Abstract
Vaginal intraepithelial neoplasia (VaIN) is a precancerous lesion for invasive vaginal cancer, which is highly malignant and difficult to treat. This study aimed to identify the risk factors for VaIN and emphasize the importance of screening and monitoring patients with a history of cervical intraepithelial neoplasia and ongoing human papillomavirus (HPV) infection. Patients suspected of having VaIN were admitted to the Department of Gynecology and Obstetrics at the Affiliated Suzhou Hospital of Nanjing Medical University between February 2014 and August 2022. Medical records of these patients were retrospectively reviewed. A total of 274 patients underwent colposcopy due to suspected VaIN, and the diagnosis was confirmed in 167 patients based on biopsy pathology reports. Multivariate analysis in which odds ratios (ORs) with a 95% confidence interval (95% CI) were calculated revealed that vaccine-related HPV-infected patients (OR = 4.30, 95% CI: 1.75–10.58), and postmenopausal patients (OR = 5.94, 95% CI: 1.95–18.09), had a significantly increased risk of developing high-grade VaIN. However, patients with prior surgical treatment had a reduced risk (OR = 0.58, 95% CI: 0.34–0.99), which may be attributed to regular monitoring. HPV16, 58, 52, and 33 were among the most prevalent HPV types, with HPV16 positivity associated with higher-grade VaIN. Patients with concurrent cervical high-grade squamous epithelial lesions also exhibited high-grade VaIN, indicating a potential link between cervical and vaginal intraepithelial lesions. Our study highlights the complex interplay between risk factors such as prior surgical treatment, human papillomavirus infection, and menstrual status in the progression of VaIN. This underscores the importance of comprehensive evaluation and management strategies for VaIN.
Similar content being viewed by others
Introduction
Vaginal intraepithelial neoplasia (VaIN) refers to a group of premalignant lesions that develop in the vaginal mucosa. According to the 5th edition of the 2020 World Health Organization (WHO) two-tier classification system of female reproductive system tumors, VaIN can be categorized into low-grade squamous epithelial lesion (LSIL) and high-grade squamous epithelial lesion (HSIL)1. VaIN 1 is classified as LSIL, representing a benign manifestation of human papillomavirus (HPV) infection, with a low risk of progressing to vaginal cancer. Conversely, VaIN 2/3 falls under HSIL, with an approximate 12% risk of evolving into invasive vaginal cancer. Despite its usually asymptomatic nature, the incidence rate of VaIN is relatively low in clinical practice, around 0.2–0.3/100,000, accounting for 0.4–1.0% of all lower genital tract epithelial neoplasms2.
The American Society for Colposcopy and Cervical Pathology (ASCCP) cervical cancer screening guidelines in 2012 incorporated high-risk HPV testing and cervical cytology into the screening system3. The ASCCP interim guidelines in 2019 advocated the use of HPV testing alone for primary screening4. Most recently, since the WHO guidelines in 2021 endorsed HPV DNA as the primary screening method and Chinese guidelines in 2023 recommended HPV nucleic acid as primary screening, the sensitivity of HPV testing has surpassed that of cytology, consistent with the International Agency for Research on Cancer (IARC) perspective on cervical cancer screening5. This advancement has resulted in expanded screening coverage, heightened awareness of vaginal diseases among colposcopists, and improved chances of detecting VaIN. A study conducted by Fudan University, Shanghai, China, revealed that 6.8–18.6% of patients with high-grade squamous intraepithelial lesions exhibited signs indicative of potential cancer2.
This study retrospectively analyzed the clinical statistics of 274 VaIN-suspected patients, depicted their clinical characteristics, and revealed the correlation between risk factors such as the history of cervical intraepithelial neoplasia (CIN) or cervical cancer, hysterectomy history, exfoliative cytology results, HPV subtype testing, and VaIN grade, as determined by colposcopy-guided vaginal biopsy. The objective of this study is to propose effective screening methods for the early detection of VaIN, mitigate instances of missed diagnoses, and furnish valuable diagnostic and treatment insights for clinical practice.
Materials and methods
Data collection
This study analyzed data from 274 patients who visited the Department of Gynecology and Obstetrics at the Affiliated Suzhou Hospital of Nanjing Medical University between February 2014 and August 2022. These patients underwent vaginal wall biopsy guided by colposcopy due to abnormal results in cervical cancer screening. Among the 274 patients suspected of VaIN, 167 were diagnosed with VaIN based on pathological results. The ages of the patients ranged from 18 to 68 years, with a mean age (standard deviation [SD]) of 44.7 (11.8) years. Among these patients, 79 were diagnosed with VaIN 1, 87 with VaIN 2/3, and 1 with vaginal squamous cell carcinoma (SCC). Inclusion criteria included clear vaginal colposcopy pathology results and approval of the study by the hospital ethics committee. Exclusion criteria were patients with concurrent malignant diseases of the female reproductive system or other systems, except malignant diseases of the lower genital tract, and patients with incomplete clinical data. All data were collected from the Department of Gynecology and Obstetrics of our hospital. All methods of this study were carried out in accordance with relevant guidelines and regulations and approved by the Ethics Committee(KL 901461). All patients were informed about the study and provided written consent before undergoing vaginal colposcopy and gave informed consent to waive the study through the Ethical Committee of Suzhou Municipal hospital.
Methods
Liquid-based cytology testing
the patient’s cervix exposed, and the surface secretions were wiped with a cotton swab. The brush head of the collection device was inserted fully into the cervix, rotating it clockwise for five turns. Then, the collection device was removed, and the brush head was stored in BD liquid-based preservation solution. Liquid-based cytology testing technology provided by BD company (USA) was utilized for examination.
The diagnostic criteria adhered to the 2001 Bethesda System (TBS) reporting system, which included assessing sample adequacy. Unsatisfactory samples were subject to re-collection for accurate testing. Satisfactory samples could be classified as negative, atypical squamous cells of undetermined significance (ASC-US), atypical glandular cells (AGC), atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion (ASC-H), LSIL, HSIL, SCC, and adenocarcinoma (AC). For the purposes of this study, ASC-US and above were considered positive. No cases of adenocarcinoma were included in this study.
HPV testing
Cervical exfoliated cell samples were used in this study. After DNA extraction using nucleic acid extraction reagents, the presence of HPV DNA was determined using the hybrid capture method (Digene Hybrid Capture II HPV DNA Test, Qiagen, USA). Test outcomes were categorized as positive or negative based on the identification of high-risk HPV types, including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82. Patients who tested positive for HPV were subsequently divided into those positive for HPV16/18 and those positive for other high-risk HPV types.
Colposcopy and biopsy
Colposcopy was performed by two experienced physicians with more than 5 years of work experience. The following three categories of patients underwent colposcopic biopsy: Patients with positive HR-HPV and cytological results indicating ASC-US; exfoliative cytology results indicating ASC-H, LSIL, HSIL, AGC, or SCC; and negative cytological results but positive for HPV16/18. The colposcopy utilized an integrated photoelectric colposcope. Initially, 0.9% normal saline was used to clean the cervical and vaginal secretions, followed by a 3–5% acetic acid-soaked cotton ball applied to the cervix, fornix, and vagina for 1 min to observe the appearance of acetowhite epithelium, mosaic patterns, and abnormal vessels on the cervix and vaginal wall. Finally, a compound iodine solution was applied to the cervix, fornix, and anterior and posterior vaginal walls to observe iodine-negative areas. Multiple biopsies were taken from the suspicious lesion areas. The pathological results were classified based on the depth of epithelial involvement, ranging from normal to VaIN 1, VaIN 2, VaIN 3, or vaginal SCC.
Lower genital tract diseases usually mean benign and malignant lesions in the cervix, vagina, vulva, and anus. In our study, we defined lower genital tract lesions as benign and malignant lesions in the cervix, vagina, and vulva.
Statistical analysis
Statistical analysis was performed using SPSS version 27.0.1 software. Continuous variables are expressed as mean (SD) and were compared using t-tests. Categorical variables are expressed as frequencies and percentages and were compared using chi-square tests. Multivariate logistic regression analysis was used to identify independent risk factors for VaIN 2/3. A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
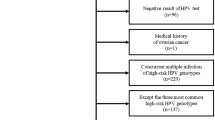
From February 2014 to August 2022, a total of 2951 lower genital tract lesions were detected under colposcopy in the Department of Obstetrics and Gynecology at our institution. Among these, VaIN cases accounted for 5.7% (167/2951) of all lower genital tract lesions, which included 2895 CIN lesions, 167 VaIN lesions, and 29 vulvar intraepithelial lesions (VIN). We analyzed data from 274 patients who had colposcopically suspected VaIN lesions (Fig. 1) when observing the iodine-negative areas, acetowhite epithelium, mosaic patterns, and abnormal vessels on the vaginal wall. These patients underwent subsequent pathologic evaluation of vaginal wall biopsy, resulting in VaIN diagnosis being confirmed in 167 patients (Fig. 2). The clinical characteristics of the 274 patients are detailed in Table 1. The majority of these patients were middle-aged females, with a mean (SD) age of 44.7 (11.8) years. Of the 274 patients, 157 were cycling females, and the remaining 117 were either naturally or artificially menopausal. Interestingly, 207 patients had a history of cervical erosion, whereas 67 did not. Additionally, cervical exfoliative cytology results were negative in 126 patients and abnormal in 148 patients.
Of the patients with suspected VaIN lesions, 70.4% had a history of CIN and 1.8% had a history of cervical cancer. Among all patients diagnosed with VaIN, 44.2% had concurrent CIN lesions and 1.5% had concurrent VIN lesions. Among the 274 patients with suspected VaIN, 68.2% had undergone loop electrosurgical excision procedure (Leep), whereas 4.0% had undergone total hysterectomy. Moreover, nine-valent vaccine-related HPV subtypes (HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 subtypes) infection was present in 74.5% of the VaIN-suspected patients.
Histopathological images of patients suspected VaIN. (A) Hematoxylin-eosin staining images for patients with VaIN0, VaIN1, VaIN2 and VaIN2-3. (B) Immunohistochemistry images indicate that P16 was positive and Ki67 was positive in lower 2/3 part of the vagina in VaIN 2 patients. (C) Immunohistochemistry images indicate that P16 was positive and Ki67 was positive in lower 2/3 to full thickness of the vagina in VaIN 2–3 patients.
Univariate/multivariate logistic regression analysis
Univariate logistic regression analysis comparing patients with VaIN 1 ( < = VaIN 1) versus VaIN 2/3 showed that prior surgical treatment (Leep or hysterectomy) for patients diagnosed with cervical HSIL based on pathological results before receiving treatment for VaIN and HPV infection were associated with high-grade VaIN (Table 2). After adjusting for confounding factors such as age, multivariate logistic regression analysis using the input method (Fig. 3) revealed that patients infected with the HPV subtypes covered by the nine-valent vaccine (vaccine-related HPV-infected patients) had a significantly increased risk of developing high-grade VaIN compared with those without vaccine-related HPV infection (OR = 4.30, 95% CI: 1.75–10.58). Postmenopausal patients were also more likely to develop high-grade VaIN than premenopausal patients (OR = 5.94, 95% CI: 1.95–18.09). In contrast, patients with prior surgical treatment had a reduced risk of high-grade VaIN (OR = 0.58, 95% CI: 0.34–0.99). This unexpected result may require further investigation with a larger sample size to confirm its validity. We speculated that patients with prior surgical treatment may be more vigilant in monitoring their condition, potentially influencing their disease progression.
HPV infection
Multivariate logistic regression analysis using the input method revealed a strong correlation between nine-valent vaccine-related HPV infection and VaIN grade. Subsequently, a multivariate logistic regression analysis using the forward (LR) method was performed. As Table 3 illustrates, patients infected with nine-valent vaccine-related HPV subtypes exhibited higher VaIN grade than those not infected with nine-valent vaccine-related HPV subtypes infection, with a significant difference between the two groups (p = 0.004).
Table 4 summarizes the genotypic distribution of HPV infection among the 274 patients suspected of VaIN. The most prevalent types were HPV16, HPV58, HPV52, and HPV33. Chi-square analysis demonstrated that patients with HPV16 infection had a higher VaIN grade than those without HPV16 infection (48.2% vs. 32.3%, p < 0.001), whereas infection with other HPV subtypes showed no significant association with VaIN grade.
Concurrent cervical intraepithelial neoplasia
Given that numerous studies have revealed a strong correlation between concomitant cervical HSIL lesions and VaIN grade, we further analyzed the relationship between concurrent CIN and VaIN grade. Table 5 shows that patients with concomitant cervical HSIL lesions exhibited a higher VaIN grade than those without coexisting CIN or with concurrent cervical LSIL lesions (X2 = 6.314, p = 0.043).
Discussion
VaIN represents a rare premalignant lesion of the lower genital tract. Risk factors for VaIN include a history of cervical or vaginal dysplasia, CIN or cervical cancer, and hysterectomy; low education level; condylomas; immunosuppression; and infection with high-risk HPV types6,7. In 2023, the European Society of Gynecologic Oncology (ESGO), International Society for the Study of Vulvovaginal Diseases (ISSVD), European College for the Study of Vulvovaginal Diseases (ECSVD), and European Federation for Colposcopy (EFC) proposed a consensus on the treatment of VaIN. The management of VaIN varies based on the lesion grade. VaIN 1 often regresses without specific treatment, requiring only observation and follow-up. However, VaIN 2/3 carries a 10% risk of progressing to invasive cancer and, therefore, necessitates aggressive management, such as topical imiquimod ointment, laser ablation, and localized vaginal mucosal resection. Traditional treatments such as topical trichloroacetic acid and 5-fluorouracil ointment are discouraged by the consensus8,9. For cases of VaIN 3 following hysterectomy for CIN, surgical radical treatment is recommended due to the inability of laser vaporization and topical medications to reach buried epithelial cells in the vaginal folds10,11. VaIN commonly occurs in the upper third of the vagina. Research by Mahalakshmi suggests that this location may be linked to the extension of the cervical transformation zone to the vaginal fornix in 1–4% of women, making incompletely matured Müllerian cells at this site more susceptible to high-risk HPV infection, leading to VaIN lesions12.
In China, HPV infection shows a bimodal peak. Women around the age of 20 who are sexually active have the highest incidence of HPV infection. The second peak of HPV infection occurs in women aged 45 to 54 years and more than 55 years, with VaIN predominantly concentrated in this age group13,14. Our study aligns with previous research indicating a high prevalence of HPV infection among patients with VaIN15. In our study, the most common HPV types in patients with VaIN were HPV16, HPV58, HPV52, and HPV33. Notably, the nine-valent vaccine, currently being promoted in our country, covers these genotypes. While HPV genotype distribution varies across studies due to geographic and population differences, there is consensus that HPV16 is the predominant genotype in VaIN cases, consistent with our findings16,17,18. Additionally, our study revealed a high prevalence of HPV infection in patients with low-grade VaIN lesions.
These results reveal the close association between VaIN grade and HPV infection, particularly HPV16. Therefore, screening for HPV16 infection should be emphasized in VaIN patients19,20. Beyond preventing CIN, surveillance for vaginal lesions during colposcopy and HPV screening during postoperative follow-up are crucial for the early detection and treatment of VaIN21,22. According to the American College of Obstetricians and Gynecologists, prophylactic HPV vaccination targeting oncogenic HPV16 and 18 has the potential to reduce vulvar cancer by approximately one-third and vaginal cancer by one-half. The high prevalence of HPV16 in VIN and VaIN cases further underscores the role of HPV vaccines in mitigating precancerous lesions23,24,25. A Danish cohort study observed a lower incidence of VaIN 2/3 in vaccinated women aged 17–26 years than in their unvaccinated counterparts26.
While numerous studies have indicated correlations between liquid-based cytology results and VaIN grade, our study did not observe this phenomenon27,28,29,30. We hypothesized that inadequate sampling of the cervix and vagina is a contributing factor. In cases where patients with existing CIN still retained their cervix, the shedding of cervical cells into the vagina could occur. Therefore, the identification of low-grade squamous cells, metaplastic cells, or glandular cells did not necessarily signify the presence of VaIN. Consequently, cytological examination alone was not considered the primary screening method in our study.
In our study, patients with current high-grade CIN were more likely to have high-grade VaIN (VaIN 2/3), suggesting that VaIN stems from residual CIN lesions31,32. Thus, aggressive focus on vaginal lesions in patients diagnosed with CIN is recommended to prevent underdiagnosis. However, Aho M suggested that VaIN originating from cervical HSIL has a higher rate of spontaneous recurrence than that not originating from cervical HSIL (91% vs. 67%), indicating different biological behaviors33.
As a retrospective study, our findings have limitations. The mean age of VaIN patients in our study was relatively low (less than 45.5 years for VaIN 1 and 42.2 years for VaIN 2/3), contrary to the commonly observed onset of VaIN in the perimenopausal period (ages 45–55 years)34. Several reasons may account for this discrepancy: First, lower VaIN detection rates in postmenopausal women may be due to vaginal epithelial atrophy caused by estrogen decline, as suggested by Helen et al., who proposed preoperative treatment with estrogen cream to enhance vaginal mucosa elasticity and improve VaIN detection rates35. Second, increased diagnosis rates of VaIN in younger women due to HPV testing and liquid-based cytology popularity may skew admission rates. Finally, the relatively small sample size of our study could have contributed to these findings. Additionally, several limitations merit consideration. The retrospective design may introduce selection bias due to reliance on historical data completeness. As a single-center study from a tertiary hospital, our cohort may reflect institutional referral patterns, potentially limiting generalizability to broader populations. The modest sample size reduces statistical power for subgroup analyses and increases type II error risk. These constraints underscore the need for prospective multicenter validation with larger cohorts. Our findings establish HPV16 infection as a key etiological factor for VAIN, supporting HPV vaccination as an effective preventive strategy. This aligns with Danish population data demonstrating a 16% reduction in VAIN incidence among vaccinated young women following national immunization program implementation24.
Conclusions
In conclusion, it is recommended that women with persistent high-risk HPV infection undergo a thorough evaluation of the entire vagina to prevent potential underdiagnosis of VaIN. Our study emphasizes the importance of screening and monitoring patients with a history of high-grade CIN and ongoing HPV16 infection. Additionally, pre-treatment vaginal assessment before addressing cervical lesions is crucial in determining the surgical procedures. Postoperative follow-up should include diligent screening for HPV to facilitate early detection and treatment of VaIN.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Cree, I. A., White, V. A., Indave, B. I. & Lokuhetty, D. Revising the WHO classification: female genital tract tumours. Histopathology 76, 151–156 (2020).
Cong, Q. et al. A retrospective study of cytology, high-risk HPV, and colposcopy results of vaginal intraepithelial neoplasia patients. BioMed Res. Int. 1–6 (2018).
Saslow, D. et al. American Cancer society, American society for colposcopy and cervical pathology, and American society for clinical pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 62, 147–172 (2012).
Perkins, R. B. et al. 2019 ASCCP Risk-Based management consensus guidelines for abnormal cervical Cancer screening tests and Cancer precursors. J. Low Genit. Tract. Dis. 24, 102–131 (2020).
Bouvard, V. et al. The IARC perspective on cervical Cancer screening. N Engl. J. Med. 385, 1908–1918 (2021).
Kim, M. K., Lee, I. H. & Lee, K. H. Clinical outcomes and risk of recurrence among patients with vaginal intraepithelial neoplasia: a comprehensive analysis of 576 cases. J. Gynecol. Oncol. 29, e6 (2018).
Zhang, L., Zhang, X., Sui, L. & Cong, Q. Risk factors for vaginal squamous intra-epithelial lesions in women with high-grade cervical lesions. Int. J. Gynecol. Cancer. 34, 1344–1348 (2024).
Kesic, V. et al. The European society of gynaecological oncology (ESGO), the international society for the study of vulvovaginal disease (ISSVD), the European college for the study of vulval disease (ECSVD), and the European federation for colposcopy (EFC) consensus statement on the management of vaginal intraepithelial neoplasia. Int. J. Gynecol. Cancer. 33, 446–461 (2023).
Yang, Y. et al. Effect of photodynamic therapy on vaginal intraepithelial neoplasia grade one: A retrospective cohort study. Photodiagnosis Photodyn Ther. 51, 104486 (2025).
Kim, J. H. et al. Risk factor and treatment of vaginal intraepithelial neoplasia after hysterectomy for cervical intraepithelial neoplasia. J. Low Genit. Tract. Dis. 26, 147–151 (2022).
Cao, D., Wu, D. & Xu, Y. Vaginal intraepithelial neoplasia in patients after total hysterectomy. Curr. Probl. Cancer. 45, 100687 (2021).
Gurumurthy, M., Leeson, S., Tidy, J. & Cruickshank, M. E. UK National survey of the management of vaginal intraepithelial neoplasia. J. Obstet. Gynaecol. 40, 694–698 (2020).
Li, K., Li, Q., Song, L., Wang, D. & Yin, R. The distribution and prevalence of human papillomavirus in women in Mainland China. Cancer 125, 1030–1037 (2019).
ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in China. Summary Report. https://Hpvcentre.Net/Statistics/Reports/CHN.Pdf?T=1674053488539
Yu, D., Qu, P. & Liu, M. Clinical presentation, treatment, and outcomes associated with vaginal intraepithelial neoplasia: A retrospective study of 118 patients. J. Obstet. Gynaecol. Res. 47, 1624–1630 (2021).
Qiu, C., Zhao, B., Wang, D. & Tang, H. Relationship between HPV-16 infection and the progression of vaginal intraepithelial neoplasia. Am. J. Transl Res. 15, 2049–2054 (2023).
Zhang, J., Chang, X., Qi, Y., Zhang, Y. & Zhang, S. A retrospective study of 152 women with vaginal intraepithelial neoplasia. Int. J. Gynecol. Obstet. 133, 80–83 (2016).
Zhou, F. Y. et al. Types and viral load of human papillomavirus, and vaginal microbiota in vaginal intraepithelial neoplasia: a cross-sectional study. Ann. Transl Med. 8, 1408–1408 (2020).
Ye, Y. et al. Characteristics of high-risk HPV infection in women with vaginal intraepithelial neoplasia in Beijing, China. J. Med. Virol. 95, e29267 (2023).
Lu, M. et al. Clinical characteristics and risk factors to high-grade vaginal intraepithelial neoplasia: a single-institution study. BMC Womens Health. 25, 44 (2025).
Zhou, Q. et al. Application of 2011 international federation for cervical pathology and colposcopy terminology on the detection of vaginal intraepithelial neoplasia. Cancer Manag Res. 12, 5987–5995 (2020).
Cong, Q., Fu, Z., Zhang, D. & Sui, L. Importance of colposcopy impression in the early diagnosis of posthysterectomy vaginal Cancer. J. Low Genit. Tract. Dis. 23, 13–17 (2019).
Smith, J. S., Backes, D. M., Hoots, B. E., Kurman, R. J. & Pimenta, J. M. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet. Gynecol. 113, 917–924 (2009).
Preti, M. et al. Human papillomavirus genotyping in high-grade vaginal intraepithelial neoplasia: A multicentric Italian study. J. Med. Virol. 96, e29474 (2024).
Shen, Y. et al. Human papillomavirus genotype attribution and integration in High-Grade vaginal intraepithelial neoplasia. J. Low Genit. Tract. Dis. 29, 60 (2025).
Bertoli, H. K. et al. Time trends in the incidence and survival of vaginal squamous cell carcinoma and high-grade vaginal intraepithelial neoplasia in Denmark – A nationwide population-based study. Gynecol. Oncol. 158, 734–739 (2020).
Sopracordevole, F. et al. Abnormal pap smear and diagnosis of High-Grade vaginal intraepithelial neoplasia: A retrospective cohort study. Med. (Baltim). 94, e1827 (2015).
Smeltzer, S., Yu, X., Schmeler, K. & Levison, J. Abnormal vaginal pap test results after hysterectomy in human immunodeficiency Virus-Infected women. Obstet. Gynecol. 128, 52–57 (2016).
Stuebs, F. A. et al. Cytology and HPV Co-Testing for detection of vaginal intraepithelial neoplasia: A retrospective study. Cancers 15, 4633 (2023).
Chen, Y. et al. Clinical characteristics and detection sensitivity of cervical Cancer screening in vaginal intraepithelial neoplasia. J. Low Genit. Tract. Dis. 28, 137 (2024).
He, Y. et al. Clinical analysis of cervical intraepithelial neoplasia with vaginal intraepithelial neoplasia. Med. (Baltim). 96, e6700 (2017).
Zhang, Y. Y., Xia, R., Chen, D. & Zhang, X. Analysis of related factors of cervical intraepithelial neoplasia complicated with vaginal intraepithelial neoplasia. Clin. Transl Oncol. 24, 902–908 (2022).
Aho, M., Vesterinen, E., Meyer, B., Purola, E. & Paavonen, J. Natural history of vaginal intraepithelial neoplasia. Cancer 68, 195–197 (1991).
Dong, H. et al. Clinical analysis of 175 cases of vaginal intraepithelial neoplasia. Eur. J. Obstet. Gynecol. Reprod. Biol. 287, 232–236 (2023).
Rhodes, H. E., Chenevert, L. & Munsell, M. Vaginal intraepithelial neoplasia (VaIN 2/3): comparing clinical outcomes of treatment with intravaginal Estrogen. J. Low Genit. Tract. Dis. 18, 115–121 (2014).
Funding
This study was supported by Natural Science Foundation of JiangSu Province of China (Number: BK20230222).
Author information
Authors and Affiliations
Contributions
Lu Shen: Conceptualization, Methodology, Writing-original draft preparation, Funding acquisition; Liuxuanning Zhou: Writing, Visualization; Xiaoxue Xi: Data curation, Manuscript editing and review; Shunyu Hou: Supervision, Funding acquisition; All authors approved the final of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This retrospective study was approved by Ethical Committee of Suzhou Municipal hospital (KL 901461). The informed consent template was uploaded.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, L., Zhou, L., Xi, X. et al. Retrospective analysis of 274 cases suspected vaginal intraepithelial neoplasia. Sci Rep 15, 17506 (2025). https://doi.org/10.1038/s41598-025-02629-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02629-0