Abstract
Slaughterhouse sludge, a byproduct of meat processing, poses significant environmental risks if not properly treated, with potential impacts including water contamination and land pollution. Anaerobic digestion (AD) of this high-organic-content sludge offers a sustainable solution by facilitating biogas production, reducing reliance on fossil fuels, and enabling resource recovery. However, the complex nature of sludge necessitates pretreatment to enhance its biodegradability. In this study, the Fenton process, utilizing hydroxyl radicals (•OH) for oxidative breakdown of organic matter, was employed to improve the digestibility of slaughterhouse sludge. A response surface methodology (RSM)-based optimization approach, specifically the central composite design (CCD), was applied to investigate the effects of key operational parameters—pH, ferrous ion (Fe2+) dosage, and hydrogen peroxide (H2O2) dosage—on sludge disintegration. The response variables analyzed were soluble chemical oxygen demand (sCOD) and volatile suspended solids (VSS) reduction. The optimal conditions were identified as a Fe2+ dosage of 7.2 mg/g total solids (TS), a H2O2 dosage of 130.4 mg/g TS, and a pH of 3. Under these conditions, sCOD and VSS degradation increased by 37.5% and 40.5%, respectively, resulting in a 31% increase in methane yield over a 20-day AD period compared to untreated sludge. These findings demonstrate that Fenton pre-treatment enhances the biodegradability of slaughterhouse sludge, thereby improving the efficiency of AD and contributing to more sustainable waste management practices.
Similar content being viewed by others
Introduction
The slaughter industry is one of the largest industries with economic significance for every nation in the livestock sector. India is the fifth-largest meat exporter globally among the many nations producing meat1. According to the Ministry of Food Processing, the country has 3600 recognised slaughterhouses, 9 modern butcheries, and 171 meat processing plants, which slaughter over 121 million livestock like sheep, pigs, goats and poultry and 36.9 million buffaloes annually for local use as well as for export2,3. Slaughterhouses have serious problems for the environment in terms of soil, water, and land pollution. Each slaughtering wastes 15 L of water on average, which equates to 630 million gallons of water annually in India. Various cleaning processes like washing the blood of slaughtered animals, sterilization of the equipment, packaging etc., the type of animal slaughtered and the processing procedure result in enormous water consumption every day4,5. As a result, excessive discharge of organics from effluent streams of slaughterhouses becomes a peculiar problem6,7. The presence of pathogenic microorganisms, along with elevated concentrations ofnitrogen, phosphorus, chlorides, suspended solids, and colloidal substances, has attracted significant research attention toward the effective treatment of slaughterhouse wastewater. These contaminants substantially contribute to increased eutrophic pollution levels, posing serious risks to both human and animal health8,9.
The treatment of municipal and industrial wastewater predominantly relies on biological processes. Although these methods effectively reduce pollutants, they generate significant quantities of waste activated sludge (WAS) that require proper handling and disposal10,11. Handling and disposal account for 30–40% of capital costs and 50–60% of operational costs in wastewater treatment facilities12. The inherent challenges associated with WAS include its high organic content, odour, and microbial contamination, necessitating pre-treatment before final disposal. Activated sludge systems convert dissolved and suspended organic contaminants into biomass and gases, but producing substantial sludge volumes daily. Biological sludge stabilization methods, such as anaerobic digestion (AD), suffer from significant limitations, including prolonged retention periods (30–40 days) and low digestion efficiencies, with volatile suspended solids (VSS) reductions typically limited to 40–50%13. The presence of extracellular polymeric substances (EPS) in WAS significantly hampers sludge hydrolysis, the rate-limiting stage in AD, and contributes to the formation of gel-like structures that complicate dewatering14,15. Despite efforts to enhance dewaterability and reduce sludge volume through chemical, electrochemical, sonication, and thermal pre-treatments, these methods often exhibit inconsistent efficacy and high costs16.
Advanced oxidation processes (AOPs) have emerged as promising technologies for addressing the limitations of conventional sludge treatment methods. AOPs leverage the high oxidative potential of hydroxyl radicals (OH) to degrade organic contaminants non-selectively, bypassing the hydrolysis stage of anaerobic digestion17. Among AOPs, the Fenton reaction has garnered considerable attention due to its environmental benefits, ability to reduce sludge volume, and potential for improving sludge biodegradability18. The Fenton process employs iron (II) to catalyze hydrogen peroxide decomposition, generating hydroxyl radicals that disrupt sludge flocs, degrade bacterial cells, and solubilize organic matter19. Studies have demonstrated the efficacy of Fenton pre-treatment in enhancing sludge solubilization and methane production. For instance, Erden and Filibeli reported superior sludge solubilization with Fenton-treated thermophilic anaerobic digestion, achieving a 26.8% higher reduction in volatile solids compared to controls20. Two-stage digestion systems incorporating Fenton pre-treatment produced 1.3 times more methane than single-stage thermophilic systems21. Additionally, Pilli et al. (2016) highlighted a 3.1-fold increase in net energy and a 15% boost in methane generation with Fenton pre-treatment22. However, despite these promising outcomes, the optimization of operational parameters—including pH, Fe2+ and H2O2 dosage less explored, limiting the widespread adoption of the Fenton process in sludge management, particularly for complex waste streams like slaughterhouse wastewater. There is a pressing need to develop more sustainable methods that minimize chemical usage while maximizing sludge digestibility.
Given the complexities involved in pre-treatment processes, optimization is essential to maximize their efficiency and sustainability. Response Surface Methodology (RSM) and Central Composite Design (CCD) are powerful statistical tools for optimizing multi-variable systems. Siddiqui et al. (2023) also demonstrated the effectiveness of RSM in optimizing Fenton pre-treatment conditions for effluent treatment plant (ETP) sludge from slaughterhouses19. To address these gaps, the current study focuses on applying the Fenton process as a pre-treatment for slaughterhouse sludge, optimizing key operational parameters (pH, Fe2+ dosage, and H2O2 dosage) using the response surface methodology (RSM) tool in the Design-Expert software. The design of experiments (DOE) tool, is increasingly prominent in the environmental and water sectors. Traditional experimental design and optimization methods like One Factor at a Time (OFAT) have several significant issues, such as inadequate resource allocation and challenges in identifying interaction effects among the variables23. By investigating its effect on sludge disintegration and biodegradability, the study aimed to provide valuable insights into enhancing anaerobic digestion efficiency for slaughterhouse wastewater. The effects of individual Fenton process parameters were evaluated both independently and in combination, with a particular focus on their influence on soluble chemical oxygen demand (sCOD) and volatile suspended solids (VSS) in sludge. The study assessed not only the isolated impact of each parameter but also explored the interactive effects, providing a comprehensive understanding of how these factors contribute to the degradation and stabilization of sludge components. This study also emphasizes the need for further research on pathogen reduction and the environmental sustainability of sludge management systems.
Materials and methods
Materials and chemicals
Waste activated sludge (WAS) was collected from the return pipe of a secondary clarifier sludge hopper of the sewage treatment facility at Al-Hamd Agro Foods Products Pvt. Ltd, Aligarh. Before usage, the sludge samples were settled and kept at 4 °C in a cold chamber to reduce biological and chemical reactions. An hour oxidation period was taken into account when conducting the studies. The maximum storage period was one week. High-purity grade hydrogen peroxide (H2O2), sodium hydroxide (NaOH), sulfuric acid (99%), and ferrous sulphate (FeSO4), were purchased from Advent Chembio Pvt. Ltd. The characteristics of the raw samples are presented in Table 1. Standard methods were used to determine total solids, volatile solids, and dissolved solids (APHA 22nd Edition)24. The sCOD (soluble chemical oxygen demand) was examined using the standard methodology (5220 B-APHA, 2005)25. Membrane filters were used to collect and filter the supernatant. The filtrate was analyzed for sCOD. Chemical oxygen demand was determined by the closed reflux titrimetric method. pH was measured using the pH meter, while TDS was measured using the HQ30 d Portable Meter LBOD 10,101 probe.
Experimental procedure and design
Fenton pre-treatment
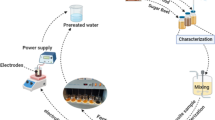
A preliminary study of WAS was conducted for the Fenton process to determine the working ranges of operating factors followed by the optimization of the Fenton process. Response surface methodology (RSM) based on central composite design (CCD) was applied taking into account the interactive impacts of process variables on two chosen responses, namely the elimination of VSS and the concentration of sCOD. Pre-treatment was applied using an appropriate effective range. Design-Expert software was used for the pre-treatment to determine the optimum operating conditions by considering the interactive effects of different variables and responses in pre-treatment for enhancing sludge digestibility and disintegration. The main operational factors that were optimised using RSM-based CCD were the dosages of H2O2, Fe2+, and pH. The CCD/RSM is used for experimental design, to obtain the interactive effects of variables and for the optimization of the process. The details regarding the process is well reported in our previous studies19,23.
A total of 42 experimental runs were carried out on the experimental set-up for the Fenton process as shown in Fig. 1. To find the ideal sludge pre-treatment operating conditions, empirical second-order polynomial models were fitted with data. Table 2 shows the coded values of process parameters and their ranges. The Fenton process coded values for pH(A), mg H2O2/g TS (B) and mg (Fe2+) g TS (C) were set at 3 levels as per the design chart obtained by RSM as shown in the Table 2. The sludge sample of 0.5 L was taken for Fenton pre-treatment. By using H2SO4 and NaOH, the pH of the sludge was first adjusted to carry out the process. The Fe2+ was added in specific quantities in the second stage. After that, the sample received additions of various H2O2 concentrations. For 60 min, the combined sample was agitated at 100–500 rpm. Ferrous (FeSO4.7H2O), the source of Fe2+ in the Fenton tests, was of analytical grade. H2SO4 (98–99%) and a stable hydrogen peroxide solution (30% w/w) were both used. Effects of individual Fenton process parameters were evaluated individually as well as their interactive effects on the sCOD and VSS of sludge. Completely mixed anaerobic digestion was carried out in two similar batch digesters on the pretreated and untreated WAS to assess each digester’s efficiency in terms of biogas output.
Biochemical methane potential (BMP)
Based on the BMP assay, the impact of Fenton pre-treatment on the anaerobic biodegradability of slaughterhouse sludge was assessed. For comparison, the BMP test was run on both untreated (R) and treated (S) samples. In 1000 mL bottles with a 300 mL reaction volume, BMP tests were conducted. To create anaerobic conditions, all bottles were purged with a gas combination that was 75% N2 and 25% CO2 for 3–4 min. To prevent gas leaking from the bottles, rubber stoppers and screw caps were used. The bottles were placed in a water bath that was kept at a constant 37 °C. Daily biogas gas productions were monitored using the liquid displacement method with distilled water and 3% NaOH (w/v)26. Biogas production in the bottles served as a gauge for the sludge anaerobic digesting performance. High anaerobic digestion performance of the sludge samples was indicated by high biogas outputs in the bottles.
Anaerobic digestion of raw and pre-treated WAS
The 20-day monitoring of cumulative methane production in serum bottles reveals that pre-treating sludge may be a viable option for enhancing anaerobic degradation. Only 600 mL of methane was produced by the raw sludge, whereas 785 mL was collected after the 20-day incubation period for the treated sludge with the recommended dosage. In this case, the Fenton-treated sludge produced over 31% more methane than the untreated sludge. The initial biodegradability of the sludge determines the rise in methane generation, with greater effects on hardly biodegradable sludge, and has been correlated linearly with sludge COD solubilization. The improvement in biogas generation from pre-treated sludge demonstrated that pre-treatment could lessen the impact of the rate-limiting phase. Due to pre-treatment WAS hydrolysing a large amount of organic waste into soluble forms, it was used right awayin the anaerobic digestion process. Pre-treatment could cause the release of organic matter from inner to outer portions, speeding up the hydrolysis of granular organics and increasing digestive effectiveness.
Results and discussion
Analysis of variance (ANOVA)
ANOVA was used to evaluate the “goodness of fit” of the data. The F-test at the 5% level of confidence found that the models for VSS removal and sCOD were significant. The fitted regression model was developed to describe the effects of factors on pre-treatment. If the p-values are less than 0.05, the relevant factors significantly affect the responses27. The two pre-treatment responses are statistically significant in the models (p < 0.05). Higher p-valued interaction terms have little to no effect on the model23. It is clear that the goodness-of-fit value for the regression models, R2, 0.9944, is suitable. A high R2 value confirms that the obtained model adequately described the pre-treatment process. Table 3 shows the analysis of variance for the response variable sCOD. Table 4 shows the fit statistics for COD with an R2 value of 0.9944 which indicates a good model. The predicted R2 of 0.9418 and the adjusted R2 of 0.9928 are reasonably in agreement; that is, the difference is less than 0.2. The Lack of Fit is not significant which is favorable to the model. Furthermore, the Adeq Precision value is greater than the desired value (A.P. > 4) which further confirms the obtained quadratic model is significant23. Similarly, Table 5 shows the analysis of variance for the response variable VSS. The Model F-value of 1161.93 suggests that the model is significant. When the P-value is less than 0.0500, model terms are deemed significant27. The lack of fit is not significant. The discrepancy between the Predicted R2 of 0.9875 and the Adjusted R2 of 0.9961 is less than 0.2, which is considered to be a reasonable agreement as evident from Table 6.
Response 1: ANOVA for sCOD
The final equation of sCOD in terms of actual factors
sCOD = 2192.08 + −202.656 * pH + 13.4754 * H2O2 + 83.5287 * Fe2 + + 0.100821 * pH * H2O2 + 2.4737 * pH * Fe2 + + 0.0359853 * H2O2 * Fe2 + + 13.2735 * pH^2 + −0.0544267 * H2O2^2 + −6.71644 * Fe2+^2.
Response 2: ANOVA for VSS
The final equation in terms of actual factors
VSS = 9710.11 + 378.03 * pH + −39.0643 * H2O2 + −261.427 * Fe2+ + −1.2252 * pH * H2O2 + −6.08223 * pH * Fe2+ + −0.116162 * H2O2 * Fe2 + + 11.8748 * pH^2 + 0.163414 * H2O2^2 + 20.0171 * Fe2+^2.
Effect of process variables
It was observed that with increasing H2O2 dosage at lower pH values, the sCOD concentration increased up to a certain extent as indicated by the red zone of the contour plot shown in Fig. 2a. This is due to more hydrolysis caused by •OH, whereas the further increase of dosage reduced the sCOD level because a higher amount of generated •OH caused complete mineralization of organic matter released from the cells of WAS. However, it is clear from the contour plot shown in Fig. 2b that initially increasing the values of both factors resulted in higher sCOD, however later on further increase caused reduction of sCOD values. This can be due to the inhibitory effect of hydroxyl radicals, which is found in correlation with past research works28,29. The 3D surface plots (Fig. 4) show that as the pH values were raised the sCOD levels decreased. The main cause of this reduction was the decreased amount of free Fe3+ brought on by the production of Fe (OH)3 molecules, which has a slow reaction rate with H2O2 during the process. Additionally, as the sludge pH increased, the production of oxidized free •OH radicals decreased. However, the impact of acidification on the disintegration of sludge was only marginal30. Because of this, the ideal pH for the greatest sludge separation with the least amount of chemical consumption to adjust the sludge pH was discovered to be 3, even though the maximum sCOD concentration was attained at pH 2.531. It has been seen that sCOD concentration increased when H2O2 was delivered in low dosages but decreased when H2O2 dosage was increased. The hydroxyl radicals in the process damaged organic compounds and microorganisms in the biomass by oxidizing cell walls and dissolving organic matter at doses less than 130 g H2O2/kg TS. The amount of sCOD in the liquid phase increased as a result of the dissolved organic matter being released into it. However, a decrease in the concentration of sCOD dosages above 130 H2O2/kg TS in the liquid phase can be explained by the presence of •OH radicals, which have a high oxidation potential and are capable of converting organic matter into water and carbon dioxide while also preventing the disintegration of sludge. Previous studies have also demonstrated that hydroxyl radicals have an inhibiting effect28.
In the Fenton reaction, the Fe2+ ion immediately interacts with H2O2 to produce •OH. As the concentration of iron ions increases, so does the rate of disintegration. Over a certain concentration, the rate of disintegration remains quite low. The sCOD value increases as the iron concentration increases up to a certain level. However, further increases in iron levels create a negative impact on •OH radical generation, which resulted in a significant declination in sCOD values. The iron dosages were discovered to be 7.2 mg/g TS after taking into account these findings. A diagnostic plot (Fig. 3) comparing the anticipated versus actual values can be used to evaluate the model’s effectiveness. The idealised trend’s linear distribution of the points shows that the anticipated values are fairly close to the corresponding observed values. This is also confirmed by the significant model term with a p-value less than 0.0001.
As can be seen in 3D surface plots in Fig. 4, there was an initial decrease followed by an increase in sCOD concentration as the H2O2 dosage was increased. The cytoplasm that was eluted from the decomposing microbe was degraded, and the surplus sludge was then solubilized by hydroxyl radicals, which caused the decrease in sCOD. Cell lysis contributed to the organic loading and the increase in sCOD concentration by releasing cell contents into the sludge slurry32. Additionally, excessive amount of H2O2 can lead to its auto-decomposition into water and oxygen (Eq. 1) and the recombination of •OH, which lowers the concentration of •OH and decreases the efficiency of degradation. Also due to the mineralization of released organic material at higher doses overall efficiency decreases.
Due to its ability to catalytically break down H2O2 and produce •OH, Fe2+ is a crucial part of the Fenton process. Because initial solubilization depends on Fe2+ concentration, raising the Fe2+ dosage raises sCOD concentration. However, if there is an excess of Fe2+ it starts to compete with organic molecules for the •OH, which reduces efficiency22. At increased Fe2+ dose, the coagulation process is also started, generating more sludge and raising the effluent’s total dissolved solids (TDS). The initial pH, which affects iron solubility, complexation, and redox cycling between states (II) and (III), limits the influence of Fe2+ dosage on the treatment. Because Fe2+ functions as a catalyst to speed up the conversion of H2O2 into •OH, its viability therefore depends on the pH of the solution. A lower concentration of Fe2+ has the opposite effect, decreasing the efficiency of solubilisation33. The concentration of sCOD increased somewhat when the initial pH was increased from 2.5 to 4.0, but it decreased significantly when the initial pH was elevated above 4.0. Two causes are identified as the root of the problem: first, lower pH values with less •OH restrict the effectiveness of Fenton oxidation, and second, higher pH values with more •OH reduce the activity of the Fenton reagent34. It was observed from the contour plots shown in Fig. 5a, that increasing H2O2 dosage at lower pH values, caused more degradation of VSS content up to a certain limit (Blue zone of contour plot), due to more oxidation and hydrolysis of WAS floc. However, a further increase in dosage reduces the VSS degradation levels. Also, at higher pH values the efficiency of the Fenton reagent decreases resulting in lesser VSS degradation as shown in 3D plots (see Fig. 7). Similar results were found in past study34,35. Furthermore, as the dosage of both H2O2 and Fe2+ increases, the degradation of VSS content increases as shown in Fig. 5b. However, after reaching at highest degradation, VSS content starts increasing with a further increase of both factors. This might be due to inhibition of sludge disintegration. Here, the actual vs. predicted plot from Fenton oxidation for VSS (Fig. 6) shows that the points were all well distributed and close to the fitted line. It was observed that there is no difference between the actual response and the predicted values by the model. This is also confirmed by the significant model term in Table 5 with a p-value less than 0.0001 (Fig. 7).
Process optimization
When there are numerous responses, it is possible to graphically depict the ideal operating conditions where all parameters concurrently match the desired response criteria36. The regions that meet the optimization requirements are shaded in the graphic representation of the area of a viable response value in the factor space. Response limitations were chosen as the least allowable values for each parameter near their achieved efficiency to obtain a relatively precise optimum zone – VSS elimination 40.5% and sCOD 2915 mg/L for Fenton oxidation. The values of the variables and responses following optimization are shown in Fig. 8.
Conclusion
The present study was performed to enhance the anaerobic digestion of slaughterhouse sludge. Fenton pre-treatment was employed to improve the solubilization and disintegration of waste activated sludge. By using CCD/RSM, the role of process variables with the overall impact of Fenton pre-treatment on waste activated sludge (WAS) was optimized in terms of VSS removal and sCOD responses. Based on optimization and result analysis, 130.4 mg H2O2/g TS and 7.2 mg Fe2+/g TS dosage at pH 3 were found to be the best suitable operating conditions showing 31% enhancement of methane production as compared to raw WAS. However, due to chemical & energy consumption, the Fenton process alone may not be a sustainable method of sludge treatment due to which combination of methods, such as electrochemical peroxidation and electro-Fenton, are also employed so as to overcome these drawbacks along with increasing solubilization and disintegration and targeting higher recovery of methane. Furthermore, the generation of Fenton sludge which is one of the drawbacks of the process must be controlled and considered in future studies. To quantify and characterise the generated sludge in order to evaluate the overall process performance.
Data availability
The datasets generated and/or analyzed will be available from the corresponding author onreasonable request.
References
Kumar, V. et al. Production of biodiesel and bioethanol using algal biomass harvested from fresh water river. Renew. Energy https://doi.org/10.1016/j.renene.2017.10.016 (2018).
Mach, N., Bach, A., Velarde, A. & Devant, M. Association between animal, transportation, slaughterhouse practices, and meat pH in beef. Meat Sci. https://doi.org/10.1016/j.meatsci.2007.06.021 (2008).
Salminen, E. & Rintala, J. Anaerobic digestion of organic solid poultry slaughterhouse waste—A review. Bioresour Technol. https://doi.org/10.1016/S0960-8524(01)00199-7 (2002).
Azam, R. et al. Production of algal biomass for its biochemical profile using slaughterhouse wastewater for treatment under axenic conditions. Bioresour. Technol. https://doi.org/10.1016/j.biortech.2020.123116 (2020).
Barrera, M. et al. Photolytic treatment of organic constituents and bacterial pathogens in secondary effluent of synthetic slaughterhouse wastewater. Chem. Eng. Res. Des. https://doi.org/10.1016/j.cherd.2011.11.018 (2012).
Del Hoyo, P., Moure, F., Rendueles, M. & Díaz, M. Demineralization of animal blood plasma by ion exchange and ultrafiltration. Meat Sci. https://doi.org/10.1016/j.meatsci.2006.06.014 (2007).
Alfonso-Muniozguren, P. et al. Single and combined electrochemical oxidation driven processes for the treatment of slaughterhouse wastewater. J. Clean. Prod. https://doi.org/10.1016/j.jclepro.2020.121858 (2020).
Vidal, J., Carvajal, A., Huiliñir, C. & Salazar, R. Slaughterhouse wastewater treatment by a combined anaerobic digestion/solar photoelectro-Fenton process performed in semicontinuous operation. Chem. Eng. J. https://doi.org/10.1016/j.cej.2019.122097 (2019).
Savin, M. et al. Antibiotic-resistant bacteria and antimicrobial residues in wastewater and process water from German pig slaughterhouses and their receiving municipal wastewater treatment plants. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2020.138788 (2020).
Mininni, G., Laera, G., Bertanza, G., Canato, M. & Sbrilli, A. Mass and energy balances of sludge processing in reference and upgraded wastewater treatment plants. Environ. Sci. Pollut Res.https://doi.org/10.1007/s11356-014-4013-2 (2015).
Tomei, M. C. et al. Techno-economic and environmental assessment of upgrading alternatives for sludge stabilization in municipal wastewater treatment plants. J. Clean. Prod. https://doi.org/10.1016/j.jclepro.2015.10.017 (2016).
Banu, J. R., Uan, D. K., Kaliappan, S. & Yeom, I. T. Effect of sludge pretreatment on the performance of anaerobic/anoxic/oxic membrane bioreactor treating domestic wastewater. Iran J. Environ. Heal Sci. Eng. 8, 2 (2011).
Appels, L., Baeyens, J., Degrève, J. & Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. https://doi.org/10.1016/j.pecs.2008.06.002 (2008).
Tiehm, A., Nickel, K., Zellhorn, M., Neis, U. & Tiehm, A. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Res. https://doi.org/10.1016/S0043-1354(00)00468-1 (2001).
Dursun, D. & Dentel, S. K. Toward the conceptual and quantitative understanding of biosolids conditioning: The gel approach. Water Sci. Technol. https://doi.org/10.2166/wst.2009.202 (2009).
Chen, Z., Zhang, W., Wang, D., Ma, T. & Bai, R. Enhancement of activated sludge dewatering performance by combined composite enzymatic lysis and chemical re-flocculation with inorganic coagulants: Kinetics of enzymatic reaction and re-flocculation morphology. Water Res. https://doi.org/10.1016/j.watres.2015.06.026 (2015).
Stasinakis, A. S. Use of selected advanced oxidation processes (AOPs) for wastewater treatment—A mini review. Glob Nest J. https://doi.org/10.30955/gnj.000598 (2008).
Amudha, V., Kavitha, S., Fernandez, C., Adishkumar, S. & Banu, J. R. Effect of deflocculation on the efficiency of sludge reduction by Fenton process. Environ. Sci. Pollut Res. https://doi.org/10.1007/s11356-016-7118-y (2016).
Siddiqui, M. I., Farooqi, I. H., Basheer, F., Rameez, H. & Isa, M. H. Pretreatment of slaughterhouse effluent treatment plant sludge using electro-fenton process for anaerobic digestion. Sustain https://doi.org/10.3390/su15043159 (2023).
Erden, G. & Filibeli, A. Effects of Fenton pre-treatment on waste activated sludge properties. Clean. Soil. Air Water https://doi.org/10.1002/clen.201000199 (2011).
Erden, G. & Filibeli, A. Improving anaerobic biodegradability of biological sludges by Fenton pre-treatment: effects on single stage and two-stage anaerobic digestion. Desalination https://doi.org/10.1016/j.desal.2009.09.144 (2010).
Pilli, S., More, T. T., Yan, S., Tyagi, R. D. & Surampalli, R. Y. Fenton pre-treatment of secondary sludge to enhance anaerobic digestion: Energy balance and greenhouse gas emissions. Chem. Eng. J. 283, 285–292. https://doi.org/10.1016/j.cej.2015.07.056 (2016).
Mahtab, M. S., Farooqi, I. H., Khursheed, A., Siddiqui, M. I. & Zhang, L. Perspectives on sustainable process control optimization through reusability of non-regenerated Fenton sludge in landfill leachate treatment. J. Water Process Eng. https://doi.org/10.1016/j.jwpe.2024.105205 (2024).
APHA & WEF, A. W. W. A. Standard methods for examination of water and wastewater. Washingt Am. Public. Heal Assoc, (2012).
Yadvika, A. K., Yadav, T. R., Sreekrishnan, S., Satya, & Kohli, S. A modified method for estimation of chemical oxygen demand for samples having high suspended solids. Bioresour Technol. https://doi.org/10.1016/j.biortech.2005.04.013 (2006).
Biodegradation of. Selected Azo dyes under methanogenic conditions. Water Sci. Technol. https://doi.org/10.1016/S0273-1223(97)00508-8 (1997).
Rouder, J. N., Engelhardt, C. R., McCabe, S. & Morey, R. D. Model comparison in ANOVA. Psychon Bull. Rev. https://doi.org/10.3758/s13423-016-1026-5 (2016).
Tokumura, M., Katoh, H., Katoh, T., Znad, H. T. & Kawase, Y. Solubilization of excess sludge in activated sludge process using the solar photo-Fenton reaction. J. Hazard. Mater. https://doi.org/10.1016/j.jhazmat.2008.06.026 (2009).
Tokumura, M., Sekine, M., Yoshinari, M., Znad, H. T. & Kawase, Y. Photo-Fenton process for excess sludge disintegration. Process. Biochem. https://doi.org/10.1016/j.procbio.2006.11.010 (2007).
Sahinkaya, E. et al. Biotreatment of As-containing simulated acid mine drainage using laboratory scale sulfate reducing upflow anaerobic sludge blanket reactor. Min. Eng.https://doi.org/10.1016/j.mineng.2014.08.012 (2015).
Pham, T. T. H., Brar, S. K., Tyagi, R. D. & Surampalli, R. Y. Optimization of Fenton oxidation pre-treatment for B. thuringiensis-based production of value added products from wastewater sludge. J. Environ. Manag. https://doi.org/10.1016/j.jenvman.2010.03.007 (2010).
Bolto, B. & Gregory, J. Organic polyelectrolytes in water treatment. Water Res. https://doi.org/10.1016/j.watres.2007.03.012 (2007).
Gulsen Akbay, H. E., Dizge, N. & Kumbur, H. Evaluation of electro-oxidation and Fenton pretreatments on industrial fruit waste and municipal sewage sludge to enhance biogas production by anaerobic co-digestion. J. Environ. Manag. https://doi.org/10.1016/j.jenvman.2022.115711 (2022).
Liu, S. T. et al. Microwave enhanced Fenton process for the removal of methylene blue from aqueous solution. Chem. Eng. J. https://doi.org/10.1016/j.cej.2012.11.003 (2013).
Feki, E., Battimelli, A., Sayadi, S., Dhouib, A. & Khoufi, S. High-rate anaerobic digestion of waste activated sludge by integration of electro-Fenton process. Molecules https://doi.org/10.3390/molecules25030626 (2020).
Ba, D. & Boyaci, I. H. Modeling and optimization i: Usability of response surface methodology. J. Food Eng. https://doi.org/10.1016/j.jfoodeng.2005.11.024 (2007).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, investigation, writing—original draft and review of the final manuscript, Mohsin Anwer, Mohd Ahmed Naim Shaikh, Mohd Salim Mahtab & Saif Ullah Khan; formal analysis, Mohd Ahmed Naim Shaikh, Mohd Salim Mahtab & Mohsin Anwer; resources, supervision and review, Izharul Haq Farooqi & Mohammad Hadi Dehghani; visualization, Mohammad Hadi Dehghani and Sayedali Mirkhalafi; review and editing, Mohd Salim Mahtab & Saif Ullah Khan. Allauthors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anwer, M., Shaikh, M., Khan, S. et al. Response surface modelling of Fenton pre-treatment of slaughterhouse sludge for enhanced anaerobic digestion. Sci Rep 15, 31584 (2025). https://doi.org/10.1038/s41598-025-02731-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02731-3