Abstract
Loss to follow-up (LTFU) poses a major challenge to achieving the joint United Nations Programme on HIV/AIDS 95–95–95 targets and ending the HIV epidemic by 2030. Despite government efforts, high LTFU rates in the test-and-treat era underscore the need for updated strategies. This study aimed to identify incidence and predictors of lost to follow-up among children receiving antiretroviral therapy (ART) in Amhara region. A multicenter facility-based retrospective follow-up study was conducted on 486 children receiving ART in Amhara Region Comprehensive Specialized Hospitals from August, 2014, to March, 2023. A systematic random sampling technique was used to select the study participants. Data were collected using national antiretroviral intake and follow-up forms through the KoBo Toolbox. Data analysis was done using STATA version 17. Descriptive analyses were summarized using the tables, and figures were used to present. Both bivariable and multivariable competing regression model were fitted to identify predictors of LTFU. Finally, adjusted sub-hazard ratio with 95% Confidence Interval (CI) was computed, and variables having a p-value < 0.05 were considered as statistically significant predictors of LTFU. Among 455 (93.62%) patient charts were included in the final analysis, 13.19% and 6.81% of the individuals LTFU and death within the follow-up period respectively. In this study, the overall incidence of LTFU was found to be 3.67 per 100 child-year observations (95% (CI): 2.85, 4.73). HIV-infected children age less than five years [adjusted sub-hazard ratio (aSHR): 2.95 (95% CI: 1.34, 6.49)], rural residence [aSHR: 3.39 (95% CI: 2.02, 5.73)], no regimen change [aSHR: 1.98 (95% CI: 1.16, 3.38)], and ART side effect [aSHR: 1.92 (95% CI: 1.13, 3.24)] were predictors for LTFU. The incidence of LTFU among HIV-infected children remains high, with younger age, rural residence, regimen changes, and ART side effects identified as key predictors. Strengthening counseling services, monitoring and managing ART side effects, and implementing an ART outcome evaluation program could help reduce LTFU.
Similar content being viewed by others
Introduction
Human immunodeficiency virus (HIV) is a virus that causes acquired immunodeficiency syndrome (AIDS) by reducing the body’s immune system1. Starting from epidemic of HIV/AIDS 40.4 million people were dead due to AIDS related disease and recently 39.9 million people were living HIV/AIDS. Globally, it has been estimated that out of 1.3 million children living with HIV, 57% of them received ART, 48% of them had viral suppression, and 76,000 children dying from HIV related causes at the end of 20232. The progression of HIV infection in children is especially rapid in the absence of HIV care ART3.
Antiretroviral therapy determined impact and outcome could be achieved when the children on ART had good adherence to regular follow-ups, and lead to healthy and productive lives4. Loss to follow-up in ART is defined as the failure to remain engaged in the continuum of care for 90 days (3 months) after the last scheduled appointment5.
LTFU is a major challenge following the initiation of antiretroviral therapy. Globally, 14–28% of children discontinue ART within the first two years, while in Sub-Saharan Africa it increases, an estimated 20–40% face LTFU6,7,8. The incidence of LTFU varies between countries. In Myanmar, it is reported at 4.7 per 100 child-years observations (CYO)9, while in Asia and Africa, it stands at 4.1 per 100 CYO8. In South Africa, the rate ranges from 7.5 to 10.8 per 100 CYO10,11, and in Ethiopia it ranges from 3.3 to 9.12 per 100 CYO12,13,14,15,16,17.
Several factors contribute to LTFU among HIV-infected children on ART. These include younger age, advanced stages of HIV, presence of opportunistic infections, poor adherence to ART drugs, rural residency, non-disclosure of HIV status, and malnutrition14,17,18,19,20.
Loss to follow-up is a significant barrier to achieving the joint United Nations Programme on HIV/AIDS 95–95–95 targets by 2025 and ending the HIV epidemic by 20306. Children who discontinue ART face increased risks of side effects, including the development and spread of drug-resistant HIV strains. This resistance undermines future treatment options and the effectiveness of HIV programs, disease progression, decline quality of life, increases mortality, and health care system challenges to public health goals2. Therefore, it is a crucial to improving health outcome and ensuring the success ART programs in children.
The Ethiopian ministry of health implementing strategies to reduce follow-up rates, including monitoring and evaluations, treating opportunistic infections, reliable outcome, early mortality reductions, un-interrupted drug supplies, non-toxic ART regimen, and decentralization of care2,5,21.
Despite government efforts, the rate of loss to follow-up (LTFU) remains high in the test-and-treat era, highlighting the need for updated information to improve strategies. While many studies have examined LTFU and its predictors, accurate estimates are crucial, particularly when accounting for death as a competing event. However, most studies overlook this factor, potentially leading to misleading results. This study aims to estimate the incidence and identify predictors of LTFU, considering death as a competing risk, in Comprehensive Specialized Hospitals in the Amhara region, Northwest Ethiopia.
Methods
Study design, study setting and period
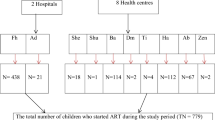
A multicenter hospital-based retrospective follow-up study was employed. The study was conducted among children aged under-15 years who were attending ART follow-up at ART center in comprehensive specialized Hospitals of Amhara region, Ethiopia, from August 2014 to March 2023. Amhara region is located in the Northwestern, North eastern and North-central parts of Ethiopia with an estimated area of 159,173.66 square kilometers, and the recent estimated population in this region is 30,848,98822.
The region covers 159,173.66 square kilometers, has 858 Health Centers, 3560 Health Posts, and 81 hospitals, and eight comprehensive specialized hospitals (CSHs). Study was conducted on these CSHs are University of Gondar, Felege Hiwot, Debre Markos, Debre Tabor, Dessie, Woldia, and Debre Birhan except Tibebe-Ghion CSH. These hospitals provide multidimensional care, including surgical, medical, pediatrics, and maternal health services. Since 2005, these hospitals have provided free ART services as part of the National AIDS Control Programme.
Study participants
The records of all HIV-infected children, whoever started ART at Amhara region comprehensive specialized Hospitals, were the source population. The records of all HIV-infected children receiving ART between July 2014 to March 2023, and whose charts were available during the data collection period were our study population. Children who had at least one month of ART follow-up during the study period were included whereas, children who had incomplete baseline records, unknown date of the outcomes variable, and transferred in from other health institutions without baseline information were excluded. From the total of 486 sample size, 31 medical charts were excluded due to incompleteness.
Sample size determination, sampling procedures, and sampling technique
The sample size was determined using double population proportion formula by considering alpha 5%, power 80% using Epi Info version 7.2 considering different predic(Table 1:).
P1: is the percent of exposed with the outcome, P2: is the percent of non-exposed with the outcome, Zα/2: is taking CI 95%, Zβ: 80% power and r is the ratio of non-exposed to exposed 1:1. Then the largest sample size was 486, so we considered this as the final sample. The sample was allocated proportionally for those Comprehensive Specialized Hospitals of the Amhara region, and records were selected using systematic random sampling techniques.
Variables of the study
The study variable was incidence of LTFU. The independent variables were socio-demographic variables were age of the child, sex of the child, residence, and marital status of the caregiver, educational status of the caregiver, HIV disclosure status, and parental status for the child. Baseline clinical characteristics, Anthropometric indices and laboratory tests variables were WHO clinical staging, CD4 count/percentage, hemoglobin level, viral load, functional and developmental status, weight for age (WFA), height for age (HFA), and weight for height (WFH)), and baseline OIs. ART and other medications-related variables included baseline ART regimens, duration of ART, ART side effects, presence regimen change, treatment failure, taking TPT, taking CPT, Neverapine and Zidovidine contained ART drugs, adherence to ART, and initiation of ART.
Operational definition
LTFU (Event) is defined as not taking ART refill for 3 months or longer from the last attendance for a refill and not yet classified as “dead” or “transferred-out”21.
The competing event was death which was recorded as the death of the child.
Censored: individuals who were formally transferred- out to other health institutions after initiating ART and or individuals who remain active on ART follow-up at the end of the study. ART adherence levels: Good adherence is a compliance of 95% or higher or ≤ 3 missed doses per month, Fair adherence is a compliance between 85% and 94% or between 4 and 8 missing doses per month, poor adherence is compliance below 85%, or ≤ 9 more missed doses per month as documented by the ART health personnel5 .
Data collection tool, and procedures, data quality control
The data were retrieved from the ART intake and follow-up form, and children`s charts using the data extraction tool adopted from Ethiopian ART guidelines and registered at ART clinics during the period of July, 2014 to March, 20235. The most recent clinical and laboratory tests at ART initiation were considered as baseline information.
The data extraction tool was pretested on 5% of the sample size before the actual data collection period at UoG Comprehensive Specialized Hospital. During this pretest, 24 medical charts were reviewed to assess data completeness and ensure the clarity of the variables. Based on the findings, amendments were made to improve the tool and process. New variables, such as the HIV status of the parents and disclosure status of the child were added to enhance the comprehensiveness of the data. Additionally, correction were made how to supervisor the data collector, the confidentiality, data via KoBo toolbox. Additionally, corrections were made to improve the supervision of data collectors, strengthen confidentiality measures, and optimize data collection using the KoBo Toolbox platform. One-day onsite training was given. Data were collected using the KoBo toolbox which was prepared with relevant restrictions by trained nurses working in the Hospitals.
Data processing and analysis
The data was coded, and entered in to KoBo tool box and then exported to STATA version 17 for final analysis. Descriptive statistics such as proportions, tables, and charts was done to describe the characteristics of the study participant.
The follow-up time or time at risk was calculated from the date of ART initiation to either the occurrence of an event (lost to follow-up), computing variable (death) or the censoring date, defined as the end of the study period. The total follow-up time was expressed in both child-month observations (CMO) and child-year observations (CYO). CMO was calculated by summing the total months each child remained in the study, while CYO was derived by dividing the total follow-up time in months by 12. The total number of lost to follow up cases per 100 person-year observations or person month observation was calculated and labeled as the incidence.
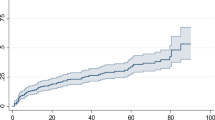
The cumulative incidence function (CIF) was estimated non-parametrically using Gray’s test and a graphic representation. Following model fitting, the proportional sub-distribution hazard assumption was also verified using the Schoenfeld residual test and the plot of log (- log (1-CIF)) against the log of time to failure for each covariate by interacting each covariate with time. Proportional hazards assumption was checked using Schoenfeld residuals or Global Test (p = 0.808); no significant violation was found.
Bivariable competing regression analysis was fitted to identify factors associated with LTFU. Those variables with a p-value of < 0.25 in the bivariable analysis were again fitted to the multivariable competing risk regression analysis. Both crude and adjusted sub-distribution hazard ratio with the corresponding 95% CI was calculated to show the strength of association. In multivariable analysis, variables with a P-value of < 0.05 were considered statistically significant.
Incomplete data were handling and managed through multiple imputations using multivariable chained equations (MVCE) were carried out to handle and manage missing data. The variables missing at random (MAR) are clarified by the “Little test” results for continuous variables and graphical patterns for categorical variables, making the application of multiple imputations straightforward. Furthermore, sensitivity analysis was performed utilizing both descriptive and inferential statistical techniques to confirm if a significant difference was detected between the outputs of the original and imputed data.
Ethical consideration
Ethical approval was obtained from the Institutional Review Board (IRB) of the School of Nursing, College of Medicine and Health Science, University of Gondar with reference No. (Ref.no.SN/102/2015 E.C). The IRB of the School of Nursing, College of Medicine and Health Science has waived informed consent for the medical records of the children. The letter of permission was obtained from each Comprehensive Specialized Hospital clinical director and head of the unit. To maintain confidentiality personal identifiers were not recorded. All of the procedures were carried out by considering the Declaration of Helsinki.
Results
Baseline socio-demographic characteristics
A total of 455 (93.62%) patient charts were included in the analysis. Nearly 60% (266) of the study participants were males. 29% of the children were in the age group of < 5 years, 58.46% of the children were males. On parent characteristics, 54.95% were married, one third of them had no formal education, and 70.99% of them were alive. One-fifth of children had CD4 cell count below the threshold, and about 15.6% had anemia; 30.57% and 48.79% nutritional statuses were wasted and stunted, respectively. In this study, the magnitudes of good adherence to ART, intake of TPT, and CPT during the follow-up period by the children were 70.55%, 63.96%, and 82.64%, respectively (Table 2).
The incidence of LTFU
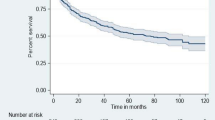
Four hundred and fifty-five HIV-infected children on ART had a follow-up time from 1 to 93 months. The observation of total time at risk was 19602.00 child-per year observations (CMO) or 1633.5 child-year observations (CYO). From the total enrolled HIV-infected children on ART, during the follow-up 13.19% (95% CI: 10.4–16.6%) developed the event of interest, and 6.81% (95% CI: 4.8–9.5%) were death. (Fig. 1)
The overall incidence of LTFU and death were 3.67 (95% CI; 2.85, 4.73) and 1.9 (95% CI; 1.33, 2.7) per 100 CYO.
Predictors for incidence of LTFU among HIV-infected children
In the bivariable competing risk regression analysis, variables under study were predictors such as age, residence, parental status of the care givers, baseline WHO clinical staging, CD4 count, functional & developmental status, regimen change, treatment failure, ART side effect, stunting, and previous OI. However, in the multivariable competing risk regression analysis, only factors such as age, residence, ART regimen change, and ART side effect were found to be significant predictors for LTFU at 5% level of significance.
In our study keeping other variables constant the sub-hazard of LTFU among children presented with age less than five years was 3 times [aSHR: 2.95 (95% CI: 1.34, 6.49)] higher than as compared to those children with ≥ 10 years. Additionally, the sub-hazard of LTFU among children came from rural residence was 3.39 times [aSHR: 3.39 (95% CI: 2.02, 5.73)], more likely as compared to urban residence. The sub-hazard of LTFU is 1.98 times higher among children who had not regimen change compared to those counter parts [aSHR: 1.98 (95% CI: 1.16, 3.38)].
Regarding ART side effect, sub-hazard of LTFU is 1.9 times higher among children who had side effect compared to their counter parts [aSHR: 1.92 (95% C: 1.13, 3.24)] (Table 3):
Discussion
This study identified the incidence and predictors of LTFU among HIV-positive children receiving ART using a multicenter facility-based retrospective cohort study in Amhara region comprehensive specialized hospitals. At the end of follow-up, about 13.19% of patients were LTFU. The overall incidence of LTFU of this study was 3.67 per 100 CYO (95% CI: 2.85, 4.73), which is aligning with findings from various regions: 4.1 and 4.7 LTFU per 100 CYO in Asia and8 and Myanmar9 respectively, in Debre Markos Ethiopia17 and in southern Ethiopia23.
Conversely, our study finding is much lower than studies conducted in India (14.4 per 100 CYO)24, South Africa (10.8 per 100 CYO)11, Tanzania (18.2 per 100 CYO)25, multi-study in Kenya, Mozambique, Rwanda, and Tanzania 14.2 per 100 CYO26, Malawi (12.6 per 100 CYO)27, in Ethiopia (6.2, 6.3, and 5.2 per 100 CYO)12,14,23.
The variations might be due to the different interventions implemented by Ethiopian government, different sample size, study setting, length of follow up11, and measurement variability in LTFU. The current guideline recommends frequent visits with advanced support and family involvement care such as adherence support through phone calls, case tracing through home visits, proactive use of health extension workers5,28. Another possible reason could be clinical characteristics of study participants, in our study only 20% of study participants are CD4 below the threshold at baseline, 18% have advanced HIV diseases, and 82.64% of study subjects taken ART prophylaxis. Dolutegravir-based combination therapy significantly lowers the risk of detectable virological replication relative to older regimens, and subsequently contributes to a reduced incidence of lost to follow-up29. Furthermore, this might be the introduction of dolutegravir (DTG) has revolutionized ART outcomes due to its improved tolerability, lower risk of resistance, and overall effectiveness. These advancements have indirectly influenced the outcomes variables30.
In this study, the sub-hazard of LTFU among children presented with age less than five years was 3 times higher than as compared to those children with ≥ 10 years. This finding is consistent with studies conducted in Ethiopia12,13,19,31,32, Botswana, Nigeria33, Indonesia34, Thailand35, and Asia36, Spain37. This might be due to Young children rely entirely on caregivers for accessing healthcare services, including ART follow-ups, Poor communication between healthcare providers and caregivers can lead to misunderstandings about the importance of ART adherence and regular follow-up visits, due to more risk for malnutrition and OIs that increase disease progression rapidly and lastly causes LTFU5. Other might be due to clinical characteristics of study participants.
The risk of LTFU among children came from rural residence was 3.39 more likely as compared to urban residence. This finding is supported by study conducted in Ethiopia13,16,19, Malawi38, and Nigeria33, Asia and Africa8. Rural residence is affecting their practice on the regular ART follow-up and health seeking behavior due to the absence of nearby ART centers and lack of transportation costs39,40. Additionally, might be due to limit their family access to information41 and absence of services for chronic diseases conditions may also contribute to LTFU among rural residence, may be due to it takes extra time devoted to waiting for different diagnosis and treatment services, commonly seek faith healing or traditional therapy42,43.
Additionally, children whose regimen was not change were two times more at risk to LTFU compared to those whose regimen was changed. The finding is consistence with study conducted in Ethiopia12,44, India24. This might be due to most of old regimen have side effects that causes advanced diseases and complications42. A lack of regimen change can affect LTFU because it may signals to patients that their needs or challenges are not being addressed, reducing their confidence and commitment to continuing care.
Furthermore, the risk of LTFU is 1.9 times higher among children who had side effects compared to those who do not, as supported by study conducted in Ethiopia44. In our study Skin rash, abdominal pain, anemia, peripheral neuropathy and diarrhea were the most common encountered side-effect of antiretroviral therapy, which lead to treatment discontinuation5,45. Additionally, side effects may cause patients to lose confidence in the medication, leading to lost from HIV care and support services46. To overcome such side-effects in the clinical workflows shall be managed by routine side-effect monitoring, offer up-front and ongoing counseling about potential side effect, develop individualized plans to managed side effect, create a system where patients can report side effect, and ensuring timely interventions.
Limitation of the study
The present study does have some inherent limitations. First, since the data were collected retrospectively, the study depends on the pre-existing recorded information on the type of diseases during follow-up that missed important variables. Second, as data were collected from secondary sources, issues with data completeness and loss were inevitable. These gaps may have introduced bias and reduced the robustness of the findings. Third, the retrospective nature of the study made it challenging to assess critical aspects such as clinical and immunological responses during the follow-up period. Additionally, potential confounders like social determinants of health, caregiver support, and economic factors may influence outcomes, limiting the study’s generalizability.
Though this manuscript has been release before an updated WHO definition of LTFU or IIT, we acknowledges the recent evidence, regardless of them sticking to the old definition of LTFU in the 2021 national guidelines. Future research should address these limitations by adopting prospective study designs and integrating a broader range of variables. Furthermore, studies should further explore the long-term impact of DTG on ART adherence and outcomes.
Conclusions and recommendations
Still, the incidence rate of LTFU is found to be high. Since death precludes the observation of lost to follow-up, a competing risk regression analysis was conducted to examine the predictive variables of LTFU, taking death into account as a competing event. Non-modifiable risk factor (Age less than five years and being rural residence), regimen change, and ART side effects were found to be predictors for LTFU. Enhancing counseling services, monitoring side effects, and implementing ART outcome evaluation programs can help to reduce LTFU. Community education, expanding health extension services, and raising awareness are also effective strategies.
Additionally, further qualitative studies are needed to explore contributing factors to LTFU among children. For instance, conducting interviews with caregivers or health workers could provide deeper insights into barriers related to regimen changes or side effects, explore how patients and caregivers perceive the efficacy of ART, the psychological and the social factors influencing treatment adherence.
Data availability
All data relevant to the study are included in the article.
Abbreviations
- aSHR:
-
Adjusted sub-hazard ratio
- AIDS:
-
Acquired immune deficiency syndrome
- ART:
-
Antiretroviral therapy
- CD4:
-
Cluster of differentiation 4
- CPT:
-
Cotrimoxazole preventive therapy
- CI:
-
Confidence interval
- CSHs:
-
Comprehensive specialized hospitals
- CYO:
-
Child-year-observations
- HIV:
-
Human immunodeficiency virus
- OIs:
-
Opportunistic infections
- WHO:
-
World Health Organization
References
Cohen, M. S. et al. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. J. Clin. Investig. 118(4), 1244–1254 (2008).
Orginization, W. H. HIV and AIDS. (2024).
Organization, W. H. Taking Stock: HIV in Children: the State of Affairs (World Health Organization, 2006).
Yayehirad, A. M. et al. Rate of immunological failure and its predictors among patients on highly active antiretroviral therapy at Debremarkos hospital, Northwest Ethiopia: a retrospective follow up study. J. AIDS Clin. Res. 4(5). (2013).
FMOH National Comprehensive HIV Prevention, Care and Treatment Training for Healthcare Providers, M.o. Health, Editor. Revised on August, 2021, Ethiopian Federal Ministry of Health Addis Ababa. 1–502.
Rosen, S., Fox, M. P. & Gill, C. J. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 4(10), e298 (2007).
Saumu, W. M. et al. Predictors of loss to follow-up among children attending HIV clinic in a hospital in rural Kenya. Pan Afr. Med. J., 32(1). (2019).
Leroy, V. et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. JAIDS J. Acquir. Immune Defic. Syndr. 62(2), 208–219 (2013).
Kaung Nyunt, K. K. et al. Factors associated with death and loss to follow-up in children on antiretroviral care in Mingalardon Specialist Hospital, Myanmar, 20062016. PLoS ONE. 13(4), e0195435 (2018).
Sengayi, M. et al. Predictors of loss to follow-up among children in the first and second years of antiretroviral treatment in Johannesburg, South Africa. Glob Health Action. 6, 19248 (2013).
Chandiwana, N. et al. High loss to follow-up of children on antiretroviral treatment in a primary care HIV clinic in Johannesburg, South Africa. Medicine 97(29), e10901 (2018).
Menshw, T. et al. Incidence and predictors of loss to follow-up among children attending ART clinics in northeast Ethiopia: a retrospective cohort study. HIV/AIDS-Res. Palliat. Care. 801–812 (2021).
Biru, M. et al. Rates and predictors of attrition among children on antiretroviral therapy in Ethiopia: a prospective cohort study. PloS One. 13(2), e0189777 (2018).
Fisiha Kassa, S. et al. Incidence of loss to follow-up and its predictors among children with HIV on antiretroviral therapy at the University of Gondar comprehensive specialized referral hospital: a retrospective data analysis. HIV/AIDS-Res. Palliat. Care.. 525–533 (2020).
Sifr, Z. et al. Level of attrition from antiretroviral therapy among human immune deficiency virus-infected children: the cases of Sidama zone, southern Ethiopia. HIV/AIDS-Res. Palliat. Care. 813–822 (2021).
Bankere, A. W. et al. Lost to follow-up and its predictors among human immune deficiency virus infected children on antiretroviral therapy, southern Oromia, and Ethiopia: a five year retrospective cohort study. (2022).
Hibstie, Y. T. et al. Nearly one in every six HIV-infected children lost from ART follow-up at Debre Markos referral hospital, Northwest Ethiopia: A 14-year retrospective follow-up study. PLoS One. 15(9), e0239013 (2020).
Girma, D. et al. Incidence of lost to follow up among HIV-positive children on antiretroviral therapy in Ethiopia: systematic review and meta-analysis. Plos One. 19(5), e0304239 (2024).
Birhanu, M. Y. et al. Incidence and predictors of loss to follow-up among Ethiopian children on antiretroviral therapy: a systematic review and meta-analysis. BMC Public. Health. 24(1), 169 (2024).
Alemu, G. G. et al. Incidence of loss to follow-up and its predictors among HIV-infected under-five children after initiation of antiretroviral therapy in West Amhara comprehensive specialized referral hospitals, Northwest Ethiopia: a multicenter retrospective follow-up study. BMC Pediatr. 24(1), 615 (2024).
ICAP. Facality level standard oprating procedures for HIV care and treatment service. (2018).
Federal Democratic Republic of Ethiopia Central Statistical Agency, Population Projection of Ethiopia for All Regions At Wereda Level from 2014–2017. Archived from the original on 6. 2018.June (2018).
Bimer, K. B. et al. Incidence and predictors of attrition among children attending antiretroviral follow-up in public hospitals, Southern Ethiopia, 2020: a retrospective study. BMJ Paediatr. Open. 5(1). (2021).
Alvarez-Uria, G. et al. Predictors of loss to follow-up after engagement in care of HIV-infected children ineligible for antiretroviral therapy in an HIV cohort study in India. Germs 4(1), 9–15 (2014).
McCormick, N. M. et al. Implementation and operational research: risk factors of loss to Follow-up among HIV-Positive pediatric patients in Dar Es Salaam, Tanzania. J. Acquir. Immune Defic. Syndr. 70(3), e73–83 (2015).
McNairy, M. L. et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda, and Tanzania. J. Acquir. Immune Defic. Syndr. 62(3), e70–81 (2013).
Ardura-Garcia, C. et al. Implementation and operational research: early tracing of children lost to follow-up from antiretroviral treatment: true outcomes and future risks. J. Acquir. Immune Defic. Syndr. 70(5), e160–e167 (2015).
WHO Guidelines Approved by the Guidelines Review Committee. In Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. (World Health Organization, 2016).
Parienti, J. J. et al. Forgiveness of dolutegravir-based triple therapy compared with older antiretroviral regimens: a prospective multicenter cohort of adherence patterns and HIV-RNA replication. In Open Forum Infectious Diseases. (Oxford University Press US, 2021).
Schneider, S. et al. Two-drug regimens dolutegravir/lamivudine and dolutegravir/rilpivirine are effective with few discontinuations in US real-world settings: results from the TANDEM study. Infect. Dis. Therapy. 13(4), 891–906 (2024).
Bankere, A. W. et al. Loss to follow-up and its predictors among children living with HIV on antiretroviral therapy, Southern oromia, Ethiopia: a 5-year retrospective cohort study. BMJ Open. 14(7), e078370 (2024).
Berheto, T. M., Haile, D. B. & Mohammed, S. Predictors of loss to follow-up in patients living with HIV/AIDS after initiation of antiretroviral therapy. North. Am. J. Med. Sci. 6(9), 453 (2014).
Akahara, C. et al. Assessment of antiretroviral treatment adherence among children attending care at a tertiary hospital in southeastern Nigeria. J. Trop. Med. 2017(1), 3605850 (2017).
Juergens, S. et al. Predictors of loss to follow up and mortality among children ≤ 12 years receiving anti retroviral therapy during the first year at a referral hospital in Bali. Public. Health Prev. Med. Archive. 4(2), 101–106 (2016).
Teeraananchai, S. et al. Attrition and mortality of children receiving antiretroviral treatment through the universal coverage health program in Thailand. J. Pediatr. 188, 210–216 (2017). e1.
Huy, B. et al. Impact of Orphan Status on HIV Treatment Outcomes and Retention in Care of Children and Adolescents in Asia, 227–231 (Elsevier, 2016).
Palladino, C. et al. Determinants of highly active antiretroviral therapy duration in HIV-1-infected children and adolescents in Madrid, Spain, from 1996 to 2012. PLoS One. 9(5), e96307 (2014).
Ardura-Garcia, C. et al. Implementation and operational research: early tracing of children lost to follow-up from antiretroviral treatment: true outcomes and future risks. JAIDS J. Acquir. Immune Defic. Syndr. 70(5), e160–e167 (2015).
Helova, A. et al. Health facility challenges to the provision of option B + in Western Kenya: a qualitative study. Health Policy Plann. 32(2), 283–291 (2017).
Nabukeera-Barungi, N. et al. Adherence to antiretroviral therapy and retention in care for adolescents living with HIV from 10 districts in Uganda. BMC Infect. Dis. 15, 1–10 (2015).
Hub, R. H. I. Healthcare Access in Rural Communities. (2024).
Baldé, A. et al. Risk factors for loss to follow-up, transfer or death among people living with HIV on their first antiretroviral therapy regimen in Mali. HIV Med. 20(1), 47–53 (2019).
Bilinski, A. et al. Distance to care, enrollment and loss to follow-up of HIV patients during decentralization of antiretroviral therapy in Neno district, Malawi: A retrospective cohort study. PloS One. 12(10), e0185699 (2017).
Berheto, T. M., Haile, D. B. & Mohammed, S. Predictors of loss to follow-up in patients living with HIV/AIDS after initiation of antiretroviral therapy. N Am. J. Med. Sci. 6(9), 453–459 (2014).
Deribe, K. et al. Defaulters from antiretroviral treatment in Jimma university specialized hospital, Southwest Ethiopia. Trop. Med. Int. Health. 13(3), 328–333 (2008).
Tiruneh, Y. M. et al. Retention in care among HIV-infected adults in Ethiopia, 2005–2011: A mixed-methods study. PLoS One. 11(6), e0156619 (2016).
Acknowledgements
First, we would like to express my deepest gratitude to the University of Gondar, College of Medicine and Health Sciences, School of Nursing. We would like thank all comprehensive referral hospital administration and ART clinic staff for their contribution.
Funding
Financial support was obtained from University of Gondar. The funding institution has no role in the preparation of the manuscript as well as the decision to publish.
Author information
Authors and Affiliations
Contributions
GBM worked on title selection, designing the study, being involved in proposal writing, training and supervising the data collectors, analyzing and interpreting the results, and preparing the manuscript. BAT, FDB, DKM, ME, TMA, YEA, GK, TDE, BD, and AAA played their role on critical on participating in its design, writing on result, and discussion. BTL, WTW, AK, LYB, TLE, KHS, and AAB actively engaged in critically revising the proposal, analyzing and interpreting the results, and writing the manuscript. All authors were involved in reading and approving the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mekonnen, G.B., Tilaye, B.A., Baye, F.D. et al. Incidence and predictors of lost to follow up among children receiving antiretroviral therapy a computing risk regression model. Sci Rep 15, 17447 (2025). https://doi.org/10.1038/s41598-025-02645-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02645-0