Abstract
The marine ecological environment of Hangzhou Bay and its adjacent waters ( 30°–31° N, 121.5–123.5° E ) shaped by various factors, serves as a spawning and feeding ground for diverse species of fish, shrimp and crabs, including a traditional gillnet fishing area for the gazami crab, Portunus trituberculatus. The study investigates spatiotemporal distribution and the mechanisms underlying the formation of these fishing grounds of P. trituberculatus using single bottom trawl survey data from 31 stations in Hangzhou Bay and its adjacent waters across four seasons in 2014, gillnet survey data during the period 25–29 July 2019 to 2021 and gillnet vessel fishing logs from September 2020 to January 2021. Seasonal variations and spatiotemporal distributions of P. trituberculatus abundance and body mass were analysed. A generalised additive model was applied to assess the influence of external factors identifying key drivers of fishing ground formation. Gillnet monitoring and fishing log data were used to explore inter-annual trends in population characteristics and catch per unit effort (CPUE). The findings revealed pronounced seasonal patterns in P. trituberculatus abundance and distribution, with notable seasonal differences in spatial and temporal dynamics. The primary external factors affecting abundance were season and bottom salinity, followed by latitude and surface chlorophyll a content. Bottom salinity emerged as the critical environmental factor driving the formation of the gillnet fishing grounds. Significant inter-annual differences were observed in P. trituberculatus carapace length (P < 0.05), although no significant differences were found between sexes within the same year. Monthly variation in CPUE 0.49–1.38 kg/net and carapace length 75.6–82.1 mm were also significant (P < 0.05). This study elucidates the spatiotemporal distribution patterns and environmental drivers shaping P. trituberculatus fishing grounds in Hangzhou Bay and its adjacent waters, providing insights for sustainable management and conservation of these fishery resources.
Similar content being viewed by others
Introduction
Hangzhou Bay, located in the northern East China Sea, lies near the Yangtze River estuary to the north and the Zhoushan Islands to the south, forming a funnel-like shape1,2. It is influenced the Yangtze River current, Qiantang River runoff and upwelling, among other factors. The adjacent waters outside the bay have developed into the renowned Zhoushan fishing ground3. A traditional gillnet fishing ground has also formed outside the bay’s mouth, establishing a specific fishing scale. The East China Sea, Yellow Sea and Bohai Sea represent the primary distribution areas for the gazami crab, Portunus trituberculatus, making it one of the most important crab fishing resources globally. Nearly 50% of P. trituberculatus production comes from the East China Sea, with the northern East China Sea contributing over 40% of this yield4,5,6. In recent years, the average annual of P. trituberculatus in the East China Sea has been 15 × 104 tonnes, peaking at almost 20 × 104 tonnes7. Since 2011, Zhejiang Province, a key area in the East China Sea, has accounted for over one-third of the national P. trituberculatus catch. In 2017, Zhejiang Province launched China’s first restricted fishing pilot in its northern waters, and conducted pre-fishing period (May, June, July) monitoring and quota fishing management in the traditional fishing area of P. trituberculatus8.
As one China’s most economically important crabs, P. trituberculatus has been the subject of extensive research focusing on fishing gear selectivity7,9, spatiotemporal distribution and its drivers10,11, migration pattern12, proliferation and release13, and genetic differentiation4,14. P. trituberculatus, as one of the bottom crabs, has the characteristics of short life cycle and rapid growth, which is easily affected by the changes of temperature, salinity and other marine environmental factors. The annual yield of P. trituberculatus fluctuates markedly due to multiple factors making its variation a critical research focus. Hangzhou Bay and its adjacent waters represent the traditional gillnet fishing grounds for P. trituberculatus in the northern East China Sea. However, resource distribution and dynamics remain poorly understood and the fishing ground formation mechanism is unclear.
Therefore, this study analysed the spatiotemporal distribution of P. trituberculatus abundance and body mass using single bottom trawl survey data from March, May, July and November 2014. A generalised additive model (GAM) was employed to investigate the primary drivers of fishing ground formation. Gillnet monitoring and fishing log data were used to examine population characteristics across years and monthly trends in catch per unit effort (CPUE) of the traditional gillnet fishing grounds. It was confirmed that P. trituberculatus population differed among the years, and the fluctuation of P. trituberculatus during the fishing process was clarified by using the actual production of traditional gillnet fisheries.Overall, this study aimed to elucidate the spatiotemporal distribution patterns and environmental drivers influencing the formation of P. trituberculatus fishing grounds in Hangzhou Bay and its adjacent waters, offering a reference for the sustainable management and conservation of these fishery resources.
Materials and methods
Survey sites and data collection
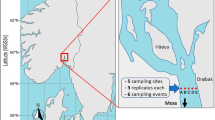
The research data were collected from three sources: trawl survey data (fishery-independent), gillnet survey data (fishery-independent) and production monitoring data (fishery-dependent). (1) Trawl survey data were obtained during seasonal fishery resource and environmental surveys conducted in winter (March), spring (May), summer (July) and autumn (November). To ensure comprehensive coverage of the study area, 31 stations were established, including 15 within Hangzhou Bay’s internal waters and 16 in the eastern adjacent waters (Fig. 1). A single bottom trawl net was used, featuring a tightened net port of 50 m, an upper line of 30 m, a lower line of 38 m and a net bag mesh of 25 mm. We use the same fishing boat to conduct investigation on the survey stations in sequence. Each station was trawled once per season. After each trawl, a multifunctional water quality metre (model: JFE- AAQ171) was employed to collect environmental data, including water depth, temperature, salinity, dissolved oxygen levels and chlorophyll content. Fish samples from each station were proportionally and randomly selected, frozen and transported to the testing room (biological measurement room of fishery resources and ecology research laboratory, zhejiang marine fisheries research institute) for biological analysis of P. trituberculatus. All stations were standardised after the survey, with trawl time set to 1 h and trawl speed at 3 knots, allowing for abundance estimates of P. trituberculatus at each station. (2) Gillnet survey data were obtained through monitoring in traditional fishing areas during the period 25–29 July 2019 to 2021, using five single gillnets with a mesh size of 140 mm (fully stretched mesh length). The length of the gillnet was 250 m and the height was 2.5 m. For data consistency nets were set at 08:00 and hauled at 16:00, the average soaking time was 8 h. After fishing, crabs were frozen and brought back to the testing room for biological analysis, including measurements of carapace length and sex-specific parameters. (3) Production monitoring data comprised fishing log data from P. trituberculatus gillnet vessels operating in traditional waters between September 2020 and January 2021. These logs included details such as latitude, longitude, nets counts and catch volume, among other parameters. All fishery resource investigations and biological analyses followed the standards outlined in the ‘Specifications for Oceanographic survey - Marine Biological Survey’ (GB/ T12763.6-2007)15.
Data processing method
Abundance
In this study, the abundance of P. trituberculatus was calculated using the swept area method, with station distributions represented by abundance values. The formula for calculating abundance is as follows:
where Ai is the abundance at station i (kg/km2), Ci is the actual catch at station i (kg), E is the escape rate (0.7)16,17, Vi is the standardised bottom trawl speed at station i (3 knots), Ti is the standardised bottom trawl time at station i (1 h) and L is the horizontal expansion width of the net port (9.9 × 10− 3 km).
GAM (generalized additive model)
GAMs are commonly used to analyse nonlinear relationships between explanatory and response variables18,19,20. The general expression of a GAM is as follows:
where g() is the link function, Y represents the response variable, α is the intercept, xj represents the explanatory variable, fj is the smoothing function for explanatory variables and ε denotes residual error.
In this study, a GAM was applied to examine the relationship between the abundance of P. trituberculatus and external factors in Hangzhou Bay and its adjacent waters. The abundance of P. trituberculatus was the response variable, whereas season, longitude (Lon), latitude (Lati), depth, surface water temperature (ST), bottom water temperature (BT), surface salinity (SS), bottom salinity (BS), surface dissolved oxygen (SO), bottom dissolved oxygen (BO), surface chlorophyll a (SChl.a) and bottom chlorophyll a (BChl.a) were considered explanatory variables. Before constructing the GAM, variance inflation factor (VIF) analysis was conducted to test for multicollinearity among explanatory variables, ensuring no collinearity issues. The VIF formula is as follows:
where \(\:{R}_{j}^{2}\) is the coefficient of determination when variable j is regressed on all other variables. A VIF value below 10 indicates no multicollinearity, allowing the variable to be retained. Variables with VIF ≥ 10 were excluded.
The GAM was constructed using the response variables and screened explanatory variables, expressed as follows:
In this formula, Abundance + 1 is used for logarithmic processing to avoid zero values, s represents natural spline smoothing and ε denotes the error. Model optimisation was performed using the Akaike information criterion (AIC) and the cumulative deviation was explained to identify the optimal model21,22, different factors were added to the model in order according to stepwise method. The optimal model was selected based on the lowest AIC value and factor significance was assessed using the F–test, with P < 0.05 considered statistically significant.
CPUE
CPUE is a critical index for evaluating resource abundance. In this study, the catch of P. trituberculatus was standardised and CPUE was used to represent monthly catches20. The formula is as follows:
where CPUEi is the catch per unit effort in month i (kg/net), Ci is the monthly catch of P. trituberculatus (kg) and n denotes the number of gillnets used.
Data visualization and significance test
Data visualisation and the Kruskal–Wallis test were conducted using Origin software. The spatial distribution of P. trituberculatus stations was visualised using Ocean Data View software. VIF testing and GAM modelling were performed using R software. The GAM model was created using the “nlme” and “mgcv” packages.
Results
Seasonal changes of abundance and body mass of P. trituberculatus
Table 1 shows abundance and body mass of P. trituberculatus in different seasons. P. trituberculatus abundance followed the trend autumn > summer > spring > winter, whereas body mass followed the trend spring > autumn > winter > summer. Significant differences were observed in abundance and body mass across seasons (P < 0.05, Kruskal-Wallis test, Fig. 2).
Spatial and temporal distribution of abundance of P. trituberculatus
In winter, P. trituberculatus was primarily distributed near Shengshan Island and the middle waters of Daishan Island–Qushan Island, with a station presence rate (percentage of stations appearing in total stations) of 32.26%. In spring, it was distributed in the north of the survey area and southeast of the Zhoushan Islands, particularly west of Sijiao Mountain, with a station presence rate of 61.29%. In summer, the species was centred around Shengshan Island, northeast of northeast Qushan Island and northeast of the Zhongjieshan Islands, with a station presence rate of 51.61%. In autumn, it was mainly distributed near Shengshan Island and the northeast side of the Zhongjieshan Islands, with a station presence rate of 67.74% (Fig. 3).
Spatial and temporal distribution of body mass of P. trituberculatus
Seasonal patterns showed that in winter, P. trituberculatus was distributed east of Sijiao Mountain–Shengshan Island. In spring, larger individuals became more prevalent, primarily in the east of Shengshan Island–Qushan Island. In summer, P. trituberculatus body weight decreased, but individuals with larger bodies were still distributed east of Shengshan Island. In autumn, P. trituberculatus body mass increased again, with distributions shifting to east of Shengshan Island and northeast of of Zhongjieshan Islands (Fig. 4).
Influence of external factors on abundance of P. trituberculatus
The multicollinearity test revealed issues with bottom water temperature and surface water temperature among the environmental variables. Therefore, these factors were excluded, and the GAM was constructed using the remaining variables optimized via the AIC. The final model expression was as follows:
The GAM explained 50.1% (R2 = 0.478) of cumulative deviation between P. trituberculatus abundance and the screening factors. Seasonal variation and bottom salinity (P < 0.001) exerted highly significant effects on abundance, with latitude and surface chlorophyll a (P < 0.05) content, P. trituberculatus abundance initially increased slowly, before accelerating as the chlorophyll a concentrations increased. In contrast, the relationship of bottom salinity and latitude with abundance were linear, showing a gradual increase in abundance with rising bottom salinity and latitude (Fig. 5).
Driving factors of formation of gillnet fishing ground of P. trituberculatus
According to the above mentioned analysis, bottom salinity is the most significant factor affecting P. trituberculatus in Hangzhou Bay and its adjacent waters, serving as the primary environmental driver for the formation of the species’ gillnet fishing ground in this area. To clarify its driving mechanism, the spatial distribution and bottom salinity of the stations in different seasons were visualized (Fig. 6). In winter, the bottom salinity range for P. trituberculatus was 14.46‰–33.90‰, with a mean of 25.63 ± 5.15‰; in spring, it was 16.52‰–33.92‰, with a mean of 26.70‰ ± 5.80‰; in summer, it was 16.01‰ – 34.36‰, with a mean of 31.16‰ ± 5.68‰; and in autumn, it was 8.83‰–33.77‰, with a mean of 26.73‰ ± 7.36‰. Significant seasonal differences were observed in bottom salinity (P < 0.05, Kruskal-Wallis test, Fig. 7).
Gillnet and production monitoring of P. trituberculatus fishing grounds
The driving factors affecting the fishing ground of P. trituberculatus were obtained in the previous study. In this part, the monitoring of gillnet in the early stage of production and the fishing situation after production in traditional gillnet fishing ground were studied to grasp the differences of P. trituberculatus in gillnet fishing ground between years and provide reference for fishery management. The data of carapace length distribution during the period 25–29 July 2019 to 2021 is shown in Table 2. The mean carapace length of P. trituberculatus was highest in 2020 and lowest in 2021.No significant difference in carapace length was observed between males and females in the same year (P > 0.05). However, there are significant differences between females and males in different years(P < 0.05).
Based on P. trituberculatus production data from two limited-catch gillnet fishing grounds in 2020, the fishing period for these areas range from September 16 to January 15. The average CPUE in September was 1.38 ± 0.53 kg/net. In October, the mean CPUE dropped to 0.96 ± 0.33 kg/net, followed by 0.91 ± 0.37 kg/net in November, 0.75 ± 0.37 kg/net in December and 0.49 ± 0.12 kg/net in January. Thus, CPUE showed a declining trend over the months.
In terms of P. trituberculatus carapace length, the mean values in September, October, November, December and January were 82.1 ± 8.8, 83.7 ± 7.9, 77.2 ± 9.0, 78.1 ± 8.8 and 75.6 ± 8.7 mm, respectively. Thus, carapace length was higher in September and October than in November, December and January. Significant differences were observed in CPUE and carapace length across months (P < 0.05, Fig. 8).
Disscussion
Temporal and spatial distribution characteristics of P. trituberculatus
This study found that the abundance and distribution of P. trituberculatus in Hangzhou Bay and its adjacent waters exhibited the highest and lowest values in autumn and winter, respectively. This trend is primarily attributed to the increase in the supplementary population of P. trituberculatus during autumn, which leads to a rise in abundance, thereby forming a feeding population12. Additionally, some juvenile crabs that grew into adults after summer gradually shifted their distribution to deeper waters with the station presence rate rising from 51.61% in summer to 67.74% in autumn, indicating a broader distribution range. In winter, as water temperatures gradually decrease, most P. trituberculatus migrate to southern Zhejiang for wintering6, markedly reducing their numbers. Consequently, P. trituberculatus abundance is lower in winter than in the other three seasons and the species’ station presence rate is the lowest in this season, mainly concentrated near islands and reefs. In the current study, P. trituberculatus body mass was found to be higher in spring and autumn, but lower in winter and summer. As shown in Fig. 4a, overwintering groups exist in the Shengshan Sea area, consistent with the findings of Song Haitang et al.,(1989)12. Spring is the peak spawning period for P. trituberculatus, characterised by larger individuals. As indicated in Fig. 4b, the the sea area east of Shengshan Island serves as a key spawning ground for P. trituberculatus in northern Zhejiang. The present study revealed the seasonal variations in P. trituberculatus abundance and body mass in Hangzhou Bay and its adjacent waters, providing valuable insights for the rational use and scientific protection of resources in the region.
Effects of environmental factors on abundance of P. trituberculatus
Seasonal changes in multiple external environmental variables markedly impact the abundance distribution of P. trituberculatus. Hangzhou Bay and its adjacent waters are located at the intersection of two major estuaries, where temperature and salinity fluctuate with the seasons, indirectly affecting P. trituberculatus migration and, in turn, the species’ abundance distribution. Salinity is a critical environmental factor influencing the survival, metamorphosis, growth and feeding of P. trituberculatus23. The current study revealed that P. trituberculatus abundance exhibits a significant linear positive correlation with bottom salinity. It is considered that bottom salinity serves as a key traditional driver for the formation of traditional gillnet fishing grounds. These findings align with the research of Li Xiaodong et al., (2021)24. As an euryhaline species, P. trituberculatus requires different salinity levels at various life stages12. Optimal growth occurs with a salinity range of 20‰–35‰ and lower salinity levels adversely affect the species’ growth and survival23,25,26. Zhang Xini et al.,(2023)23 reported that P. trituberculatus weighing 45 g ± 5 g exhibits a minimum salinity tolerance of 12‰, below which mortality begins. Furthermore, salinity affects the feeding frequency and food intake of P. trituberculatus. Shentu Jikang et al.25 observed that feeding frequency and food intake were higher with salinity values of 20‰ and 30‰ compared to 5‰ and 10‰. Therefore, salinity plays a multifaceted role in influencing P. trituberculatus. Latitude is another important geographical factor affecting P. trituberculatus. The current study found that P. trituberculatus abundance increases with latitude with higher densities observed in the northern areas of the study region. Compared to the southern region, this pattern is primarily linked to the favourable geographical environment of the northern area, which is enriched by nutrients from the confluence of the Yangtze and Qiantang river, making it more conducive to growth and reproduction of P. trituberculatus. Li Xiaodong et al.,(2021)24 identified a low-abundance area in the southeast sea area of the northern East China Sea, suggesting that this region is less suitable for P. trituberculatus survival. Although chlorophyll a does not directly impact P. trituberculatus resource distribution, it serves as an indirect food source for the species, contributing to its growth and reproduction. Chlorophyll a concentration is considered to be the most important factor affecting the fatness of male P. trituberculatus, and there is a significant positive correlation between them27. Therefore, the increase of population density of P. trituberculatus in this study may be related to the increase of fatness of male P. trituberculatus. Additionally, the number of juvenile crabs released and the fishing fleet size also influence the abundance of P. trituberculatus resources5. Therefore, P. trituberculatus abundance is affected by multiple factors and a more comprehensive analysis incorporating additional external variables is necessary to better understand the factors specifically impacting abundance in Hangzhou Bay and its adjacent waters.
Interannual difference analysis and fishery management prospect of P. trituberculatus gillnet fishery
In the fishing industry, P. trituberculatus production shows considerable annual fluctuations, with distinct “big and small year” phenomena often triggering reactions from fishermen and atttacting widespread societal attention28. P. trituberculatus is mainly caught using gillnets and crab pots with trawls and dragnets also contributing to the catch5,7. However, gillnets are the predominant method, followed by crab pots. Consequently, the “big and small year” phenomena are typically observed in traditional gillnet fisheries. As shown in Fig. 1, the traditional gillnet fishing grounds for P. trituberculatus in northern Zhejiang are primarily located at depths of 30–50 m, with a long history of development and relatively fixed fishing grounds. In terms of location and operation timing, traditional gillnet fishing in northern Zhejiang Province mainly targets the bait groups and overwintering transit groups12. Significant differences in the mean carapace length of P. trituberculatus were observed between different years, which may be attributed to external environmental factors28. During summer, bottom salinity values at stations S10, S11 and S22 were lower than 30, whereas the remaining three stations had salinity levels exceeding 30, with relatively concentrated salinity values. These findings indicate that bottom salinity significantly affects P. trituberculatus abundance, suggesting that salinity may be a primary factor driving abundance and size variations in the traditional gillnet fishing grounds of northern Zhejiang. From a fishing production perspective, the CPUE of P. trituberculatus using gillnets was highest and lowest in September and January (of the following year), respectively, with the September CPUE being 2.8 fold higher than that in January. This decrease in CPUE over time can be attributed to two factors: first, the declining catch over the fishing season; second, the migration of P. trituberculatus12. In conclusion, by analyzing the temporal and spatial distribution of P. trituberculatus and its influencing factors, and combining with the carapace length and production fishing situation of P. trituberculatus in traditional gillnet fishing ground, we believe that multiple factors contribute to the inter-annual differences observed in the gillnet fishing grounds of northern Zhejiang Province. As the fishing grounds are fixed, bottom salinity plays a crucial role in driving gillnet fishery formation. In constrast, the fishing grounds’ flexibility allows for adjustment based on current climatic conditions. Future evaluations of inter-annual differences in the P. trituberculatus fishery should integrate various fishing methods. To manage the P. trituberculatus gillnet fishery in northern Zhejiang Province effectively, it is essential to forecast the number of gillnets based on the current year’s climate regulate the number of gillnets used by fishing vessels, strictly enforce the minimum mesh size requirements and gradually implement a quota-based fishing system8.
Conclusions
Based on the resource investigation in Hangzhou Bay and its adjacent waters in spring, summer, autumn and winter, and the monitoring and production investigation in the traditional gillnet production area, this study revealed the resource density and distribution characteristics of P. trituberculatus in Hangzhou Bay and its adjacent waters, and the key driving factors affecting its distribution. The results showed that the spatial and temporal distribution of population density and individual size of P. trituberculatus in Hangzhou Bay and its adjacent waters showed obvious seasonal variation, and the distribution of P. trituberculatus showed significant correlation with seasons and bottom salinity, which suggested that the bottom salinity significantly affected the spatial and temporal distribution of P. trituberculatus in Hangzhou Bay and its adjacent waters. The population size of P. trituberculatus in traditional gillnet fishing ground has obvious interannual difference. Because of the fixed fishing grounds, bottom salinity was an important factor driving the formation of gillnet fishery. These findings can provide scientific references for understanding the population dynamics of P. trituberculatus in Hangzhou Bay and its adjacent waters, fishing ground formation mechanism and formulating fishery management measures.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. The data should not be circulated without the knowledge of the authors, because they involve the rights and interests of project funders.
References
Wang, F. et al. Application of neural network and MODIS 250 m imagery for estimating suspended sediments concentration in Hangzhou Bay, China. Environ. Geol. 56, 1093–1101. https://doi.org/10.1007/s00254-008-1209-0 (2009).
Wang, F. et al. Remote-sensing inversion model of surface water suspendedsediment concentration based on in situ measured spectrumin Hangzhou Bay, China. Environ. Earth Sci. 67, 1669–1677. https://doi.org/10.1007/s12665-012-1608-0 (2012).
Gao, S. Q., Yu, G. H. & Wang, Y. H. Distributional features and fluxes of dissolved nitrogen, phosphorus and silicon in the Hangzhou Bay. Mar. Chem. 43, 65–81. https://doi.org/10.1016/0304-4203(93)90216-B (1993).
Liu, S., Sun, J. S. & Hurtado, L. A. Genetic differentiationof Portunus trituberculatus, the world’s largest crab fishery, among its three main fishing areas. Fish Res. 148, 38–46. https://doi.org/10.1016/j.fishres.2013.08.003 (2013).
Wang, Y. B., Ye, T., Wang, X. G. & Zhou, C. Y. Impact of main factors on the catch of Portunus trituberculatus in the Northern East China sea. Pak. J. Zool. 49, 15–19. https://doi.org/10.17582/journal.pjz/2017.49.1.15.19 (2016).
Yu, C. G., Song, H. T., Yao, G. Z. & Shen, X. Y. Study on rational study or rational utilization of crab resources in the inshore water of Zhejiang. Mar. Fish. 25, 136–141. https://doi.org/10.3969/j.issn.1004-2490.2003.03.008 (2003).
Xu, G. Q., Zhu, W. B. & Xu L,X. Gillnet selectivity for swimming crab Portunus trituberculatus in the East China sea. Fish. Sci. 87, 31–38. https://doi.org/10.1007/s12562-020-01488-0 (2021).
Zhu, W. B. et al. Transition to timely and accurate reporting: An evaluation of monitoring programs for China’s first Total Allowable Catch (TAC) pilot fishery. Mar. Policy. 129, 104503. https://doi.org/10.1016/j.marpol.2021.104503 (2021).
Zhang, J., Shi, X. F., He, P. G. & Shi, J. G. Effectiveness of escape vent shape in crab pots for releasing swimming crab Portunus trituberculatus in the East China sea. Aquacult. Fish 8, 332–340. https://doi.org/10.1016/j.aaf.2021.12.007 (2023).
Liu, X. Y., Han, X. L. & Han, Z. Q. Effects of climate change on the potential habitat distribution of swimming crab Portunus trituberculatus under the species distribution model. J. Oceanol. Limnol. 40, 1556–1565. https://doi.org/10.1007/s00343-021-1082-1 (2022).
Sun, C. Y. & Wang, Y. B. Impacts of the sampling design on the abundance index Estimation of Portunus trituberculatus using bottom trawl. Acta Oceanol. Sin. 39, 48–57. https://doi.org/10.1007/s13131-020-1607-z (2020).
Song, H. T., Ding, Y. P. & Xu, Y. J. Migration distribution and population composition characteristics of Portunus trituberculatus in the offshore of Northern Zhejiang Province. Mar. Sci. Bull. 8, 66–74 (1989).
Wang, Y. B. et al. Spawner-recruit analysis of Portunus (Portunus) trituberculatus (Miers, 1876) in the case of stock enhancement implementation: A case study in Zhejiang sea area, China. Turk. J. Fish Aquat. Sci. 17, 293–299. https://doi.org/10.4194/1303-2712-v17_2_08 (2017).
Liu, Y. et al. Genetic differentiation between populations of swimming crab Portunus trituberculatus along the coastal waters of the East China sea. Hydrobiologia 618, 125–137. https://doi.org/10.1007/s10750-008-9570-2 (2009).
GB/T 12763. 6-2007; Specifications for Marine Surveys-Part 6: Marine Biological Surveys. General Administration of QualitySupervision, Inspection and Quarantine of the People’s Republic of China. Standardization Committee of the People’s Republic of China: Beijing, China (2008).
Yu, C. G., Song, H. T. & Yao, G. Z. Assessment of the crab stock biomass in the continental shelf waters of the East China sea. J. Fish China. 28, 41–46 (2004).
Xu, G. Q., Xu, K. D., Wang, H. X. & Zhou, Y. D. Spatial and temporal distribution of Portunus trituberculatus and its influencing factors in Ruian sea area, Zhejiang Province. Haiyang Xuebao. 46, 66–67. https://doi.org/10.12284/hyxb2024052 (2024).
Borchers, D. L., Buckland, S. T., Priede, I. G. & Ahmadi, S. Improving the precision of the daily egg production method using generalized additive models. Can. J. Fish Aq. Sci. 54, 2727–2742 (1997).
Wood, S. N. & Augustin, N. H. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecol. Model. 157, 157–177. https://doi.org/10.1016/S0304-3800(02)00193-X (2002).
Tian, S. Q. et al. Standardizing CPUE of ommastrephes bartramii for Chinese squid-jigging fishery in Northwest Pacific ocean. Chin. J. Oceanol. Limnol. 27, 729–739. https://doi.org/10.1007/s00343-009-9199-7 (2009).
Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. AC-19, 716–723. https://doi.org/10.1007/978-1-4612-1694-0_16 (1974).
Planque, B., Bellier, E. & Lazure, P. Modelling potential spawning habitatof sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) in the Bay of Biscay. Fish Oceanogr. 16, 16–30. https://doi.org/10.1111/j.1365-2419.2006.00411.x (2007).
Zhang, X. N. et al. Analysis of key physiological characteristics of Portunus trituberculatus in response to short-term low salinity stress. J. Ocean Univ. China 22, 746–754. https://doi.org/10.1007/s11802-023-5341-y (2023).
Li, X. D., Wang, J., Yang, C. H. & Wang Y,B. Spatiotemporal distribution of Portunus trituberculatus in the Northern East China sea based on two machine learning methods. Oceanol. Limnol. Sin. 52, 1284–1292. https://doi.org/10.11693/hyhz20210200050 (2021).
Ye, Y. F. et al. Strategy of metabolic phenotype modulation in Portunus trituberculatus exposed to low salinity. J. Agric. Food Chem. 62, 3496–3503. https://doi.org/10.1021/jf405668a (2014).
Shentu, J. K., Xu, Y. J. & Ding, Z. N. Effects of salinity on survival, feeding behavior and growth of the juvenile swimming crab, Portunus trituberculatus (Miers, 1876). Chin. J. Oceanol. Limnol. 33, 679–684. https://doi.org/10.1007/s00343-015-4218-3 (2015).
Xu, H. & Zhang, C. L. Spatiotemporal variation in the condition factor of Portunus trituberculatus and influencing factors in Haizhou Bay. J. Fish. Sci. China. 30, 492–501. https://doi.org/10.12264/JFSC2022-0443 (2023).
Zhang, H. L. et al. Spring inter-annual variation of Portunus trituberculatus abundance in the Zhongjieshan Archipelago seas. J. Zhejiang Ocean Univ. (Nat. Sci. Ed.). 41, 569–573. https://doi.org/10.3969/j.issn.1008-830X.2022.06.013 (2022).
Author information
Authors and Affiliations
Contributions
Guoqiang Xu: methodology, formal analysis, and writing—original draft preparation. Guoqiang Xu and Hongliang Zhang: software and data curation. Guoqiang Xu and Yongdong Zhou: writing—review and editing, visualization, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, G., Zhang, H. & Zhou, Y. Spatiotemporal distribution and fishing ground driving mechanism of swimming crab Portunus trituberculatus in Hangzhou Bay and its adjacent waters. Sci Rep 15, 22429 (2025). https://doi.org/10.1038/s41598-025-02748-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02748-8