Abstract
Gastric signet cell carcinoma (GSRC) is a special type of gastric cancer. Due to its high metastatic rate patients usually have a poor prognosis. Accurately predicting the survival time of these patients and selecting the best treatment strategy are urgent clinical questions. All patients included in the study were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. The demographic and clinical information of these patients was subjected to Cox regression analysis using SPSS software. The risk factors that were subsequently screened were used to construct and validate the nomogram through R software. A total of 1003 patients were included in this study. After Cox regression analysis, six variables (T stage, surgery status, chemotherapy, metastases status of bone, liver and lung) were identified as risk factors that independently influenced the patients’ prognosis. These variables were used to construct a nomogram, which was shown to have good predictive power by receiver operating characteristic curves and calibration curves. The AUC values for 3-, 6-, and 12-month OS prediction were 0.845, 0.793, and 0.751 in the training cohort, and 0.800, 0.735, and 0.693 in the validation cohort and decision curve analysis indicating that this nomogram could result in a good clinical benefit for the patient. We have successfully developed and validated a nomogram that accurately predicts 3-, 6- and 12-month overall survival in patients with metastatic GSRC. This can provide a theoretical basis for clinical practice and help clinicians to choose the best treatment strategy for their patients.
Similar content being viewed by others
Introduction
Gastric cancer is the fifth most prevalent cancer in the world, and according to the International Agency for Research on Cancer (IARC), more than one million people are diagnosed with it each year, and about 800,000 die from the disease1.Morbidity and mortality rates vary considerably around the world, with developed countries typically having lower rates and developing countries having higher rates. Among them, gastric signet ring cell carcinoma (GSRC) is a rare but highly aggressive malignant tumor of the gastrointestinal tract2. It is characterized by the presence of large amounts of mucin in the tumor cells, causing the nucleus to be pushed to the edge of the cell, giving it the characteristic imprinted cell morphology for which it is named3. It is highly invasive, easily penetrates the gastric wall and develops early peritoneal metastasis, which leads to advanced stage at diagnosis, insensitivity to conventional chemotherapy, high drug resistance due to the complex tumor microenvironment, and a significant risk of postoperative recurrence.
Since gastric signet ring cell carcinoma (GSRC) often has no obvious symptoms in the early stage and is highly aggressive, it is often detected at an advanced stage. This leads to the poor prognosis of gastric impression cell carcinoma. Generally speaking, patients with early diagnosis and treatment of GSRC have a better prognosis, with a five-year survival rate of more than 50%4. However, due to its invasiveness, often advanced stage at the time of diagnosis and resistance to conventional treatment, the prognosis of patients with advanced or metastatic gastric signet ring cell carcinoma is poor and early death is common.
In terms of treatment, the therapeutic strategy for GSRC usually needs to be determined based on the clinical stage of the tumor and the overall condition of the patient. For early-diagnosed GSRC, surgical resection is the treatment of choice, and surgical procedures such as local excision or total gastrectomy can be chosen according to the location and size of the tumor. For advanced or metastatic GSRC, chemotherapy and radiotherapy are usually used in combination with surgery to prolong patients’ survival time and improve their quality of life5.
Nomogram is a graphical tool for predictive modelling and clinical decision making6,7. It helps physicians predict the probability of specific outcomes for individual patients, such as survival, disease recurrence, or response to treatment, by integrating multiple variables into a simple scoring system. Nomogram is intuitive and easy to understand, which helps physicians better assess patient risk, develop individual treatment plans, and make it easier to apply in the clinic than traditional statistical models.
There is a lack of short-term survival prediction tools for metastatic GSRC, and existing models mostly focus on long-term prognosis. This study assessed the risk of early death in patients with metastatic GSRC by predicting the probability of survival at 3-, 6- and 12- months. It aims to provide a reliable reference for clinicians to select more appropriate treatment strategies for patients with metastatic GSRC.
Methods
Data collection
All patient demographic and clinical information in this study was extracted from the Surveillance, Epidemiology, and End Results (SEER) database (version 8.4.0.1). The study was ethically exempted as no private patient information was involved.
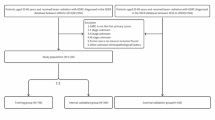
Inclusion criteria were as follows: (1) Diagnosed with gastric signet ring cell carcinoma (ICD-O-3 code: 8490/3) between 2000 and 2015 (2) AJCC (AJCC 7th edition) M stage of M1 Exclusion criteria were as follows: (1) survival time and survival status unknown (2) AJCC T-stage of T0 (3) other clinical information (e.g., age, sex, surgical status, etc.) unknown. A total of 1003 patients were included in the study.
Statistical analysis
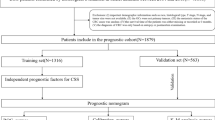
The total cohort was randomly divided into a training set and a validation set in a 7:3 ratio. Cox proportional hazards regression was used for multivariate analysis to identify independent prognostic factors in thyroid cancer patients who did not undergo surgery.
The COX risk regression model is a semiparametric survival analysis method for assessing the effect of multiple covariates on the risk of an event occurring. In the training set, univariate and multivariate Cox regression analyses were conducted to identify risk factors affecting postoperative overall survival (OS). Using R software, A nomogram to predict 3, 6, and 12-month OS was developed based on the multivariate Cox regression results. Receiver operating characteristic (ROC) curves were generated, and the area under the curve (AUC) was calculated to assess the model’s discrimination ability. Calibration curves and decision curve analysis (DCA) was performed for 3, 6, and 12 months to evaluate predictive accuracy and clinical utility. X-tile software was used to determine the optimal cutoff point for the nomogram score, categorizing patients into three risk groups. Kaplan-Meier survival curves were plotted based on these risk groups. All statistical analyses and charting were conducted using SPSS (version 25.0) and R software (version 4.3.1). A two-tailed P-value of less than 0.05 was considered statistically significant.
Results
Demographic and clinical information characteristics of patients
After screening, a total of 1003 patients were included in the study (Fig. 1). These patients were randomized in a 7:3 ratio into training cohort and validation cohort by R software. Clinical information of these patients was included in the study, including survival time, survival status, age, sex, race, AJCC TNM stage, surgery status, radiotherapy, chemotherapy, tumor grade, metastasis status of bone, brain, and tumor grade. metastasis status of bone, brain, liver and lung (Table 1).
Univariate and multivariate cox survival regression analyses
After univariate Cox regression analysis, some of the variables were screened as independent influences that may affect 3-, 6-, and 12-month OS in GSRC patients with metastasis. All the variables were shown in Table 1, age, sex, T stage, N stage, surgery status, chemotherapy, metastasis status of bone, liver and lung. The results of this study are summarized in the Table 2, which shows that overall survival was significantly associated with T stage (HR = 0.822, 95% CI 0.676–0.998; P = 0.048 for “T4”), surgery status (HR = 2.152, CI 1.699–2.726; P < 0.01 For “Yes”), chemotherapy (HR = 3.199, CI 2.675–3.825; P < 0.01 For “Yes”), metastasis status of bone (HR = 0.750, CI 0.590–0.952; P = 0.018 For “Yes”), liver (HR = 0.775, CI 0.617–0.973; P = 0.028 For “Yes”) and lung (HR = 0.727, CI 0.552–0.957; P = 0.023 For “Yes”). survival (P < 0.05). The above variables were included in multivariate Cox regression analyses to exclude confounding between variables. Finally, T stage, chemotherapy, surgery status, metastases status of bone, liver and lung proved to be independent risk factors affecting 3-, 6-, and 12-month OS in GSRC patients with metastasis(Table 2).
Construction and validation of nomogram predicting OS of patients with GSRC
Six independent risk factors (T stage, chemotherapy, surgery status, metastasis of bone, liver and lung) demonstrated by univariate and multivariate Cox regression analyses were used to construct a 3-, 6-, and 12-month prediction model for OS of GSRC patients with metastasis (Fig. 2). As shown in Fig. 3, the AUC of the 3-, 6-, and 12-month prediction models all showed good results in the training cohort (0.845, 0.793, 0.751) and validation cohort (0.800, 0.735, 0.693). The calibration curves yielded a better fit which close to 45° (Fig. 4). These indicate that this model has an accurate predictive performance. The DCA curve proves that patients can get a high benefit from this predictive model (Fig. 5).
Hazard stratification
Based on the patients’ nomogram scores, we classified the patients into three risk strata: low (< 419), middle (419–475) and high (> 475). The stratification criteria were determined by X-tile software, and the Kaplan-Meier curves indicated a significant difference in the prognosis of patients in different risk groups (Fig. 6).
Discussion
Gastric cancer is the fifth most prevalent cancer and the third most deadly cancer in the world8. Among them, gastric signet ring cell carcinoma has been confirmed by many studies as one of the independent influences on the poor prognosis of gastric cancer9,10. Although the overall survival of GSRC patients is gradually increasing with the development of therapeutic strategies, the prognosis of GSRC patients with metastases is still poor. How to accurately predict the prognosis of these patients in order to individual treatment strategies is an urgent clinical problem.
It has been noted that overall survival time in patients with gastric signet ring cell carcinoma is not statistically different between gender, which is consistent with our findings9. However, it has also been suggested that estrogen has a facilitating role in the migration of GSRC to the uterus or ovaries, which may contribute to the higher rate of high tumor distant migration in female GSRC patients11. Interestingly, our study points out that age is not one of the independent risk factors affecting short-term prognosis in patients with metastatic GSRC. This suggests that younger patients with metastatic GSRC also possess a higher probability of early death. In a study on the effect of age on the prognosis of GSRC patients, the investigators noted that although the HR was lower in the younger group (< 45) than in the older group (> 74), patients in the younger group demonstrated a higher rate of metastasis12. This may explain why age is not an independent risk factor in patients with metastatic GSRC; although older patients are in poorer general health, younger patients with metastatic GSRC tend to have more advanced tumor progression. The effect of age on the prognosis of GSRC patients is still unclear, and this may need to be confirmed by more studies13.
The principles of radiotherapy and chemotherapy for GSRC patients remain highly controversial. Some investigators have pointed out the important role of hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of GSRC patients, especially when surgery is performed after continued multimodal chemotherapy until cytology is negative, which is effective in prolonging the overall survival of the patients14,15. However, a retrospective study also noted that chemotherapy was not statistically significant for survival in perioperative GSRC patients16. For radiotherapy, a retrospective study noted that perioperative radiotherapy was significant in prolonging overall survival in patients with advanced GSRC17,18,19. But the same researchers note that perhaps not all patients benefit from chemotherapy20. In our study, patients with metastatic GSRC who received chemotherapy had a significantly better prognosis than those who did not receive chemotherapy, while this result was not statistically significant for patients who received radiotherapy. This suggests that in GSRC patients with distant tumor metastasis, chemotherapy may provide a significant effect in prolonging survival while radiation therapy has a more limited therapeutic effect. This may provide new ideas for the future development of chemotherapy and radiotherapy principles for GSRC patients.
Liver metastases are very common in patients with advanced gastric cancer, with statistical data showing that approximately 14% of gastric cancer patients develop liver metastases21. Although chemotherapy with surgery has a recognized treatment strategy for patients with liver metastases from gastric cancer. However, the therapeutic effect is still very limited and the prognosis of these patients is worrisome22. Lung metastases also accounted for a high percentage of metastatic GSRC patients and often coexisted with liver metastases, which may be due to the metastasis of cancer cells to the liver and then to the lungs via the circulatory system23. Gastric cancer rarely metastasizes to bone, but when it does it usually suggests a very poor prognosis because these metastases are usually osteolytic16,24. Even less common in comparison is the occurrence of brain metastases, which occurred in only 11 of our included patients (Table 1). At the same time, some researchers have pointed out that brain metastases are more often combined with liver, lung, and bone metastases, which indicates a worse prognosis25. Therefore, we believe that the statistical bias is great in this case. Whether brain metastases should be considered as an independent influence on the prognosis of patients with metastatic GSRC remains to be studied more.
In addition to the above mentioned influencing factors, the prognosis of patients with signet ring cell carcinoma is also affected by some other factors, such as tumor size and lymph node metastasis. Some studies have shown that patients with larger tumors and lymph node metastasis have poorer prognosis. In response to the poor prognosis of patients with gastric signet ring cell carcinoma, some studies have been devoted to finding new therapeutic strategies and targeted drugs to improve the prognosis of patients. For example, studies targeting the tumor microenvironment and immune escape mechanisms are expected to bring new breakthroughs in the treatment of gastric signet ring cell carcinoma. Some targeted therapeutic agents are beginning to be applied to the treatment of SRCC, for example, targeted agents against HER2 have shown some efficacy in some HER2-positive SRCC patients. In addition, some molecular biological indicators such as mutation or deletion of TP53, APC, CDH1, Notch-1 and other genes in tumor tissues are associated with poor prognosis26. Gene therapy will be a future research hotspot, e.g. miR-935 targeting the Notch-1 gene has been shown to inhibit GSRC cell migration27. This will provide more therapeutic strategies for patients with metastatic GSRC. Although there are still some challenges and controversies in the research of signet ring cell carcinoma, with the continuous development of molecular biology and targeted therapy technology, it is believed that in the future, the understanding of signet ring cell carcinoma will be more in-depth, and the treatment strategy will be more individualized and precise, providing patients with better therapeutic effects and survival rates.
This study introduces the first validated nomogram predicting 3-, 6-, and 12-month survival in metastatic gastric signet ring cell carcinoma (GSRC), addressing a critical gap in prognostic tools focused on long-term outcomes or generalized cohorts. By integrating tumor stage, metastasis patterns (bone, liver, lung), and treatment responses, the model provides dynamic risk stratification for this high-risk population. Its visualization enables rapid clinical application without specialized software, while robust validation (AUC > 0.70) supports reliability despite SEER limitations. Future integration of molecular biomarkers (e.g., HER2) and external validation could refine precision, guiding personalized therapies and bridging prognostic gaps.
However, we must recognize that our study has some limitations. First, bias is unavoidable due to the insufficient sample size of the population, the nature of retrospective studies and the lack of external validation. We will further expand the clinical data content to include patient samples from more hospitals in subsequent study programs. Second, skin and breast metastases of gastric signet ring cell carcinoma are equally important, but we did not conduct a relevant study due to the lack of relevant data. Despite these shortcomings, it is undeniable that this predictive model still has a high clinical value, which can help clinicians to choose the best treatment strategy for patients as well as provide a theoretical basis for researchers.
Conclusion
We have successfully developed and well validated a nomogram that accurately predicts 3-, 6-, and 12-month OS in patients with metastatic GSRC. The nomogram has been shown to have high predictive performance and to provide excellent clinical benefits to patients, helping clinicians to choose the best clinical strategy for their patients.
Data availability
The dataset from the SEER database that was generated and/or analyzed during the current study is available in the SEER dataset repository (https://seer.cancer.gov/). The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GSRC:
-
gastric signet ring cell carcinoma
- OS:
-
overall survival
- SEER:
-
Surveillance, epidemiology, and end results
- ROC:
-
receiver operating characteristic curves
- AUC:
-
area under the curve
- DCA:
-
decision curve analysis
References
Ilic, M. & Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 28 (12), 1187–1203 (2022).
Zhao, W. et al. Single-cell analysis of gastric signet ring cell carcinoma reveals cytological and immune microenvironment features. Nat. Commun. 14 (1), 2985 (2023).
Arai, T. Where does signet-ring cell carcinoma come from and where does it go? Gastric cancer: official. J. Int. Gastric Cancer Association Japanese Gastric Cancer Association. 22 (4), 651–652 (2019).
Bramati, C. et al. Early diagnosis of oral squamous cell carcinoma May ensure better prognosis: A case series. Clin. Case Rep. 9 (10), e05004 (2021).
Mengardo, V., Treppiedi, E., Bencivenga, M., Dal Cero, M. & Giacopuzzi, S. Tailored treatment for signet ring cell gastric cancer. Updates Surg. 70 (2), 167–171 (2018).
Wu, J. et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. (London England). 40 (7), 301–312 (2020).
Jin, C. et al. A nomogram for predicting the risk of invasive pulmonary adenocarcinoma for patients with solitary peripheral subsolid nodules. J. Thorac. Cardiovasc. Surg. 153 (2), 462–469e461 (2017).
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 136 (5), E359–386 (2015).
Yu, J. M. et al. Clinical characteristics and prognostic analysis of patients with signet ring cell gastric carcinoma. Technol. Cancer Res. Treat. 19, 1533033820983812 (2020).
Yokota, T. et al. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J. Exp. Med. 186 (2), 121–130 (1998).
Ur Rahman, M. S. & Cao, J. Estrogen receptors in gastric cancer: advances and perspectives. World J. Gastroenterol. 22 (8), 2475–2482 (2016).
Ren, J. et al. Effect of age on prognosis of gastric Signet-Ring cell carcinoma: A SEER database analysis. Med. Sci. Monitor: Int. Med. J. Experimental Clin. Res. 24, 8524–8532 (2018).
Pernot, S. et al. Signet-ring cell carcinoma of the stomach: impact on prognosis and specific therapeutic challenge. World J. Gastroenterol. 21 (40), 11428–11438 (2015).
Badgwell, B. et al. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann. Surg. Oncol. 24 (11), 3338–3344 (2017).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355 (1), 11–20 (2006).
Messager, M. et al. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann. Surg. 254 (5), 684–693 (2011). discussion 693.
Wei, F., Lyu, H., Wang, S., Chu, Y. & Chen, F. Postoperative radiotherapy improves survival in gastric Signet-Ring cell carcinoma: a SEER database analysis. J. Gastric Cancer. 19 (4), 393–407 (2019).
Gong, H., Chu, Y., Hu, Q. & Song, Q. Preoperative radiotherapy is associated with significant survival benefits for patients with gastric signet ring cell carcinoma: A SEER-Based approach. Technol. Cancer Res. Treat. 19, 1533033820960746 (2020).
Tian, H. K. et al. Clinicopathological characteristics and prognosis of gastric signet ring cell carcinoma. World J. Clin. Cases. 10 (29), 10451–10466 (2022).
Ling, C. R., Wang, R., Wang, M. J., Ping, J. & Zhuang, W. Prognosis and value of preoperative radiotherapy in locally advanced rectal signet-ring cell carcinoma. Sci. Rep. 7, 45334 (2017).
Roh, H. R. et al. Outcome of hepatic resection for metastatic gastric cancer. Am. Surg. 71 (2), 95–99 (2005).
Cui, J. K., Liu, M. & Shang, X. K. Hepatectomy for liver metastasis of gastric cancer: A Meta-Analysis. Surg. Innov. 26 (6), 692–697 (2019).
Riihimäki, M., Hemminki, A., Sundquist, K., Sundquist, J. & Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget 7 (32), 52307–52316 (2016).
Park, J. M. et al. Bone recurrence after curative resection of gastric cancer. Gastric Cancer: Official J. Int. Gastric Cancer Association Japanese Gastric Cancer Association. 16 (3), 362–369 (2013).
Lai, M. Y. et al. The relationship between brain metastasis and HER2 expression status in gastric cancer. Clin. Translational Oncology: Official Publication Federation Span. Oncol. Soc. Natl. Cancer Inst. Mexico. 26 (3), 765–773 (2024).
Puccini, A. et al. Molecular profiling of signet-ring-cell carcinoma (SRCC) from the stomach and colon reveals potential new therapeutic targets. Oncogene 41 (26), 3455–3460 (2022).
Yan, C. et al. miR-935 suppresses gastric signet ring cell carcinoma tumorigenesis by targeting Notch1 expression. Biochem. Biophys. Res. Commun. 470 (1), 68–74 (2016).
Author information
Authors and Affiliations
Contributions
DX H and ZP Z conceived and designed the study. DX H and X L performed the literature search. DX H and YL H generated the figures and tables. DX H and X C analyzed the data. DX H wrote the manuscript and ZP Z critically reviewed the manuscript. DX H and ZP Z supervised the research. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were carried out by relevant guidelines and regulations. Data extraction and usage have been approved by the SEER Program. All the data can be found in the SEER dataset: https://seer.cancer.gov/seerstat/.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, D., Li, X., Huang, Y. et al. Individualized prediction tool for patients with metastatic gastric signet cell carcinoma. Sci Rep 15, 33163 (2025). https://doi.org/10.1038/s41598-025-02671-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02671-y