Abstract
This study delved into the impact of aging on the properties of five common microplastic types, including polymethylmethacrylate (PMMA), polystyrene (PS), polyvinyl chloride (PVC), polyethylene (PE), and polypropylene (PP). The aging process significantly altered the particle size distribution: PS, PP, and PMMA underwent a contraction, with average sizes decreasing by 6.8%, 3.2%, and 1.7%, respectively, whereas PE and PVC experienced an expansion, with increases of 3.1% and 1.7%. Notably, aging generally increased the specific surface area of all microplastics by more than 20%, a change that could influence their environmental interactions. Scanning electron microscopy revealed marked surface cracks and depressions in aged PE and PVC, in contrast to minor surface alterations in PS and PMMA. Fourier transform infrared spectroscopy further indicated modifications in the characteristic peaks of aged PMMA, PP, and PE microplastics. Exposure experiments demonstrates that increasing microplastic concentrations from 100 mg/L to 5000 mg/L accelerated mortality rates in clams, with juveniles exhibiting slower mortality onset compared to adults. Prolonged exposure led to rising mortality rates across all groups, suggesting a cumulative toxic effect from long-term microplastic exposure. These findings underscore the environmental ecological risks associated with the altered physicochemical properties of aged microplastics, particularly for the Philippine clam. This study provides an essential insight for advancing our understanding of microplastic behavior and their ecological impacts, highlighting the need for further research to mitigate these environmental threats.
Similar content being viewed by others
Introduction
With the acceleration of industrialization, plastics have been widely utilized in packaging, manufacturing, agriculture, healthcare, and daily life due to their light weight, durability, and low cost1. However, the extensive production and use of plastics have led to the accumulation of plastic waste in the environment. In nature, plastic waste degrades into tiny particles, known as microplastics, through physical, chemical, and biological processes2. Currently, microplastic pollution has become a global environmental issue. Microplastics have been detected in oceans, freshwater, soil, and even the air, not only in remote polar regions but also in urban water bodies and soils2. The global distribution of microplastic types areas follows: 36% polyethylene (PE), 21% polypropylene (PP), 12% polyvinyl chloride (PVC), < 10% polyethylene terephthalate (PET), < 10% polyurethane (PUR), and < 10% polystyrene (PS)3. A report by the U.S. Environmental Protection Agency on global marine microplastic monitoring revealed that 90% of microplastics are concentrated in the upper 12 m of seawater, while about 40% are found below 20 m4. Among these, PE, PP, PS, and PES are the predominant polymer types in seawater, beaches, and subtidal sediments5.
Microplastics can be ingested by marine animals, and studies have documented their ingestion by over 200 species6. Microplastics can also transfer across trophic levels. For instance, in freshwater food chains, PS nanoparticles transfer from algae to zooplankton, and subsequently to species such as Oryzias sinensis and Zacco temminckii7. The accumulation patterns of microplastics vary among marine organisms. In fish, microplastics primarily accumulate in the liver, intestines, and gills8,9,10, while in shellfish, they tend to accumulate in the digestive gland, circulatory system, and gills11. In crustaceans such as shrimp and crabs, microplastics accumulate in the gills, muscles, and hepatopancreas12,13. Since microplastics are non-nutritive, their ingestion may divert energy from reproduction to survival, potentially affecting subsequent generations14. High concentrations of PS microplastics can disrupt the normal development of copepods, leading to developmental delays and significant downregulation of the Usp gene associated with molting and metamorphosis15. Under the influence of PET microplastics, oysters exhibit reduced egg production and sperm quality, leading to lower larval development levels16. Furthermore, microplastics may adsorb heavy metals, organic compounds, and microorganisms, thereby increasing their potential harm to ecosystems and organisms17,18,19. The transfer and accumulation of microplastics through the food chain ultimately pose significant threats to human health. Studies have shown that microplastics entering the human body may affect various organs, the nervous system, and the endocrine system20,21,22,23,24.
However, the aging process of microplastics in natural environments also presents new challenges to marine biological safety. Research indicates that ultraviolet (UV) radiation, heat, oxygen, and microorganisms are the primary drivers of microplastic degradation. UV radiation initiates polymer chain breakage through photo-oxidation, while heat accelerates oxidation and decomposition25. Microorganisms further degrade microplastics by secreting enzymes26. Aged microplastics fragment into smaller particles (e.g., nanoplastics), which can more easily penetrate biological barriers such as cell membranes and intestinal walls, entering marine organisms and causing more severe ecotoxicological effects27. Studies have shown that aged microplastics ingested by marine diatoms lead to cell membrane damage, mitochondrial dysfunction, and oxidative stress, ultimately triggering apoptosis or necrosis28. In marine mussels, aged microplastics may activate the immune system, inducing excessive inflammatory responses that result in tissue damage or chronic inflammation29. In freshwater shrimp, aged microplastics may interfere with the endocrine system, affecting the development and function of reproductive organs, leading to reduced reproductive capacity or abnormal embryonic development30. Aging causes the surface oxidation of microplastics, forming functional groups such as hydroxyl and carboxyl groups, which may enhance interactions with biomolecules, leading to cell damage or inflammatory responses in organisms like Spirulina31. The increased surface area of aged microplastics makes them more prone to adsorb persistent organic pollutants (e.g., polycyclic aromatic hydrocarbons, pesticides) and heavy metals, forming “pollutant-microplastic complexes” that amplify their toxicity32. During aging, additives (e.g., plasticizers, antioxidants) and monomers (e.g., bisphenol A, styrene) in microplastics may be more readily released into the environment. These additives and monomers pose potential hazards such as endocrine disruption, carcinogenicity, and reproductive toxicity33. Aged microplastics are more likely to transfer through the food chain, leading to bioaccumulation and biomagnification in organisms34. Therefore, the aging process of microplastics not only threatens the stability of marine ecosystems but also poses potential risks to the quality and safety of marine products and human health.
Mollusks play a crucial role in marine ecosystems, serving as intermediaries in the food chain that connect primary producers to top predators while maintaining environmental balance through their biofiltration processes35. The Philippine curtain clam, a small marine bivalve, is widely distributed along the coastlines of China, Japan, Korea, and North Korea. Due to its suitability for high-density aquaculture and its economic value in human diets, this species is widely used as a model organism for monitoring and assessing marine pollution. The filter-feeding nature of clams makes them ideal carriers of microplastic particles and trace pollutants. Studies have shown that wild clams contain various types of microplastic particles, including fibers, fragments, films, and granules36, with the most common types being PE, PET, PP, and PS37. These microplastics primarily accumulate in key tissues such as the digestive tract and gonads of clams, potentially causing toxic effects38. Ingested microplastics can also concentrate chemicals and persistent organic pollutants, posing high risks of cancer and debilitating diseases to humans through the food chain39,40,41. Although there are extensive literatures on the aging and toxicity of PS42, reports on the toxicity of aged microplastics such as PE, PP, PVC, and polymethyl methacrylate (PMMA) in clams are limited. This is significant because microplastics derived from synthetic polymers with different chemical and physical properties may induce varying toxic effects.
This study focuses on the Philippine curtain clam, a species that plays a vital role in marine ecosystems and is also widely farmed for economic purposes. The research investigates the toxic effects and distribution patterns of five common plastics (PP, PS, PMMA, PVC, and PE) in natural seawater on both adult and juvenile Philippine curtain clams. The findings not only elucidate the degradation mechanisms of microplastics but also provide critical scientific insights for assessing their ecological safety in marine organisms. These results contribute to a deeper understanding of microplastic pollution and offer theoretical support for developing effective mitigation strategies.
Materials and methods
Preparation of microplastics
The method employed to prepare five sets of microplastic samples was described by Wang et al.43, each composed of different polymers: polypropylene (PP) (A.R., Yi En Chemical Technology Co., Ltd., Shanghai, China), poly-methyl methacrylate (PMMA) and poly-vinyl chloride (PVC) (A.R., Aladdin Biochemical Science and Technology Co. Ltd., Shanghai, China), polystyrene (PS) and polyethylene (PE) (A.R., Sigma Aldrich Trading Co., Ltd., Shanghai, China).
Two agate milling jars, each containing 6 g of plastic pellets and 30 ml of a 0.05% bovine serum protein (BSA) solution (A.R., McLean Biochemical Technology Co. Ltd., Shanghai, China) as a stabilizer, were sealed and subjected to 16 h of planetary ball milling (Xinnuo Instrument Group Co., LTD, Shanghai, China) to create a plastic suspension. The suspension was then balanced, weighed, and centrifuged at 10,000 rpm and 4 °C for 20 min. The supernatant was analyzed for particle size using a laser diffraction meter (Mastersizer 3000E, Spectrum Instrument System Co. Ltd, Shanghai, China) and filtered to obtain moist microplastic samples, which were dried at 50 °C for 20 min, yielding microplastics with a particle size range of 0.8–1.0 μm.
Aging of microplastics
The design of the experimental seawater aging apparatus for microplastics is depicted in Figure S1. The main component of the device is a transparent PMMA tube with dimensions of 20 cm in length, 5 cm in outer diameter, and 4.6 cm in inner diameter. Both ends of the tube are sealed using hydrophilic polytetrafluoroethylene (PTFE) filter membranes, which have a pore size of 0.45 μm. The method for sealing the filter membranes and their dimensions is detailed in reference44. Waterproofing around the ends and the body of the device is achieved using PTFE waterproof tape, and the apparatus is finally secured with stainless steel pipe clamps. The finished device is shown in Figure S1.
500 mg of each kind of plastic was taken into a beaker respectively, and 100 ml of seawater (sourced from the Aquatic Experimental Farm of Jimei University, Xiamen, China) was added. After thorough mixing and ultrasonic treatment, the mixture evenly dispersed the plastics into the seawater. The treated suspension was then added into the seawater aging device, which had been designed in the previous section, sealed, and placed into a transparent EVA bucket equipped with a 1 mm mesh seal (Figure S2). The bucket was filled with seawater and submerged into the seawater storage tank at a depth of approximately 1 m for natural aging. To avoid adverse factors such as waves, reefs, and the passage of fishing boats, which could potentially cause damage to the experimental setup or pollution, the experimental location was selected to be the seawater storage tank within the aquatic experimental site of Jimei University in Xiamen, China.
After 30 days of seawater aging, the device was removed from the seawater pool. The plastic was then separated from the seawater using a filtration device. It was washed with ultrapure water and separated again by filtration, with the washing process being repeated three times to obtain clean moist plastic powder. The wet plastic powder was dried in an oven at 50 ºC for 1 h to obtain dry plastic powder.
Microplastic staining
Rhodamine B (Rh B) was selected as the dyeing agent. Rh B working solution was made at a concentration of 200 mg/L using anhydrous ethanol and ultrapure water as solvents, respectively. 500 mg of each of the microplastics prepared in the Sect. 2.1 was taken and placed on a PTFE membrane. Rh B stained more effectively with anhydrous ethanol than with ultrapure water as the solvent45,46. However, PMMA is easily corroded after contact with anhydrous ethanol47, so in this experiment PE, PS, PP, and PVC were dyed using working solution with anhydrous ethanol as solvent, and PMMA was dyed using working solution with ultrapure water as solvent. Each kind of plastic was dropped into 3 mL of working solution, and after 30 min of dyeing, it was washed into a clean 500 mL beaker with ultrapure water, and then filtered, dried and continued to repeat the above dyeing operation until a uniformly dyed microplastic powder was obtained (Figure S3).
Microplastics characterization
The particle sizes of the aging samples were measured using a laser diffraction particle size distribution meter (Malvern ms3000, Malvern Panalytical, Worcestershire, UK). When a laser beam passes through a dispersed particle sample, the particle size was measured by measuring the intensity of scattered light, and then the data was analyzed to calculate the particle size distribution that forms the scattering spectrum. The obscuration was set to 5–20%, and the test was begun. The sample cell was cleaned once with ethanol and rinsed twice with distilled water before use. After the cleaning process was completed, the sample cell was vacuum-degassed to remove bubbles and dissolved gases, preventing interference with laser scattering and ensuring accurate measurements. After the sample was added, the microplastic sample was stirred evenly dispersed in ultrapure water through the dispersion head, ensuring that the obscuration remained at 5−20% before the measurement was started.
The sample obtained was directly dropped onto a single crystal wire silicon wafer (1 cm×1 cm) which was then completely dried in a vacuum drying oven at 60℃. Following this, gold spraying was carried out and, after the gold spraying, the samples were photographed by scanning electron microscopy. The working distance of the detector was set at about 5 mm and the acceleration voltage was adjusted to 5 kv.
Each microplastic sample powder was mixed with potassium bromide at a ratio of 1:100 under infrared light and was then pressed into a uniform transparent film by a manual tablet press. ATR-FTIR measurements were made by an infrared spectrometer (Tensor 27, Brewer Co., Ltd., Germany). In the FTIR software, the advanced measurement was selected, with air as the measurement background, and each measurement was performed at a resolution of 4 cm−1 in the 4500–400 cm−1 area, with 130 scans per sample. The membrane sample was placed into the membrane and the test was started after the background was determined. A blank was made for every two samples that were analyzed.
Surface area and porosimetry analysis using the N2-BET method with a Nova 3200e analyzer (Quantachrome Instruments, Boynton Beach, USA) was employed to characterize the component polymers, surface micro-morphology, specific surface area, and pore volume of the particles.
Exposure experiments
Collection of Philippine curtain clams
Three hundred juvenile Philippine curtain clams with intact shells of similar size (shell length 1 ± 0.20 cm, shell height 0.5 ± 0.20 cm) were collected off the coast of Nandi Park, Longzhou Road, Jimei District, Xiamen City, Fujian Province (N 24°56’26’’, E 118°09’61”). They were brought to the laboratory and cleaned using seawater filtered by 0.45 µm millipore filter membrane (Jinteng Experimental Equipment Co., LTD, Tianjin, China). Philippine clam seedlings were placed in a plastic incubator (15 cm×10 cm×10 cm), which was renewed daily, kept aerated and fed with dilute starch solution. They were ready to be used for experiments after 24 h of incubation.
Adult Philippine curtain clams (shell length 4 ± 0.50 cm, shell height 2 ± 0.50 cm) were purchased from the Guankou Farmers Market, Jimei District, Xiamen City, Fujian Province, and were cleaned using ultrafiltered seawater. They were cleaned with ultra-filtered seawater and placed in a plastic bucket (36 cm × 35 cm × 27 cm). The temporary culture method was the same as above.
Toxicological experiments on microplastics in Philippine curtain clams
Each original or aged microplastic was prepared with 0.45 μm-filtered seawater in a clean beaker to form 1 L mixtures with four different concentrations of 100 mg/L, 500 mg/L, 1000 mg/L, and 5000 mg/L, respectively, and the microplastic samples were evenly dispersed in the solvent by ultrasonic treatment before use. Six Philippine curtain clams were cultured in each beaker. And control groups was also set up. Each group was set up with four replicates. Observations were made continuously for 168 h, during which time the behavioral signs, survival and mortality of the clams were recorded (Fig. 1). The salinity, pH, and dissolved oxygen were maintained at 2.50 ± 0.07%, 8.01 ± 0.12, and 7.00 ± 0.18 mg/L, respectively. The oxygenation was intermittently supplemented during the experiment.
The distribution of microplastics in Philippine curtain clams
Five microplastic samples stained with Rh B were added to 0.45 μm-filtered seawater in clean beakers to make a suspension of 1000 mg/L, and the microplastic samples were dispersed evenly in the solvent by ultrasonic treatment. Four adult Philippine curtain clams were cultured in each beaker. All experiments were completed in four parallel groups. After 48 h of continuous exposure, the clams were rinsed three times with ultrapure water to remove surface-adhered microplastics. The clams were then shelled and dissected to obtain the gills, digestive glands, and the remaining tissues. Subsequently, tissue homogenization was performed on the gills and digestive glands, and the biological samples were treated with a 10% KOH digestion method at 60 °C for 24 h48,49 and centrifuged at a speed of 4500 r/min for 5 min. The supernatant was collected for the measurement of fluorescence intensity using a fluorescence spectrophotometer (Shimadzu Corporation, Japan), and a spiked recovery experiment was performed to ensure accuracy.
Statistical analysis
Statistical tests were conducted using the SPSS Statistical Analysis Software Program version 19. Independent sample t-Test was used to analysis the particle size characterization, specific surface area, mortality rates differences between the new and old groups and between adult and juvenile samples. One-way analysis of variance (ANOVA) confirmed tissue distribution, mortality rates concentration-dependent differences across all tested levels and mortality rates material-related differences. A probability level of P < 0.05 was considered statistically significant.
Results and discussions
Particle size characterization
A detailed analysis was conducted on the particle size distribution of five types of microplastics (PS, PP, PMMA, PE, and PVC), and the impact of aging on their particle size characteristics was investigated (Table 1). The results indicate that aging exerts varying degrees of influence on the particle size distribution of microplastics. After aging, the particle sizes of PS, PP, and PMMA significantly decreased (P < 0.05), with PS’s DX(90) reducing from 1.189 μm to 1.109 μm, PP’s DX(90) from 0.948 μm to 0.917 μm, and PMMA’s DX(90) slightly from 1.191 μm to 1.177 μm. In contrast, the particle size distribution of PE and PVC expanded after aging, particularly notable in PE, where the DX(90) significantly increased from 0.984 μm to 1.116 μm (P < 0.05). These findings suggest that the effect of aging on particle size distribution are material-specific and vary across microplastic types.
Specific surface area analysis
Based on the data provided in Table 2, we can observe the changes in the specific surface area (m2/g) of different microplastics before and after aging treatment. The aging process generally significantly enhanced the specific surface area of all microplastics (P < 0.001). Specifically, the specific surface area of PS increased by 26.0%, PP by 22.0%, PMMA by 23.9%, PE by 26.0%, and PVC by 24.9%. These changes suggest that the aging process may have led to alterations in the surface morphology of the microplastics, such as an increase in surface roughness or a reorganization of the microstructure, thereby increasing their specific surface area. The statistical significance of particle size characterization and specific surface area analysis indicates that the particle surfaces have been eroded or the particles have undergone fragmentation, resulting in a decrease in particle size and an increase in the exposed surface area. An increase in specific surface area could potentially influence the interactions of microplastics with other substances in the environment, such as enhanced adsorption capacity, which may increase the ecological risks associated with microplastics.
SEM analysis
The SEM analysis of different microplastics samples is presented in Fig. 2. PS microplastics are uniform microspheres with a slightly rough surface and shallow cracks and dents before aging (Fig. 2(a)). The molecular structure of PS contains a benzene ring, which provides stability and results in relatively good aging resistance for PS. After aging, the surface of PS microplastics showed cracks and dents compared to those without aging, and some PS microplastics were cracked and deformed into irregularly shaped fragments (Fig. 2(b)). No significant changes in PP microplastics after aging were observed (Fig. 2(c-d)). PP microplastics are formed through the polymerization and connection of numerous PP fragments. The surface is uneven and irregularly shaped, and due to the molecular structure of PP containing stable carbon-carbon single bonds and carbon-hydrogen bonds, these bonds are not easily broken under conventional conditions. The methyl side group (-CH3) of PP is non-polar, which makes PP highly tolerant to many chemicals and less susceptible to chemical reactions.
PMMA microplastics and PMMA fragments bond and polymerize with each other. Although the arrangement of non-aged PMMA microplastics is disorderly, it is evident that the surface is smooth, with fewer cracks, and the microplastics are relatively full and round(Fig. 2(e)). The molecular structure of PMMA includes stable carbon-carbon single bonds and carbon-hydrogen bonds, which are not easily broken under conventional conditions. Additionally, although the carbonyl group (C = O) of PMMA has a certain polarity, it does not readily undergo chemical reactions under most environmental conditions. However, after continuous exposure to seawater, some PMMA microplastics displayed some cracks (Fig. 2(f)).
The surface of PE microplastics before aging is smooth and uniform, with fewer cracks and a more full appearance(Fig. 2(g)). After aging, the surface of PE microplastics exhibited obvious cracks and depressions (Fig. 2(h)). This may be due to PE being a non-polar hydrocarbon polymer, where oxidation reactions occur, leading to the formation of hydroxyl groups on the molecular chain, reducing the toughness of PE and causing surface cracking.
Unaged PVC microplastics are very round and smooth, with almost no cracks on the surface, appearing very full, and closely resembling spherical shapes(Fig. 2(i)). In contrast to non-aged PVC microplastics, those that have undergone aging show cracks on the surface, and some exhibit significant depressions (Fig. 2(j)). Under the influence of ultraviolet light and high temperatures, PVC may undergo dechlorination reactions, resulting in a decline in material properties, and the appearance of slight cracks and surface depressions.
In summary, PE and PVC samples showed obvious cracks and depressions after aging. PS and PMMA samples exhibited slight cracks and depressions post-aging. No significant aging marks were observed on PP samples, which may be related to the hydrophobicity and insufficient aging time of PP powder. These findings are consistent with those of previous studies50,51,52,53,54.
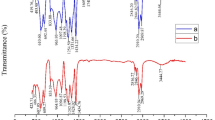
FTIR analysis before and after aging
Ultraviolet radiation and oxygen exposure are the two main factors that induce the aging process of MPs in aquatic environment. In the FTIR spectra of PS (Figure. 3 (a-b)), the O-H contraction vibration peak appeared in the 3500 cm−155. The absorption peak nearly at 2400 cm−1 is usually associated with the stretching vibration of the carbon-hydrogen (C-H) bond on the benzene ring in PS56.The adsorption band nearly in the 1700 cm ̶1 was attributed to the C = C stretching vibration in the aromatic structure in PS57.
After the aging of PP samples (Figure. 3 (c-d)), superficial signals were observed at 1650–1850 cm−1 and 3250–3600 cm−1, which were determined to be the expansion vibrations of carbonyl groups (i.e., ketones, esters and lactones) and hydroxyl groups (i.e., alcohols and hydroperoxides), respectively58,59. These infrared spectral data showed that the oxidation, hydrolysis, and cracking of PP samples occurred during the aging process, which led to the formation of some new functional groups. Compared with of PMMA samples (Figure. 3 (e)), hydrolytic reaction or oxidation reaction may have led to the formation of a hydroxyl group during the aging process (the characteristic peak at nearly band 3500 cm ̶1 (Figure. 3 (f))60.
After the PE sample aging (Figure. 3(g-h)), a new O-H stretching vibration peak appeared near the band 3500–4000 cm ̶1, indicating that chemical reactions such as oxidation or hydrolysis occurred during the aging process of the PE sample. The formation of new functional groups on the MPs surface, such as vinyl, carbonyl, and hydroxyl/hydroxyl peroxides, resulted in a significant increase in the carbonyl index of MPs, which is consistent with previous studies61.
In the FTIR spectra of PVC (Figure. 3 (i-j)), similar to the FTIR spectra of PS, the O-H contraction vibration peak appeared in the 3500 cm−155. The absorption peak nearly at 2400 cm−1 might exist C = O absorption band in PVC62. The adsorption band nearly in the 1700 cm ̶1 was attributed to the C = O stretching vibration peak appeared in PVC51, and the CH2 deformation appeared in the 1400 cm ̶1 band in PVC63. After aging, the characteristic peaks of the samples did not change, and the shape of the characteristic peaks was basically the same. This indicated that no new functional groups appeared in the aging process of these microplastics, which may have been caused by the short duration and low degradation degree of the aging process of microplastics.
Exposure experiment results and analysis
Analysis of microplastic lethality
In the 100 mg/L microplastic exposure experiment depicted in Fig. 4, significant disparities in mortality rates over time were observed between unaged and aged microplastics, as well as among different types of microplastics (PS, PMMA, PVC, PE and PP). Adult Philippine clams exposed to non-aged PS, PMMA, and PVC began to show individual deaths after continuous feeding for 72 to 86 h, while those exposed to PE and PP began to die after 100 to 132 h. In the PS, PMMA, and PVC exposure groups, the mortality rate of clams reached 50% between 110 to 120 h, while in the PE group, it was observed at 158 h, and in the PP group, the clam mortality rate remained at 20% from 145 h onwards. Adult Philippine clams exposed to aged PS, PMMA, and PVC began to show individual deaths after continuous feeding for 72 to 86 h, while those exposed to PE and PP began to die after 96 to 120 h. In the PS, PMMA, and PVC exposure groups, the mortality rate of clams reached 50% at 115 to 122 h, while in the PE group, it was observed at 145 h, and in the PP group, the clam mortality rate was 40% at 160 h. In the adult clam group, both non-aged and aged PS, PMMA, and PVC exhibited strong toxicity. Aged PE and PP exhibited stronger toxicity compared to the non-aged group, while aged PS, PMMA, and PVC showed a certain degree of toxicity reduction compared to the non-aged group.
Juvenile Philippine clams exposed to non-aged PS, PMMA, and PVC began to show individual deaths after continuous feeding for 108 to 120 h, while those exposed to PE and PP began to die after 132 to 146 h. In the PS, PMMA, and PVC exposure groups, the mortality rate of clams reached 50% at 145 to 150 h, while at 160 h, the clam mortality rate was 40% in the PE group and 20% in the PP group. Juvenile Philippine clams exposed to aged PS, PMMA, and PVC began to show individual deaths after continuous feeding for 132 to 146 h, while those exposed to PE and PP began to die after 132 h. From the onset of individual deaths until 160 h, in the PS, PMMA, and PVC exposure groups, the mortality rate of clams reached 50% at 155 to 160 h, while in the PE and PP groups, the clam mortality rate was 40% at 160 h. In the juvenile clam group, both non-aged and aged PS, PMMA, and PVC exhibited strong toxicity. Aged PE and PP exhibited stronger toxicity compared to the non-aged group, while aged PS, PMMA, and PVC showed a certain degree of toxicity reduction compared to the non-aged group.
From the above analysis, it is evident that PS, PMMA, and PVC have a stronger toxic effect on both adult and juvenile Philippine clams compared to PE and PP. This might be due to the significantly higher specific surface area of PE and PP before and after aging (Table 1), which allowed them to more effectively adsorb inorganic or organic contaminants in seawater64,65, providing a certain degree of purification to the seawater. Compared to the original microplastics, the toxic effect of aged PS, PMMA, and PVC on both adult and juvenile clams showed a slight reduction, but aged PE and PP exhibited significantly stronger toxicity. This might be due to the formation of new groups in aged PE and PP, such as highly hydrophilic -OH groups, which can induce lipid peroxidation reactions, protein and DNA denaturation in clams66, leading to cellular damage and functional impairment. Although PMMA also formed highly hydrophilic groups during aging, it did not show significant toxicity enhancement, possibly because most PMMA remained hydrophobic after aging67. The mortality rate of juvenile Philippine clams was lower than that of adults under the same conditions, possibly because the daily food intake of juvenile clams was much lower than that of adults, making it more difficult for microplastics to accumulate in their bodies. Additionally, due to their smaller size, juvenile clams might have more difficulty ingesting the gradually floating plastic samples compared to adults. And their overall lower metabolic rate may have influenced how rapidly they accumulated and reacted to toxins.
Mortality of Philippine Curtain Clam in exposure experiments with 100 mg/L concentration of microplastic suspensions: (a) Adult Philippine curtain clams exposed to unaged microplastic; (b) Adult Philippine curtain clams exposed to aged microplastic; (c) Juvenile Philippine curtain clams exposed to unaged microplastic; (d) Juvenile Philippine curtain clams exposed to aged microplastic.
Figure 5 illustrates that in the exposure experiment with a microplastic sample concentration of 500 mg/L, the initiation of mortality in adult Philippine clams exposed to unaged PS, PMMA, and PVC commenced after 60 h of continuous feeding. Conversely, in the exposure experiments involving PE and PP, mortality onset occurred between 84 and 108 h. Within the PS, PMMA, and PVC exposure groups, 50% clam mortality was noted between 110 h and 120 h, whereas in the PE group, it transpired at 142 h, and in the PP group, a 35% mortality rate was observed after 160 h of culture. In adult Philippine clams subjected to aged PS, PMMA, and PVC, mortality was detected after 60 h of continuous feeding, and between 84 and 108 h in the PE and PP exposure experiments. The 50% mortality point for clams in the PS and PMMA exposure groups was reached between 110 h and 118 h, in the PVC group at 132 h, in the PE group at 140 h, and in the PP group, a 35% mortality rate was recorded after 160 h of culture.
Juvenile Philippine clams exposed to unaged PS, PMMA, and PVC exhibited mortality after 84 h of continuous feeding, and between 108 and 120 h in the PE and PP exposure experiments. The 50% mortality thresholds for clams in the PS, PMMA, and PVC exposure groups were achieved at 130 h, 122 h, and 145 h respectively, in the PE group at 150 h, and in the PP group, a 35% mortality rate was documented after 160 h of culture. Similarly, juvenile Philippine clams exposed to aged PS, PMMA, and PVC showed mortality after 84 h of continuous feeding, and between 108 and 120 h in the PE and PP exposure experiments. In the PS, PMMA, and PVC exposure groups, 50% clam mortality was attained at 128 h, 135 h, and 148 h respectively, in the PE group at 150 h, and in the PP group, a 32% mortality rate was recorded after 160 h of culture.
The analysis reveals that, akin to the 100 mg/L exposure group, both juvenile and adult clam populations exposed to unaged and aged PS, PMMA, and PVC exhibited greater toxicity compared to those exposed to PE and PP. Elevating the microplastic sample concentration to 500 mg/L resulted in a marked shift in the mortality timing of Philippine clams, with both the onset of mortality and the time to 50% mortality significantly shortened for both adult and juvenile populations, suggesting a positive correlation between concentration changes and microplastic enrichment in Philippine clams. Nonetheless, there was no discernible difference in mortality rates between the aged and unaged groups across the five materials, possibly due to the high microplastic concentration overshadowing the nuances in plastic types. Future investigations should delve deeper into the specific effects of aging processes on plastic toxicity and assess the contributions of microplastic attributes such as shape, size, and surface roughness to overall toxicity.
Mortality of Philippine Curtain Clam in exposure experiments with 500 mg/L concentration of microplastic suspensions: (a) Adult Philippine curtain clams exposed to unaged microplastic; (b) Adult Philippine curtain clams exposed to aged microplastic; (c) Juvenile Philippine curtain clams exposed to unaged microplastic; (d) Juvenile Philippine curtain clams exposed to aged microplastic.
As depicted in Fig. 6, within the experimental framework involving a 1000 mg/L microplastic suspension, adult Philippine clams subjected to non-aged PS, PMMA, and PVC exhibited initial mortality after sustained ingestion periods ranging from 36 h to 60 h. Conversely, those exposed to PE and PP displayed mortality onset between 72 h and 96 h. Notably, the 50% mortality threshold was reached in the PS, PMMA, and PVC groups at 120 h, 132 h, and 145 h, respectively, with the PE group at 145 h and the PP group at 160 h, registering a mortality rate of 35%. In the context of aged PS, PMMA, and PVC exposure, adult Philippine clams mortality commenced between 48 h and 60 h, with PE and PP exposures initiating mortality between 72 h and 96 h. The 50% mortality mark was achieved in the PS, PMMA, and PVC groups at 138 h, 142 h, and 150 h, respectively, with the PE group at 158 h and the PP group at 160 h, indicating a mortality rate of 40%.
Juvenile Philippine clams exposed to non-aged PS, PMMA, and PVC began to perish after 84 h of continuous feeding, whereas those exposed to PE and PP experienced mortality onset between 108 h and 120 h. The 50% mortality was observed in the PS, PMMA, and PVC groups at 119 h, 132 h, and 144 h, respectively, with the PE group at 144 h and the PP group at 160 h, showing a mortality rate of 35%. In the scenario of aged PS, PMMA, and PVC exposure, juvenile Philippine clams mortality initiation occurred after 84 h of feeding, with PE and PP exposures resulting in mortality onset between 108 h and 120 h. The 50% mortality threshold was met in the PS, PMMA, and PVC groups at 158 h, 140 h, and 148 h, respectively, with the PE group at 150 h and the PP group at 160 h.
The experimental outcomes underscore that non-aged PS, PMMA, and PVC exert a heightened toxicity on both adult and juvenile Philippine clams, manifesting as an earlier mortality onset and a quicker attainment of the 50% mortality threshold. In contrast, PE and PP exhibit a diminished toxicity, with a delayed mortality onset and a protracted timeline for reaching the 50% mortality mark. Aged PS, PMMA, and PVC appear to intensify their toxicity towards adult Philippine clams, evidenced by an accelerated mortality onset and a hastened 50% mortality occurrence. However, the toxicity dynamics of aged PS, PMMA, and PVC towards juveniles are incongruent; certain plastic types (e.g., PMMA) demonstrate a reduction in toxicity, while others (e.g., PS) reveal an escalation in toxicity. This divergence suggests that the aging process may exert varied influences on the toxicity profiles of different plastic types. Under congruent exposure parameters, adult Philippine clams typically exhibit greater susceptibility to plastic toxicity, characterized by an earlier mortality onset and a higher 50% mortality rate. This heightened sensitivity may be attributable to the physiological states, metabolic capacities, and toxic substance tolerances of adults. As the duration of exposure lengthens, the mortality rates of clams across all exposure cohorts escalate, albeit at disparate paces contingent upon the plastic type and aging condition. This variability may be attributable to the bioavailability, bioaccumulation, and specific toxicological mechanisms of plastics on organisms. At a concentration of 1000 mg/L, non-aged PS, PMMA, and PVC demonstrate a more pronounced toxicity towards adult Philippine clams, with an earlier mortality onset and a more rapid attainment of the 50% mortality threshold. This observation implies that the toxicological impacts of plastics may amplify with escalated exposure concentrations.
Mortality of Philippine Curtain Clam in exposure experiments with 1000 mg/L concentration of microplastic suspensions: (a) Adult Philippine curtain clams exposed to unaged microplastic; (b) Adult Philippine curtain clams exposed to aged microplastic; (c) Juvenile Philippine curtain clams exposed to unaged microplastic; (d) Juvenile Philippine curtain clams exposed to aged microplastic.
As shown in Fig. 7, in the exposure experiment with 5000 mg/L microplastic samples, adult Philippine curtain clams exposed to unaged PS, PMMA, and PVC began to die after continuous feeding for 48 h, while individual deaths in the PE and PP exposure experiments started between 72 h and 108 h. The 50% mortality rate in the PS, PMMA, PVC, PE, and PP exposure groups occurred between 142 h and 158 h. Adult Philippine curtain clams exposed to aged PS, PMMA, and PVC began to die after continuous feeding for 36 to 48 h. Individual deaths in the aged PS, PMMA, and PVC exposure groups started between 36 and 48 h, and in the PE and PP exposure experiments, individual deaths occurred between 72 and 108 h. The 50% mortality rate in the PS, PMMA, PVC, PE, and PP exposure groups occurred between 132 h and 155 h.
Juvenile Philippine curtain clams exposed to unaged PS, PMMA, and PVC began to show individual deaths after continuous feeding for 84 h, and in the PE and PP exposure experiments, individual deaths occurred between 108 h and 120 h. Individual deaths were observed at 120 h. The 50% mortality rate in the PS, PMMA, PVC, PE, and PP exposure groups for adult clams occurred between 140 h and 158 h. Philippine curtain clams larvae exposed to aged PS, PMMA, and PVC showed individual deaths after continuous feeding for 84 h, and in the PE and PP exposure experiments, individual deaths occurred between 108 h and 120 h. The mortality rate of the clams increased stepwise from the onset of individual deaths to 160 h. In the PE and PP exposure experiments, the mortality rate of the clams began to rise from 108 to 120 h. The 50% mortality rate in the PS, PMMA, PVC, PE, and PP exposure groups for juvenile clams occurred between 140 h and 158 h.
At a concentration of 5000 mg/L, the onset of mortality in Philippine curtain clams did not advance as it did in other concentration exposure groups, which may suggest that the enrichment rate of microplastics in Philippine curtain clams approached its limit when the concentration was 1000 mg/L. The similar sensitivity of adult and juvenile Philippine curtain clams to microplastics, reaching a 50% mortality rate at similar time points, further indicates that the enrichment rate of microplastics has approached its limit.
The comprehensive analysis indicates that there are differences in the toxicity of various types and conditions (aged versus non-aged) of microplastics to Philippine curtain clams, and factors such as microplastic concentration, clam age, and exposure duration all influence toxicity manifestations.
There were significant differences in the lethality rates of new and aged microplastics at concentrations ranging from 100 to 1000 mg/L (P < 0.05), while there were no significant differences in the values of new and aged microplastics at a concentration of 5000 mg/L. This may be caused by the fact that the excessively high content of microplastics may have masked the original differences between new and aged microplastics. The filtration feeding system and metabolic mechanism of juvenile clams have not been fully developed. At a concentration of 100–500 mg/L, they are more vulnerable to the effects of microplastics. The relatively larger ratio of the surface area to the volume of juveniles makes them have more opportunities to come into contact with microplastics, resulting in significant differences in the mortality rates (P < 0.05). However, at high concentrations of 1000–5000 mg/L, a large amount of microplastics cause serious damage to the physiological functions of both juvenile and adult clams, exceeding the differences in their tolerances, leading to no significant differences in the mortality rates. For the same material at different concentrations, there were certain differences in the toxicity to adult and juvenile clams. Regarding aged microplastics and adult clams, the mortality rate of PP at high concentrations was significantly higher than that at low concentrations. For example, except for the concentration of 5000 mg/L, there were significant differences in the mortality rates among different groups of PE; there was a positive correlation between the concentration of PS and the mortality rate. For juvenile clams, the overall mortality rate caused by aged microplastics was 0%, but there were differences among specific concentrations of some materials.
Mortality of Philippine curtain clams in exposure experiments with 5000 mg/L concentration of microplastic suspensions: (a) Adult Philippine curtain clams exposed to unaged microplastic; (b) Adult Philippine curtain clams exposed to aged microplastic; (c) Juvenile Philippine curtain clams exposed to unaged microplastic; (d) Juvenile Philippine curtain clams exposed to aged microplastic.
The distribution of microplastics within Philippine curtain clams
Upon exposure to stained microplastic suspensions at a concentration of 1000 mg/L for 48 h, the tissue distribution within the Philippine curtain clams was depicted in Figure S4 and S5. In the PMMA exposure group, the soft shell, gills, and digestive glands of the Philippine curtain clams were found to have been stained pink, with the digestive glands exhibiting the deepest coloration, indicating that PMMA had been accumulated predominantly in these tissues, with the highest concentration in the digestive glands (a). The PP exposure group revealed a white soft shell, with minimal stained microplastics having been detected in the gills and digestive glands, suggesting a minor enrichment of PP in these organs (b). Following exposure to PE, the soft shell of the Philippine curtain clams was observed to have taken on a light pink to orange tint, while the gills and digestive glands were notably dark pink, signifying that PE enrichment had been primarily localized to these tissues (c). In the PS exposure group, the soft shell was lightly pink, the gills exhibited a more intense pink, and the digestive glands were comparatively pale, indicating that PS accumulation had occurred across these tissues, with the gills demonstrating the highest concentration (d). The PVC exposure group presented a white soft shell, with the gills and digestive glands having been stained light pink, suggesting a minor presence of PVC in these tissues (e).
Quantitative fluorescence analysis revealed tissue-specific accumulation patterns of microplastics in the Philippine curtain clams, consistent with previous studies11. The distribution characteristics exhibited significant plastic-type dependency and tissue specificity (Figs. 8 and S5). PS showed the highest accumulation in gill tissue (6.25 µg/g, 28.92%), significantly exceeding other tissues, likely due to the affinity of its benzene ring structure for gill mucosal surfaces, while maintaining substantial levels in the digestive gland (5.67 µg/g, 26.24%). PMMA demonstrated predominant enrichment in the digestive gland (6.6 µg/g, 35.00%, P < 0.05 versus other tissues), aligning with its hydrophilic properties that facilitate filter-feeding uptake. PE exhibited a dual accumulation pattern in both gills (4.68 µg/g, 32.03%) and digestive gland (4.39 µg/g, 30.04%), with significant inter-tissue differences (P < 0.05) reflecting its moderate hydrophobicity-mediated trans-tissue transport. PVC showed comparable distribution between gills (3.82 µg/g, 35.03%) and digestive gland (3.93 µg/g, 36.03%, P > 0.05), whereas polypropylene (PP) accumulated predominantly in the digestive gland (2.15 µg/g, 38.91%) and gills (2.03 µg/g, 36.74%), both exhibiting limited tissue penetration due to high hydrophobicity. Notably, distinct tissue distribution patterns emerged among plastic types ( P < 0.05): PMMA and PP showed the highest relative proportions in the digestive gland (35.00% and 38.91%, respectively), while PS and PVC maintained balanced gill-digestive gland distributions, attributable to synergistic effects between physicochemical properties (e.g., hydrophilicity/hydrophobicity, functional groups) and biological uptake pathways (filter-feeding, respiration).
The results demonstrate that microplastic distribution is jointly regulated by physicochemical properties and uptake mechanisms68. PMMA’s hydrophilicity promotes digestive gland enrichment via filter-feeding; PE’s moderate hydrophobicity enables gill capture followed by digestive gland transfer; PS’s benzene ring enhances gill adhesion; while Pp and PVC’s high hydrophobicity restricts tissue permeation, with PVC’s density further reducing bioavailability. Crucially, all tested microplastics were detected in gills, confirming respiration as a major uptake route, while digestive gland dominance underscores filter-feeding’s pivotal role. These findings systematically elucidate microplastic translocation and accumulation mechanisms in aquatic filter-feeders, providing critical scientific basis for ecological risk assessment.
Fluorescence intensity of microplastics distribution in the Philippine curtain clams. The different letters inside the cumulative bars represent the significant differences in the proportion of fluorescent intensities between different organs.The letters following the hyphen (-) represent significant differences in the proportion of fluorescent intensities among different plastics within the tissue.
Conclusion
This study demonstrated that short-term aging had a significant impact on the particle size of various types of microplastics, with certain types experiencing changes in surface morphology, such as visible cracks and depressions on PE and PVC surfaces. The specific surface areas of all types of microplastics were increased by more than 20% after short-term aging, which may have enhanced their interaction with environmental pollutants and contributed to water purification, to some extent reducing their toxic effects on Philippine curtain clams. PE and PP with larger specific surface areas exhibited lower toxicity to both adult and juvenile Philippine curtain clams, whether they were aged or not. During the aging process, new functional groups such as hydroxyl and carbon-carbon triple bonds were formed in some types of microplastics, such as PP and PE, which may have caused tissue or cellular damage in Philippine curtain clams and enhanced the toxicity of aged PP and PE. Under the same exposure conditions, the mortality rate of juvenile clams was lower than that of adult clams, possibly due to differences in food intake, size, and metabolic rate, which impeded the accumulation of microplastics and the rate of toxic effects. As the concentration of microplastics increased from 100 mg/L to 5000 mg/L, the onset of mortality and the time to reach 50% mortality were significantly shortened for both adult and juvenile clams, indicating a positive correlation between microplastic concentration and the speed of toxic effects. With increasing exposure time, the mortality rate of all exposure groups increased, suggesting that long-term exposure to microplastics may have led to cumulative toxic effects. All tested microplastics were detected in the gills, confirming that respiration is the main uptake pathway. The dominant enrichment of microplastics in the digestive glands highlights the core role of filter-feeding behavior. In summary, this study preliminarily revealed that the type of microplastics, aging, concentration, and the age and size of the exposed organisms significantly influenced the toxicity and mortality rate of Philippine curtain clams. The distribution of microplastics in aquatic organisms is closely associated with their uptake mechanisms. These findings underscored the complexity and multifaceted nature of microplastic toxicity and the importance of considering multiple factors when assessing their ecological impacts.
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to requirements of the research institution.
References
Fu, Z., Chen, G., Wang, W. & Wang, J. Microplastic pollution research methodologies, abundance, characteristics and risk assessments for aquatic biota in China. Environ. Pollut. 266, 115098 (2020).
Yang, Y. et al. Plastic waste and microplastics (MPs) formation: management, migration, and environmental impact. J. Environ. Chem. Eng. 12, 112926 (2024).
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3 (7), e1700782 (2017).
Everaert, G. et al. Risks of floating microplastic in the global ocean. Environ. Pollut. 267, 115499 (2020).
Shim, W. J., Hong, S. H. & Eo, S. Marine microplastics: abundance, distribution, and composition. In Microplastic Contamination in Aquatic Environments (1–26). Elsevier. (2018). Eddy, Y. Z.
Walkinshaw, C., Lindeque, P. K., Thompson, R., Tolhurst, T. & Cole, M. Microplastics and seafood: lower trophic organisms at highest risk of contamination. Ecotoxicol. Environ. Saf. 190, 110066 (2020).
Chae, Y., Kim, D., Kim, S. W. & An, Y. J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 8 (1), 284 (2018).
Ding, J., Zhang, S., Razanajatovo, R. M., Zou, H. & Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 238, 1–9 (2018).
Su, L. et al. The occurrence of microplastic in specific organs in commercially caught fishes from Coast and estuary area of East China. J. Hazard. Mater. 365, 716–724 (2019).
Rahman, R. R. et al. Organ-specific bioaccumulation of microplastics in market fish of Dhaka and size-dependent impacts of PVC microplastics on growth of Anabus testudineus. Environ. Pollut. 361, 124807 (2024).
Du, H. et al. Quantitative assessment of in vivo distribution of nanoplastics in bivalve ruditapes philippinarum using reliable SERS tag-labeled nanoplastic models. Nanoscale 14, 7807–7816 (2022).
Seta, A. S. et al. Oxidative effects of consuming microplastics in different tissues of white shrimp Litopenaeus vannamei. Mar. Pollut. Bull. 193, 115137 (2023).
Truchet, D. M., Buzzi, N. S., Moulatlet, G. M. & Capparelli, M. V. Macroecotoxicological approaches to emerging patterns of microplastic bioaccumulation in crabs from estuarine and marine environments. Sci. Total Environ. 870, 161912 (2023).
Park, E. J. et al. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 324, 75–85 (2020).
Kim, K., Yoon, H., Choi, J. S., Jung, Y. J. & Park, J. W. Chronic effects of nano and microplastics on reproduction and development of marine copepod Tigriopus japonicus. Ecotoxicol. Environ. Saf. 243, 113962 (2022).
Sorini, R., Kordal, M., Apuzza, B. & Eierman, L. E. Skewed sex ratio and gametogenesis gene expression in Eastern oysters (Crassostrea virginica) exposed to plastic pollution. J. Exp. Mar. Biol. Ecol. 544, 151605 (2021).
Cao, Y. et al. A critical review on the interactions of microplastics with heavy metals: mechanism and their combined effect on organisms and humans. Sci. Total Environ. 788, 147620 (2021).
Nguyen, M. K. et al. Emergence of microplastics in the aquatic ecosystem and their potential effects on health risks: The insights into Vietnam. J. Environ. Manage. 344, 118499 (2023).
Nguyen, M. K. et al. A comprehensive review on ecological effects of microplastic pollution: An interaction with pollutants in the ecosystems and future perspectives. TrAC Trends Anal. Chem. 168, 117294 (2023).
Cho, Y. et al. Evaluation of size-dependent uptake, transport and cytotoxicity of polystyrene microplastic in a blood-brain barrier (BBB) model. Nano Converg. 11, 40 (2024).
Shi, L. et al. Innovative mechanisms of micro- and nanoplastic-induced brain injury: emphasis on the microbiota-gut-brain axis. Life Sci. 357, 123107 (2024).
Barceló, D., Picó, Y. & Alfarhan, A. H. Microplastics: detection in human samples, cell line studies, and health impacts. Environ. Toxicol. Pharmacol. 101, 104204 (2023).
Yee, M. S. L. et al. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 11, 496 (Basel, Switzerland, 2021).
Wu, P. et al. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 437, 129361 (2022).
Chamas, A. et al. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 8 (9), 3494–3511 (2020).
Jaiswal, S., Sharma, B. & Shukla, P. Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov. 17, 100567 (2020).
Sorasan, C. et al. Ageing and fragmentation of marine microplastics. Sci. Total Environ. 827, 154438 (2022).
Wang, S. et al. The interactions between microplastic Polyvinyl chloride and marine diatoms: physiological, morphological, and growth effects. Ecotoxicol. Environ. Saf. 203, 111000 (2020).
Pirsaheb, M., Hossini, H. & Makhdoumi, P. Review of microplastic occurrence and toxicological effects in marine environment: experimental evidence of inflammation. Process Saf. Environ. Prot. 142, 1–14 (2020).
Sun, S., Jin, Y., Luo, P. & Shi, X. Polystyrene microplastics induced male reproductive toxicity and transgenerational effects in freshwater Prawn. Sci. Total Environ. 842, 156820 (2022).
Hadiyanto, H. et al. Interactions between polyethylene and polypropylene microplastics and Spirulina Sp. microalgae in aquatic systems. Heliyon, 7(8), e07676 (2021).
Sun, Y. et al. Combined neurotoxicity of aged microplastics and Thiamethoxam in the early developmental stages of zebrafish (Daniorerio). Environ. Pollut. 348, 123853 (2024).
Luo, H. et al. Effects of aging on environmental behavior of plastic additives: migration, leaching, and ecotoxicity. Sci. Total Environ. 849, 157951 (2022).
Dahms, H. U., Hagiwara, A. & Lee, J. S. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquatic toxicology. Netherlands 101 (1), 1–12 (Amsterdam, 2011).
Rodrigues-Filho, J. L. et al. From ecological functions to ecosystem services:linking coastal lagoons biodiversity with human well-being. Hydrobiologia, 850(12), 2611–2653 (2023).
Ding, J. et al. An examination of the occurrence and potential risks of microplastics across various shellfish. Sci. Total Environ. 739, 139887 (2020).
Rosa, M., Ward, J. E. & Shumway, S. E. Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: a review. J. Shellfish Res. 37 (4), 727–746 (2018).
Sussarellu, R. et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. 113(9), 2430–2435 (2016).
Goswami, S. et al. The alarming link between environmental microplastics and health hazards with special emphasis on cancer. Life Sci. 355, 122937 (2024).
Alpaydin, A.Ö. et al. Microplastics, as a risk factor in the development of interstitial lung disease-a preliminary study. Environ. Pollut. 363, 125054 (2024).
Bora, S. S. et al. Microplastics and human health: unveiling the gut microbiome disruption and chronic disease risks. Front. Cell. Infect. Microbiol. 14, 1492759 (2024).
Martin, L. M., Gan, N., Wang, E., Merrill, M. & Xu, W. Materials, surfaces, and interfacial phenomena in nanoplastics toxicology research. Environ. Pollut. 292, 118442 (2022).
Wang, J. et al. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 254, 113024 (2019).
Chen, C.C. et al. Copper adsorption to microplastics and natural particles in seawater: a comparison of kinetics, isotherms, and bioavailability. Environ. Sci. Technol. 55 (20), 13923–13931 (2021).
Jain, R., Mathur, M., Sikarwar, S., & Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manage. 85(4), 956–964 (2007).
Radiul, S. M., Chowdhury, J., & Hazarika, S. Fluorescent H-aggregates of pure rhodamine B (RhB) in glycerol, ethylene glycol, methanol and butanol under ambient condition. J. Mol. Struct. 1275, 134606 (2023).
Haynes, W. M. CRC handbook of chemistry and physics (Ed. William, M. H.) (CRC press, 2016).
Dehaut, A. et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 215, 223-233 (2016).
Zou, Y. et al. Effects of six digestion methods on fluorescence detection of polystyrene microplastics in organisms. Environ. Sci. 40(1), 496-503 (2019).
Gao, L. et al. Sorption behaviors of petroleum on micro-sized polyethylene aging for different time in seawater. Sci. Total Environ. 808, 152070 (2022).
Ouyang, Z. et al. The photo-aging of polyvinyl chloride microplastics under different UV irradiations. Gond. Res. 108, 72–80 (2022).
Mao, R. et al. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 393, 122515 (2020).
Wu, X. et al. Photo aging of polypropylene microplastics in estuary water and coastal seawater: important role of Chlorine ion. Water Res. 202, 117396 (2021).
Kaddouri, A., Serier, B., Kaddouri, K. & Belhouari, M. Experimental analysis of the physical degradation of Polymers–The case of polymethyl methacrylate. Frattura Ed. Integrità Strutturale. 14 (53), 66–80 (2020).
Sen, P. & Pugazhenthi, G. Synergistic effect of dual nanofillers (MWCNT and Ni–Al LDH) on the electrical and thermal characteristics of polystyrene nanocomposites. J. Appl. Polym. Sci. 135 (29), 46513 (2018).
Kradjel, C. & Lee, K. A. NIR analysis of polymers. In Handbook of Near-Infrared Analysis (Eds. Donald, A. B. & Emil, W. C.) 547–586. (CRC, 2007).
Du, Y. et al. Hydrophilic modification of polycarbonate surface with surface alkoxylation pretreatment for efficient separation of polycarbonate and polystyrene by froth flotation. Waste Manag. 118, 471–480 (2020).
Brandon, J., Goldstein, M. & Ohman, M. D. Long-term aging and degradation of microplastic particles: comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 110 (1), 299–308 (2016).
Tang, C. C., Chen, H. I., Brimblecombe, P. & Lee, C. L. Morphology and chemical properties of polypropylene pellets degraded in simulated terrestrial and marine environments. Mar. Pollut. Bull. 149, 110626 (2019).
Salehuddin, S. M. F., Wahit, M. U., Kadir, M. R. A., Sulaiman, E. & Kasim, N. H. A. Mechanical and morphology properties of feather fiber composite for dental post application. Malaysian J. Anal. Sci. 18 (2), 368–375 (2014).
Li, C. et al. Aging process of microplastics in the aquatic environments: aging pathway, characteristic change, compound effect, and environmentally persistent free radicals formation. Water, 14(21), 3515 (2022).
Zhu, H. M., Jiang, X. G., Yan, J. H., Chi, Y. & Cen, K. F. TG-FTIR analysis of PVC thermal degradation and HCl removal. J. Anal. Appl. Pyrol. 82 (1), 1–9 (2008).
Ramesh, S., Leen, K. H., Kumutha, K. & Arof, A. K. FTIR studies of PVC/PMMA blend based polymer electrolytes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 66 (4–5), 1237–1242 (2007).
Shao, X. et al. Polyamide microplastics as better environmental vectors of Cr (VI) in comparison to polyethylene and polypropylene microplastics. Mar. Pollut. Bull. 186, 114492 (2023).
Agboola, O. D. & Benson, N. U. Physisorption and chemisorption mechanisms influencing micro (nano) plastics-organic chemical contaminants interactions: a review. Front. Environ. Sci. 9, 678574 (2021).
Abidli, S. et al. Effects of environmentally relevant levels of polyethylene microplastic on Mytilus galloprovincialis (Mollusca: Bivalvia): filtration rate and oxidative stress. Environ. Sci. Pollut. Res. 28, 26643–26652 (2021).
Huang, Z. & Wang, H. Study on the impact of Photoaging on the generation of very small microplastics (MPs) and nanoplastics (NPs) and the wettability of plastic surface. Environ. Sci. Pollut. Res. 30 (40), 92963–92982 (2023).
Guzzetti, E., Sureda, A., Tejada, S. & Faggio, C. Microplastic in marine organism: environmental and toxicological effects. Environ. Toxicol. Pharmacol. 64, 164–171 (2018).
Acknowledgements
The authors would like to express special thanks to the Aquaculture Experimental Farm of Jimei University (Xiamen, Fujian) for their great help in clam cultivation.
Funding
This study was financially supported by the Fujian Provincial Department of Young and Middle-aged Teachers Education Research Project Science and Technology (JAT200703), Xiamen Medical College Students’ Innovation and Entrepreneurship Training Program (X202212631001), Science and Technology Project of Xiamen Medical College (K2023-32).
Author information
Authors and Affiliations
Contributions
Conceptualization, L.Z.; Funding acquisition, L.Z.; Investigation, T.T. and J.C.; Methodology, T.T. and L.Z.; Project administration, J.C. and Y.H.; Resources, J.C. and J.Y.; Software, Y.X. and H.Y.; Validation, T.T., Y.H. and J.Y.; Writing-original draft, Y.C., H.Y. and L.Z.; Writing-review and editing, Y.X., Y.C. and F.Y. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study upholds ethical guidelines, minimizing harm to marine organisms. Humane methods were used to ensure their welfare. The research aims to contribute to sustainable development and conservation of marine ecosystems, informing effective environmental policies.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeng, L., Yang, H., Tong, T. et al. Study on the toxic effect of seawater-aged microplastics on Philippine curtain clams. Sci Rep 15, 18078 (2025). https://doi.org/10.1038/s41598-025-02823-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02823-0
Keywords
This article is cited by
-
Unraveling the adsorption behavior of Zn(II) on UV-aged PET and PP microplastics: kinetic and isotherm analyses
Environmental Science and Pollution Research (2025)