remain a significant challenge owing to their inherent complexity. Techniques such as liquid chromatography-mass spectrometry (LC-MS)-assisted glycoproteomics and time-of-flight-MS (TOF-MS)-assisted glycomics have recently advanced significantly3. However, these methods often face challenges in distinguishing glycan components, configurations, and linkage variations due to their identical mass spectrometric signatures. As a result, the confirmation of novel glycan structures still frequently relies on chemical characterization, immunochemical methods, and antibody-supported immunodetection. Additional complexity arises from the intricacies of glycan biosynthesis, which involves numerous glycan-related genes with overlapping substrate specificities1. Unlike nucleic acids or proteins, glycan structures cannot be directly predicted from genomic information alone. Therefore, direct and detailed structural analyses are essential for elucidating the roles of glycans in biological systems and disease pathogenesis. Recent methodological advances, along with improved instrumentation4,5, have significantly enhanced our understanding of aging and disease mechanisms. For example, sialic acid (Sia)-containing glycoproteins have been shown to change during aging6,7,8, whereas Sia levels are often elevated in cancer cells, likely as a means to evade innate immune surveillance mediated by Siglecs (sialic acid-binding immunoglobulin-like lectins)9. Despite the progress in instrument-assisted glycomics, certain glycans remain difficult to detect or quantify. Among them is polysialic acid (polySia), a highly functional glycan that resists conventional LC-MS or TOF-MS analysis10. Even monosialic acids (monoSia), which are known to be closely associated with aging, pose analytical challenges due to their extensive diversity, an estimated 50–80 monoSia species11. Crucial information can sometimes be overlooked during MS analysis, because the labile nature of glycans often compromises their integrity under standard analytical conditions.
PolySia is a linear polymer of Sia with a degree of polymerization (DP) ranging from 8 to 400. In vertebrates, the predominant form of polySia is α2,8-linked polyNeu5 Ac attached to the neural cell adhesion molecule (NCAM), referred to as polySia/PSA-NCAM which is primarily expressed in the brain10,12 PolySia-NCAM expression is developmentally regulated: during embryonic stages, nearly all NCAMs are modified with polySia, whereas in adulthood, polySia expression becomes restricted to specific brain regions, such as the hippocampus (HIP), olfactory bulb (OB), and suprachiasmatic nucleus (SCN). However, quantifying polySia in region-specific areas and tracking its changes during aging remains difficult due to the lack of reliable and sensitive analytical techniques.
PolySia serves two principal biological functions. First, it exhibits anti-adhesive properties by inhibiting homophilic NCAM–NCAM interactions and heterophilic interactions between NCAM and other cell adhesion molecules (CAMs)10,12,13. This function is attributed to polySia’s strong hydration capacity, which increases intercellular spacing and generates repulsive fields in the surrounding environment13. Second, polySia acts as a molecular reservoir by binding physiologically active molecules, including neurotrophins (e.g., brain-derived neurotrophic factor [BDNF]), growth factors (e.g., fibroblast growth factor 2 [FGF2]), and neurotransmitters (e.g., dopamine), thereby regulating their availability to their respective receptors10,11,12,13,14,15,16,17,18,19. These repulsive and attractive fields created by polySia collectively modulate a wide array of brain functions. Impairments in polySia structure have been implicated in various brain diseases and cancers20,21,22. Its expression is influenced by both genetic23,24,25 and environmental factors26,27, and is known to undergo dynamic regulation during neural activity. Therefore, it is imperative develop precise and robust methodologies for quantifying polySia in its native state.
Several analytical methods have been developed to assess the quantity and quality of polySia structures28,29,30, each with specific advantages and limitations. Western blotting (WB) can be used for polySia detection; however, the characteristic smear staining pattern leads to imprecise and low-sensitivity quantification. Moreover, highly glycosylated molecules such as polySia-containing glycoconjugates or mucins are challenging to immobilize uniformly on membranes31. Enzyme-linked immunosorbent assay (ELISA) offers another potential method, but it faces similar limitations, the difficulty in immobilizing highly glycosylated proteins compromises quantification accuracy. Furthermore, while existing evidence suggests that alterations in glycan epitopes are associated with aging and various diseases, precise measurement of these epitopes, particularly in the brain, remains insufficiently explored.
To address these challenges, we developed new probes and established a sensitive, quantitative sandwich ELISA for the detection and quantification of polySia, a unique glycoepitope in the brain. Using this probe-supported method, we successfully quantified the regional distribution of polySia in the brain, particularly in the context of aging. Furthermore, we applied the assay to various biological samples and uncovered novel insights into polySia.
Results
Development of polySia-specific probes
PolySia detection is typically performed using chemical methods29,30 or anti-polySia antibody-based approaches in combination with endo-N-acylneuraminidase (Endo-N), an enzyme that specifically cleaves oligo/polySia structures with DP > 510. These methods are often supplemented with α2,3/α2,6-sialidase or α2,3/α2,6/α2,8-sialidase to improve specificity, as anti-glycan antibodies can sometimes exhibit nonspecific binding. However, enzymatic treatments require acidic conditions, which can compromise polySia stability, and the enzymes used are expensive and difficult to procure. Therefore, there is a pressing need for direct polySia detection methods which do not rely on enzymatic treatment.
To overcome these limitations, we developed new recombinant antibody probes: anti-polySia-hIgG (positive, pAb) and anti-polySia-negative hIgG (a structurally identical, non-binding negative control antibody, nAb). We analyzed the interaction of pAb/nAb with polySia using a biolayer interferometry (BLI)-based method (Fig. 1A). The antibodies were immobilized on a sensor chip via a streptavidin-binding peptide (SBP), and real-time interaction with polySia was monitored (Fig. 1B). The pAb showed clear and stable binding to polySia, with a dissociation constant of 9.36 × 10−10 (± 4.55 × 10−12) M, whereas the nAb displayed only minimal and transient interaction, confirming its utility as a negative control. To further validate antibody specificity, we performed ELISA using lysates from embryonic mouse brains. Samples immobilized on plastic plates were probed with both pAb and nAb (Fig. 1C). Binding of the pAb was reduced following Endo-N treatment, though not completely abolished, while nAb binding remained undetectable—corroborating the BLI findings (Fig. 1B). Subtracting the nAb signal from the pAb signal allowed for accurate quantification of polySia levels. Next, we assessed antibody utility in blot-based detection. The pAb detected the characteristic smear pattern of polySia-NCAM, which was largely but not completely eliminated by Endo-N treatment (Fig. 1D, Supplementary Fig.S1). In contrast, the nAb produced no detectable signal. Tissue staining of mouse brain sections further supported these findings: while pAb staining occasionally exhibited non-specific signals, nAb staining did not (Fig. 1E). These results clearly demonstrate the specificity and versatility of the pAb/nAb probe pair for reliable polySia detection across diverse applications.
Establishment and characterization of recombinant positive and negative anti-polySia-hIgG. (A) Schematic of the biolayer interferometry (BLI) method used to analyze polySia–antibody interactions. Antibodies were immobilized onto a chip via their Fc region, and colominic acid (purified polySia derived from Escherichia coli K1 cell walls) was used as the analyte. (B) Chromatogram of the interaction. The positive antibody (pAb; black) and negative antibody (nAb; orange) were immobilized onto the chip, and polysialic acid (25 nM) was applied from 0–300 s. After 300 s, dissociation was analyzed in phosphate-buffered saline (PBS). (C) ELISA of mouse embryonic brain (MEB) lysate. Immobilized polySia in MEB was detected using pAb (black) or nAb (orange). As a control, immobilized polySia was digested with Endo-N and detected using pAb (light blue). (D) Western blot analysis of adult mouse brain lysate with and without Endo-N treatment. Left panel: pAb detects polySia. Right panel: nAb shows no detection. HSC70 was used as a loading control. (E) Immunohistochemistry of the hippocampus (HIP) in a mouse brain section (polySia: green; IBA1: red; DAPI: blue; scale bar: 200 μm). Left panel: pAb detects polySia-positive cells, particularly in the dentate gyrus, CA3, and CA1 regions of the HIP. Right panel: nAb fails to detect polySia. Abbreviations: polySia, polysialic acid; pAb, positive antibody; nAb, negative antibody; Endo-N, endo-N-acylneuraminidase;; DAPI, 4′,6-diamidino-2-phenylindole; IBA1, ionized calcium-binding adaptor molecule 1; HIP, hippocampus; BLI, biolayer interferometry; PBS, phosphate-buffered saline; MEB, mouse embryonic brain.
Establishment of sandwich ELISA for polysia
To address the limitations associated with conventional polySia quantification methods described in the Introduction, we established a novel sandwich ELISA using two newly developed probes, pAb and nAb, for polySia detection (Fig. 2A). In this assay, an anti-polySia IgM antibody, specific to a distinct epitope on the non-reducing terminal end of polySia, was used as the capture antibody. Following immobilization of polySia via its non-reducing terminal end, its internal structure was quantified using a combination of pAbs and nAbs. Because the pAb includes an SBP tag, streptavidin-peroxidase could be employed, facilitating the detection of samples from various animal species. We evaluated polySia expression using this sandwich ELISA system (Fig. 2B, left panel) and observed high reproducibility, with coefficients of variation (CV) of less than 10% at detectable levels. The detection limits using mouse brain homogenates from embryonic and adult animals were < 1 ng and 3 ng, respectively (Fig. 2B, middle panels). Additionally, the detection limit for purified polySia-NCAM was less than 0.3 ng/well. Thus, this sandwich ELISA method substantially outperforms conventional Western blot (WB) analysis in both sensitivity and quantification capabilities (Supplementary Fig.S2).
Establishment of the sandwich ELISA. (A) Schematic representation of the Left panel: Detection of free polySia using colominic acid (0–200 ng/well). Middle panel: Detection using mouse embryonic brain (MEB) and mouse adult brain (MAB) lysates (0–200 ng/well, based on BSA concentration). Right panel: Detection using purified polySia-NCAMs (0–200 ng/well, based on BSA) concentration. Abbreviations: polySia, polysialic acid; ELISA, enzyme-linked immunosorbent assay; MEB, mouse embryonic brain; MAB, mouse adult brain; BSA, bovine serum albumin; NCAM, neural cell adhesion molecule.
Quantification of polysia in mouse brain samples during aging
To date, no comprehensive quantitative analysis of polySia in the developing and aging brain, particularly within specific brain regions, has been conducted due to the lack of reliable techniques. Using our newly established sandwich ELISA method, we performed a detailed analysis of polySia levels across various brain regions during aging. Brain samples were collected from C57BL/6 mice at developmental and aging stages, ranging from embryonic day (E)17 to 24 months (Fig. 3A). The brain regions were dissected as illustrated in Fig. 3B. Tissue weight increased from the fetal to the adult stage in all regions. However, the degree of variation beyond 8 weeks of age differed among regions. Analysis of protein content per tissue weight revealed that while some regions exhibited changes correlated with developmental stage, others did not. The timing of substantial weight increase, also varied by region. In particular, protein content per tissue weight was reduced in the olfactory bulb (OB), cerebrum (CBR), and cerebellum (CBL), suggesting brain atrophy associated with aging. PolySia was highly expressed in all regions during the fetal period and immediately after birth, then declined as the mice matured into adulthood. However, post-adult variation in polySia expression differed by region. In the prefrontal cortex (PFC), expression gradually declined from 8 weeks to 24 months. In contrast, other regions exhibited a gradual increase in polySia expression between 4 and 12 months. Notably, in the OB, amygdala (AMG), and HIP, expression levels increased again between 18 and 24 months (Fig. 3C). These quantitative results are summarized as a heat map (Fig. 3D).
PolySia analysis of mouse brain samples. (A) Timeline of brain sample collection. (B) Brain regions selected for polySia quantification. (C) PolySia expression levels across brain regions (OB, PFC, SCN, AMG, HIP, CBR, CBL, MO) and developmental/aging stages (E17, P0, P1, 3w, 8w, 4 m, 6 m, 12 m, 18 m, 24 m). Post-hoc comparisons were performed using Tukey’s Honestly Significant Difference (HSD) test (†: 0.05 < p < 0.1, *: p < 0.05, **: p < 0.01). (D) Heatmap of polySia expression levels by region. Left panel: Expression levels compared to 8-week samples (8w = 1.0; color scale: min. 0.5, max. 5.0). The heatmaps outlined by dotted lines indicate comparisons starting from 8w (color scale: min. 0.2, max. 3.0). Right panel: Comparisons beginning from 6 m (6 m = 1.0; color scale: min. 0.2, max. 3.0). Abbreviations: polySia, polysialic acid; OB, olfactory bulb; PFC, prefrontal cortex; SCN, suprachiasmatic nucleus; AMG, amygdala; HIP, hippocampus; CBR, cerebrum; CBL, cerebellum; MO, medulla oblongata; E17, embryonic day 17; P0, postnatal day 0; P1, postnatal day 1; 3w, 3 weeks; 8w, 8 weeks; 4 m, 4 months; 6 m, 6 months; 12 m, 12 months; 18 m, 18 months; 24 m, 24 months; HSD, Honestly Significant Difference.
Analysis of changes in glycans in HIP associated with aging
Hippocampal changes during aging are of particular importance, as hippocampal atrophy is associated with cognitive decline and has been linked to Alzheimer’s disease (AD)32. A detailed analysis of polySia quantification revealed a decrease in polySia levels in the HIP at 18 months (Fig. 2C). To investigate structural changes in other glycans in the HIP during aging, we employed a lectin microarray system using 45 lectins to detect diverse glycan structures (Fig. 4A)33. Results from the lectin analysis are provided in Supplementary Figure S4. As polySia-specific probes are limited to anti-polySia antibodies, we additionally used Sia-specific probes, Maackia amurensis lectin (MAL)−1 and Sambucus nigra lectin (SNA), which recognize the α2,3-Sia and α2,6-Sia structure, respectively, but do not detect polySia. The majority Sia-related staining signals originated from SNA, indicating that α2,6-linked Sia is the predominant Sia-containing structure in the HIP (Fig. 4B). An increase in this structure was observed at 6 and 12 months, followed by a decline at 18 and 24 months. In contrast, asialo-glycans, which lack Sia, increased from 8 weeks to 4 months, followed by a decrease at 18 and 24 months.
Analyses of sialoglycoconjugates in hippocampus by lectin microarray and RNA-seq. (A) Schematic of the lectin microarray system. (B) Expression levels of sialoglycoconjugates in HIP. The net intensities for each lectin (MAL-I, SNA, and RCA120) were calculated as the mean of triplicate spots minus background signal. (C) Metabolic pathway of Sia. (D) Expression levels of Sia-related genes were visualized using publicly available RNA-seq data (GSE201029). FPKM values are shown as white bars (3 months) and gray bars (22 months). Error bars indicate the standard error of the mean (SEM). Significant differences were tested by Student’s t-Test (†: 0.05 < p < 0.1, *: p < 0.05). Abbreviations: Gne: GlcNAc-2-epimerase/ManNAc kinase; Nans: Neu5 Ac 9-phosphate synthase; Nanp: Neu5 Ac 9-phosphate phosphatase; Cmas: CMP-Neu5 Ac synthetase; Slc35a1: solute carrier family 35 member 1 (Cst); St3 gal: ST3 β-galactoside α−2,3-sialyltransferase; St6 gal: ST6 β-galactoside α−2,6-sialyltransferase; St8 sia: ST8 α-N-acetylneuraminide α−2,8-sialyltransferase; Neu : neuraminidase; HIP, hippocampus; Sia, sialic acid; FPKM, fragments per kilobase of transcript per million mapped reads; SEM, standard error of the mean; MAL-I, Maackia amurensis lectin I; SNA, Sambucus nigra agglutinin; RCA120, Ricinus communis agglutinin 120; RNA-seq, RNA sequencing.
To determine whether changes in Sia-related glycans were associated with altered transcription of the Sia-related genes, we analyzed gene expression data from RNA-seq datasets in public databases (comparing 3- and 22-months-old mice). The Sia metabolic pathways are shown in Fig. 4C. We examined the Sia-related genes and transferases involved in the synthesis of α2,3- α2,6- and α2,8-linked Sia on N-glycoproteins, as well as Sia-degrading enzymes Neu1 and Neu4 (Fig. 4D). Among the polySia-synthesizing enzymes, ST8Sia4 but not ST8Sia2 showed a significant increase. Notably, other sialyltransferases such as ST3Gal1 and ST6Gal1, responsible for synthesizing MAL-1 and SNA epitopes, also displayed increased expression. These transcriptomic results were not entirely consistent with the findings from the sandwich ELISA and lectin microarray analyses. Of note, the sialidase Neu4, which is known to cleave polySia, was downregulated, whereas Neu1, another enzyme capable of cleaving polySia did not show as decrease. Intriguingly, genes involved in the biosynthesis and transport of sialic acid, Nans, Nanp, Cmas, and Slc35a1, were downregulated, indicating that the decrease in Sia-related glycans, including polySia, was primarily due to a reduced availability of substrates rather than diminished sialyltransferase activity.
Quantification of polysia in blood samples
Identifying biomarkers in the blood that reflect the physiological state of the brain, including disease conditions, is of great importance. To investigate blood levels of polySia and explore their relationship with brain polySia, we quantified polySia using sandwich ELISA in samples collected from E17 to 24 months of age. Prior to polySia analysis, we measured total protein levels in the blood during aging. Albumin, the major blood protein and a key serum component, is believed to decline with age due to reduced liver function, potentially contributing to the overall decrease in serum protein concentration. In mouse serum, total protein levels increased until 6 months of age, then gradually declined from 6 to 24 months (Fig. 5A). However, the polySia expression profile was entirely different from that of total protein (Fig. 5A, middle panel). Similar to the pattern observed in the brain, serum polySia levels sharply decreased by 8 weeks after birth. Subsequently, a slight increase was observed at 18 months, followed by a significant decrease at 24 months. Because the blood polySia profile closely mirrored that of the brain, we hypothesized that changes in blood polySia concentration might reflect brain-related pathologies. To test this hypothesis, we analyzed polySia concentrations in the blood of ST8Sia2-knockout (KO) mice, which exhibit impaired polySia expression. ST8Sia2-KO mice display schizophrenia (SCZ)-related phenotypes and are widely used as a model for SCZ studies24. Notably, we observed for the first time that both heterozygous and homozygous KO mice showed an upregulation of polySia in the blood (Fig. 6A). Finally, we extended our analysis to human samples.
PolySia analysis of mouse blood samples. (A) Protein concentration and polySia levels measured at each developmental/aging stage (E17, P0, P1, 3w, 8w, 4 m, 6 m, 12 m, 18 m, 24 m). Post-hoc comparisons were performed using Tukey’s Honestly Significant Differences (Tukey’s HSD) test (†: 0.05 < p < 0.1, *: p < 0.05, **: p < 0.01). (B) Heatmap of polySia expression levels across stages. Left panel: expression levels normalized to 8w (8w = 1.0; color gradation: min. 0.5, max. 5.0). The heatmaps surrounded by dotted lines include only comparisons from 8w onward (8w = 1.0; color gradation: min. 0.2, max. 3.0). Right panel: expression levels normalized to 6 m (6 m = 1.0; color gradation: min. 0.2, max. 3.0). Abbreviations: polySia, polysialic acid; E17, embryonic day 17; P0, postnatal day 0; P1, postnatal day 1; 3w, 3 weeks; 8w, 8 weeks; 4 m, 4 months; 6 m, 6 months; 12 m, 12 months; 18 m, 18 months; 24 m, 24 months.
PolySia analysis of schizophrenia model mouse and patient blood samples. The amount of polySia was measured in (A) serum samples from schizophrenia model mice (St8 sia2-KO; all groups, n = 4), (B) blood samples from patients with schizophrenia (all groups, n = 16), and (C) blood samples from patients with CIDP (male controls, n = 11; male CIDP, n = 14; female controls, n = 8; female CIDP, n = 12). Post-hoc comparisons were performed using Tukey’s Honestly Significant Differences (Tukey’s HSD) test (†: 0.05 < p < 0.1, *: p < 0.05, **: p < 0.01). Abbreviations: polySia, polysialic acid; KO, knockout; CIDP, chronic inflammatory demyelinating polyneuropathy; HSD, Honestly Significant Differences.
Comparison of polysia levels in psychiatric disorders and neurodegenerative disease
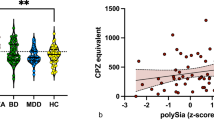
We compared polySia concentrations in the plasma of patients with schizophrenia (SCZ), representing a psychiatric disorder, and in the serum of patients with chronic inflammatory demyelinating polyneuropathy (CIDP), representing a neurodegenerative disease, alongside healthy control samples. Serum and plasma measurements produced comparable results (Supplementary Fig. S5). PolySia concentrations were notably elevated in male patients with SCZ (Fig. 6B). A similar trend was observed in female patients with SCZ; however, healthy females exhibited higher baseline polySia levels compared to healthy males. Conversely, no significant differences in polySia levels were detected in CIDP patients relative to controls, suggesting that polySia levels remain relatively stable in this neurodegenerative condition (Fig. 6C). Overall, these findings indicate that blood polySia levels change in an SCZ-dependent manner.
Discussion
All methods using the newly developed antibody demonstrated that the positive antibody (pAb) exhibited strong binding affinity toward polySia, whereas the negative mutant (nAb) showed no activity at the endpoint (Fig. 2C–E). However, according to the BLI results (Fig. 2B), the negative antibody exhibited negligible binding activity, although any bound antibody was washed out during the dissociation step. This characteristic confirms that the nAb functions effectively as a negative control at the endpoint. Conventionally, enzymatic digestion of glycan epitopes such as Endo-N digestion for polySia has been used to verify the presence of specific glycans. However, these enzymes are often expensive, difficult to procure, and may have inconsistent activity, which can result in incomplete cleavage of the target glycan. The nAb, a single amino acid variant of the pAb, retains nearly identical structural and physicochemical properties to the pAb, differing only in its specificity. This similarity allows the nAb to serve as a practical and non-enzymatic alternative for confirming glycan specificity (Fig. 2D). Its lack of enzymatic activity offers additional advantages by reducing both cost and processing time. It is anticipated that recombinant positive- and negative-pair probe systems will become a valuable tool in glycobiology research, enabling rapid and reliable glycan analysis.
This method overcomes the limitations of conventional analytical approaches for polySia and enables comprehensive quantitative analysis in the developing and aging brain. Notable differences were observed in OB, with increased polySia levels at 24 months. Normal aging in humans has been shown to reduce OB function. Furthermore, in patients with AD, the decline in olfactory function is more severe than that seen with normal aging, and there is a known correlation between age-related olfactory decline and cognitive decline34. Studies have also demonstrated a relationship between the OB and AD35,36. Considering these findings, it is plausible that the observed increase in polySia at 24 months may represent a compensatory mechanism to counteract age-related decline in olfactory function and associated cognitive deterioration. The suprachiasmatic nucleus (SCN), which plays a central role in regulating circadian rhythms, also showed an age-related decrease in polySia expression. Circadian rhythms are known to decline with age in both mice and and humans37,38. Disrupted circadian regulation is a common symptom in AD38,39, often manifesting as disturbed sleep cycles, frequent awakenings, increased daytime sleep, and sleep deprivation. Moreover, Ncam-KO polySia-deficient mice exhibit impaired circadian rhythms40. These observations suggest that the age-related decrease in polySia in the SCN may contribute to diminished SCN function and disrupted circadian rhythms with aging. AMG is associated with memory-related HIP functions, with AMG activity influencing synaptic plasticity, long-term potentiation (LTP), and long-term depression (LTD) in the HIP. AMG atrophy has been observed in the brains of patients with AD, and a negative correlation has been reported between AMG volume and emotional abnormalities in these individuals41,42. Although the fluctuations in polySia observed in the AMG in this study were less pronounced than in other regions, with a decrease only at 18 months, this pattern mirrors that of the HIP, suggesting that polySia expression changes may be involved in the coordinated functions of the AMG and HIP. Age-related decline in hippocampal neurogenesis is associated with reduced learning and memory capacity43. A reduction in polySia may impede neurogenesis and LTP, thereby disrupting normal hippocampal function. Notably, an increase in polySia at 12 months was observed before a rapid decline at 18 months, suggesting a degree of resilience. After an initial postnatal decrease, polySia expression rose again between 6 and 12 months. As noted above, we speculate that brain resilience mechanisms may be at play in these regions, acting as a system that resists the age-related decline in polySia expression.
To date, no polySia-recognizing lectins have been identified, although a lectin microarray system has been established. PolySia remains undetected by glycoproteomic or glycomic analyses. Therefore, the newly established sandwich ELISA represents the most effective method for identifying and quantifying polySia. Using this method, we were able to accurately evaluate polySia expression levels. We also focused on Sia-containing structures, particularly the terminal structures of polySia. Lectin-based histochemical staining of the HIP in 9-week- and 29-month-old rats revealed an age-related decrease in glycoproteins containing α2,3-Sia, α2,6-Sia, and α2,8-Sia in different regions of the HIP44. These findings are consistent with previous reports45,46,47, suggesting that in the HIP, the expression of Sia-containing glycans, other than polySia, declines with age (Fig. 4B). This age-related reduction also coincides with an increase in asialoglycans, likely due to the overall decline in Sia levels during aging. Focusing on Sia-related gene expression, age-related changes in sialidase genes such as Neu1 and Neu4, along with downregulation of the biosynthetic pathway excluding Gne, are thought to contribute to reduced cellular Sia concentrations (Fig. 4D). The Gne enzyme is subject to negative feedback regulation by CMP-Sia, a metabolite of Cmas and a donor substrate for all sialyltransferases48. Even at the transcript level, Gne may be regulated via a decrease in free Sia-related molecules due to this feedback mechanism. Because decreased Sia levels are detrimental to cellular function, the observed upregulation of enzymes that utilize Sia as a substrate may represent a compensatory, resilience-associated response to aging. Additionally, while expression of sialyltransferases responsible for α2,3-Sia and α2,6-Sia linkages was upregulated, expression of sialidases decreased, which is likely to result in increased levels of α2,3-Sia and α2,6-Sia. To be noted, expression of the polysialyltransferase ST8Sia4 but not ST8Sia2 was increased. However, this increase did not align with the observed decrease in polySia levels determined by the sandwich ELISA. This discrepancy suggests that gene expression levels should be interpreted in conjunction with the activity of metabolic enzymes involved in substrate synthesis and utilization.
Based on the results of polySia expression in the blood, both the SCZ model mouse (ST8Sia2-KO mice) and human patient samples showed similar increases in polySia even at young age (Fig. 6A, B). Previous studies have reported that the brains of patients with SCZ exhibit accelerated aging compared to those of healthy controls49. It is also noteworthy that, even in healthy individuals, polySia levels tend to increase with age. However, the correlation between age and polySia remains unclear. We also hypothesized that CIDP, which affects the nervous system and is characterized by inflammation, might influence polySia levels. However, compared with the control samples, no notable alterations were observed, indicating that CIDP does not influence polySia concentrations in the blood. This approach has been valuable in elucidating the relationship between polySia and various diseases. The use of animal models to investigate associated pathologies and assess therapeutic efficacy may contribute to advancing the diagnosis and treatment of these conditions. It is important to quantify various samples using a validated and standardized method, and we propose adopting this newly developed sandwich ELISA for future analysis.
Conclusion
A panel of polySia-specific antibodies, including both positive and negative binders, was generated, and a sandwich ELISA was established for the accurate and sensitive detection of polySia. This sandwich ELISA was used to quantify polySia in various mouse brain regions and serum samples. While extensive research has examined the substantial changes in polySia-NCAM during early development, there has been a lack of investigations into changes in polySia during adulthood. This study represents the first attempt to capture both age-related and region-specific alterations in polySia expression in the adult mouse brain. It is conceivable that resilience mechanisms, which counteract brain aging and help preserve neuroplasticity, are at play in neuroplastic brain regions. Using this highly sensitive method, we also analyzed human blood samples. While no significant changes were observed in conditions not involving polySia in the blood, such as CIDP, marked were found in samples from individuals with SCZ. In the future, we plan to expand the number and diversity of samples to further investigate polySia expression differences across aging, disease states, and species. This will be supported by our newly developed probe-based method.
Data availability
The datasets analysed during the current study (GSE201029) are available in the Gene Expression Omnibus repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE201029.
References
Varki, A. et al. Essentials of Glycobiology. 4th edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; (2022). https://doi.org/10.1101/9781621824213 (2022).
Magnani, J. L. et al. A monoclonal antibody-defined antigen associated with Gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J. Biol. Chem. 257, 14365–14369 (1982).
Dong, X. et al. Advances in mass spectrometry-based glycomics. Electrophoresis 39, 3063–3081. https://doi.org/10.1002/elps.201800273 (2018).
Kuno, A. et al. Evanescent-field fluorescence-assisted lectin microarray: a new strategy for glycan profiling. Nat. Methods. 2, 851–856. https://doi.org/10.1038/nmeth803 (2005).
Thaysen-Andersen, M., Kolarich, D. & Packer, N. H. Glycomics & glycoproteomics: from analytics to function. Mol. Omics. 17, 8–10. https://doi.org/10.1039/d0mo90019b (2021).
Cindrić, A., Pribić, T. & Lauc, G. High-throughput N-glycan analysis in aging and inflammaging: state of the Art and future directions. Semin Immunol. 73, 101890. https://doi.org/10.1016/j.smim.2024.101890 (2024).
Miura, Y. et al. Change in N-Glycosylation of plasma proteins in Japanese semisupercentenarians. PLoS One. 10, e0142645. https://doi.org/10.1371/journal.pone.0142645 (2015).
Miura, Y. et al. Characteristic glycopeptides associated with extreme human longevity identified through plasma glycoproteomics. Biochim. Biophys. Acta Gen. Subj. 1862, 1462–1471. https://doi.org/10.1016/j.bbagen.2018.03.025 (2018).
Morishita, S., Hane, M., Kitajima, K. & Sato, C. Substantial basis for Glyco-Assembly: Siglec7 and synthetic sialylpolymers. In Glycosignals in Cancer (eds Furukawa, K. & Fukuda, M.) (Springer, 2023). https://doi.org/10.1007/978-981-19-7732-9_3.
Sato, C. & Kitajima, K. Disialic, oligosialic and polysialic acids: distribution, functions and related disease. J. Biochem. 154, 115–136. https://doi.org/10.1093/jb/mvt057 (2013).
Angata, T. & Varki, A. Chemical diversity in the Sialic acids and related alpha-keto acids: an evolutionary perspective. Chem. Rev. 102, 439–469 (2002).
Sato, C. & Kitajima, K. Polysialylation and disease. Mol. Aspects Med. 79, 100892. https://doi.org/10.1016/j.mam.2020.100892 (2021).
Yang, P., Yin, X. & Rutishauser, U. Intercellular space is affected by the polysialic acid content of NCAM. J. Cell. Biol. 116, 1487–1496. https://doi.org/10.1083/jcb.116.6.1487 (1992).
Kanato, Y., Kitajima, K. & Sato, C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology 18, 1044–1053. https://doi.org/10.1093/glycob/cwn084 (2008).
Isomura, R., Kitajima, K. & Sato, C. Structural and functional impairments of polysialic acid by a mutated polysialyltransferase found in schizophrenia. J. Biol. Chem. 286, 21535–21545. https://doi.org/10.1074/jbc.M111.221143 (2011).
Ono, S., Hane, M., Kitajima, K. & Sato, C. Novel regulation of fibroblast growth factor 2 (FGF2)-mediated cell growth by polysialic acid. J. Biol. Chem. 287, 3710–3722. https://doi.org/10.1074/jbc.M111.276618 (2012).
Hane, M., Sumida, M., Kitajima, K. & Sato, C. Structural and functional impairments of polysialic acid (polySia)-neural cell adhesion molecule (NCAM) synthesized by a mutated polysialyltransferase of a schizophrenic patient. Pure Appl. Chem. 84, 1895–1906. https://doi.org/10.1351/pac-con-11-12-10 (2012).
Hane, M. et al. Protective effects of polysialic acid on proteolytic cleavage of FGF2 and ProBDNF/BDNF. Glycobiology 25, 1112–1124. https://doi.org/10.1093/glycob/cwv049 (2015).
Mori, A., Hane, M., Niimi, Y., Kitajima, K. & Sato, C. Different properties of polysialic acids synthesized by the polysialyltransferases ST8SIA2 and ST8SIA4. Glycobiology 27, 834–846. https://doi.org/10.1093/glycob/cwx057 (2017).
Barbeau, D., Liang, J. J., Robitalille, Y., Quirion, R. & Srivastava, L. K. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc. Natl. Acad. Sci. U S A. 92, 2785–2789 (1995).
Hane, M., Kitajima, K. & Sato, C. Comprehensive analysis of oligo/polysialylglycoconjugates in Cancer cell lines. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23105569 (2022).
Roth, J. et al. Evaluation of polysialic acid in the diagnosis of Wilms’ tumor. A comparative study on urinary tract tumors and non-neuroendocrine tumors. Virchows Arch. B Cell. Pathol. Incl. Mol. Pathol. 56, 95–102. https://doi.org/10.1007/BF02890007 (1988).
Angata, K. et al. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J. Biol. Chem. 279, 32603–32613. https://doi.org/10.1074/jbc.M403429200 (2004).
Kröcher, T. et al. Schizophrenia-like phenotype of polysialyltransferase ST8SIA2-deficient mice. Brain Struct. Funct. 220, 71–83. https://doi.org/10.1007/s00429-013-0638-z (2015).
Takahashi, Y. et al. Polysialylation in a DISC1 mutant mouse. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23095207 (2022).
Abe, C. et al. Chlorpromazine increases the expression of polysialic acid (PolySia) in human neuroblastoma cells and mouse prefrontal cortex. Int. J. Mol. Sci. 18 https://doi.org/10.3390/ijms18061123 (2017).
Abe, C., Yi, Y., Hane, M., Kitajima, K. & Sato, C. Acute stress-induced change in polysialic acid levels mediated by Sialidase in mouse brain. Sci. Rep. 9, 9950. https://doi.org/10.1038/s41598-019-46240-6 (2019).
Sato, C., Kitajima, K., Inoue, S. & Inoue, Y. Identification of oligo-N-glycolylneuraminic acid residues in mammal-derived glycoproteins by a newly developed immunochemical reagent and biochemical methods. J. Biol. Chem. 273, 2575–2582. https://doi.org/10.1074/jbc.273.5.2575 (1998).
Sato, C., Inoue, S., Matsuda, T. & Kitajima, K. Development of a highly sensitive chemical method for detecting alpha 2 -> 8-linked oligo/polysialic acid residues in glycoproteins blotted on the membrane. Anal. Biochem. 261, 191–197. https://doi.org/10.1006/abio.1998.2718 (1998).
Mori, A. et al. Combinational analyses with multiple methods reveal the existence of several forms of polysialylated neural cell adhesion molecule in mouse developing brains. Int. J. Mol. Sci. 21 https://doi.org/10.3390/ijms21165892 (2020).
Hatanaka, R. et al. Identification of a buried β-strand as a novel disease-related motif in the human polysialyltransferases. J. Biol. Chem. 300, 105564. https://doi.org/10.1016/j.jbc.2023.105564 (2024).
Rao, Y. L. et al. Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech 12, 55 (2022). https://doi.org/10.1007/s13205-022-03123-4
Ebe, Y. et al. Application of lectin microarray to crude samples: differential glycan profiling of lec mutants. J. Biochem. 139, 323–327. https://doi.org/10.1093/jb/mvj070 (2006).
Cerf-Ducastel, B. & Murphy, C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 986, 39–53. https://doi.org/10.1016/s0006-8993(03)03168-8 (2003).
Gómez-Isla, T. et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J. Neurosci. 16, 4491–4500. https://doi.org/10.1523/JNEUROSCI.16-14-04491.1996 (1996).
Murray, H. C. et al. Distribution of PSA-NCAM in normal, Alzheimer’s and Parkinson’s disease human brain. Neuroscience 330, 359–375. https://doi.org/10.1016/j.neuroscience.2016.06.003 (2016).
Chang, H. C. & Guarente, L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153, 1448–1460. https://doi.org/10.1016/j.cell.2013.05.027 (2013).
Witting, W., Kwa, I. H., Eikelenboom, P., Mirmiran, M. & Swaab, D. F. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol. Psychiatry. 27, 563–572. https://doi.org/10.1016/0006-3223(90)90523-5 (1990).
Hu, K., Van Someren, E. J., Shea, S. A. & Scheer, F. A. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: involvement of the circadian pacemaker. Proc. Natl. Acad. Sci. U S A. 106, 2490–2494. https://doi.org/10.1073/pnas.0806087106 (2009).
Shen, H. et al. Role of neural cell adhesion molecule and polysialic acid in mouse circadian clock function. J. Neurosci. 17, 5221–5229 (1997).
Wright, C. I., Dickerson, B. C., Feczko, E., Negeira, A. & Williams, D. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer’s disease. Biol. Psychiatry. 62, 1388–1395. https://doi.org/10.1016/j.biopsych.2006.11.013 (2007).
Wang, D. W. et al. Diagnostic value of amygdala volume on structural magnetic resonance imaging in Alzheimer’s disease. World J. Clin. Cases. 9, 4627–4636. https://doi.org/10.12998/wjcc.v9.i18.4627 (2021).
Gil-Mohapel, J. et al. Hippocampal neurogenesis levels predict WATERMAZE search strategies in the aging brain. PLoS One. 8, e75125. https://doi.org/10.1371/journal.pone.0075125 (2013).
Sato, Y., Kimura, M. & Endo, T. Comparison of lectin-binding patterns between young adults and older rat glycoproteins in the brain. Glycoconj. J. 15, 1133–1140. https://doi.org/10.1023/a:1006911821339 (1998).
Itakura, Y., Sasaki, N. & Toyoda, M. Qualitative and quantitative alterations in intracellular and membrane glycoproteins maintain the balance between cellular senescence and human aging. Aging (Albany NY). 10, 2190–2208. https://doi.org/10.18632/aging.101540 (2018).
Itakura, Y. et al. N- and O-glycan cell surface protein modifications associated with cellular senescence and human aging. Cell. Biosci. 6, 14. https://doi.org/10.1186/s13578-016-0079-5 (2016).
Itakura, Y. et al. Spatiotemporal changes of tissue glycans depending on localization in cardiac aging. Regen Ther. 22, 68–78. https://doi.org/10.1016/j.reth.2022.12.009 (2023).
Seppala, R., Lehto, V. P. & Gahl, W. A. Mutations in the human UDP-N-acetylglucosamine 2-epimerase gene define the disease sialuria and the allosteric site of the enzyme. Am. J. Hum. Genet. 64, 1563–1569. https://doi.org/10.1086/302411 (1999).
Constantinides, C. et al. Brain ageing in schizophrenia: evidence from 26 international cohorts via the ENIGMA schizophrenia consortium. Mol. Psychiatry. 28, 1201–1209. https://doi.org/10.1038/s41380-022-01897-w (2023).
Acknowledgements
This research was funded by Japan Agency for Medical Research and Development (AMED) grant number [18ae0101069h0003; 19ae0101069h0004; 20ae0101069h0005; 20gm6410007h0001, 21gm6410007h0002, 22gm6410007h0003, and 23gm6410007h0004] (to CS), and a Grant-in-Aid for Scientific Research(B) (21H02425, 23K21291, and 25K02224) from MEXT (to CS). This work was also supported by grant-in-Aid for Early-Career Scientists (22K15059), grant-in-Aid for Scientific Research C (24K08829) and the 34th The Naito Reserch Grant to MH. This research was also funded by Amed (JP22tm0424222) to IK and NO, JP21wm0425007; JP23ak0101215; JP22gm1410011; JP23gm1910005; JP24zf0127011 to NO. It is funded by grant-in-Aid for Early-Career Scientists (23K14751) to YF and Scientific Research(A)(JP23H00420) to MK. A part of this work is supported by J-Glyconet (JGN) and human glycome atlas project (HGA).
Author information
Authors and Affiliations
Contributions
Conceptualization M.H., C.S.; Data curation M.H., A.N., K.H., C.A.,T.N., K.K.,C.S.; Formal analysis M.H., C.S.; Funding acquisition M.H., Y.F., I. K., M.K., N.O., K.K., C.S.; Investigation M.H., A.N., K.H., C.A.,T.N., K.K.,C.S.; Methodology M.H., K.H., T.N., K.K.,C.S.; Project administration: M.H., C.S.; Resources: M.H., A.N., K.H., C.A., T.N., I.K., S.F., Y.F., K.I., K.S., S.K., M.K., N.O., K.K., C.S.; Supervision C.S.; Validation M.H., A.N., K.H., C.A.,T.N., K.K.,C.S.; Writing – original draft M.H., C.S.; Writing – review & editing M.H., A.N., K.H., C.A., T.N., I.K., S.F., Y.F., K.I., K.S., S.K., M.K., N.O., K.K., C.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hane, M., Naramura, A., Hayakawa, K. et al. A highly sensitive quantitative method of polysialic acid reveals its unique changes in brain aging and neuropsychiatric disorders. Sci Rep 15, 19033 (2025). https://doi.org/10.1038/s41598-025-02583-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02583-x