Abstract
This research presents the synthesis of BaFeO2.67/Fe2O3 nanocomposite using a simple chemical co-precipitation method without any capping agents, which distinguishes it from existing methodologies. The study provides a comprehensive investigation into its structural, morphological, and optical properties. X-ray diffraction (XRD) analysis confirmed the formation of a hexagonal crystalline structure with an average crystallite size of approximately 40.50 nm. Fourier-transform infrared spectroscopy (FTIR) identified key functional groups, further establishing the compound’s unique characteristics. Notably, surface potential measurements revealed a net surface charge of -20.85 ± 0.50 mV, indicating excellent stability, which is critical for practical applications. Scanning electron microscopy (SEM) illustrated rough surfaces with irregularly shaped agglomerates, suggesting strong interactions between barium (Ba) and iron (Fe). Energy-dispersive X-ray spectroscopy (EDX) validated the presence of Ba, Fe, and O elements. Furthermore, optical analyses demonstrated a band gap energy of 2.09 eV, positioning this nanocomposite as a promising candidate for applications in catalysis, magnetic materials, and optoelectronic devices. This work not only contributes to the understanding of BaFeO2.67/Fe2O3 nanocomposites but also opens new avenues for their application in advanced technological fields.
Similar content being viewed by others
Introduction
Nanocomposites have garnered significant attention in recent years due to their unique structural, optical, and electronic properties, which arise from the combination of different materials at the nanoscale1,2,3. Among various nanocomposite systems, metal oxides have emerged as crucial components in a wide range of applications, including catalysis, energy storage, environmental remediation, and sensing technologies4,5,6,7,8,9. The integration of metal oxides can enhance the performance characteristics of materials, making them suitable for advanced applications in fields such as electronics, photonics, and renewable energy. Barium ferrite (BaFeO2.67) and iron oxide (Fe2O3) are two prominent metal oxides known for their magnetic, electronic, and optical properties10,11,12,13. BaFeO2.67 is particularly interesting due to its potential applications in magnetic materials and catalysts13, while Fe2O3 is widely used in gas sensing, photocatalysis, and as a pigment14,15,16,17. Recent studies have shown that the combination of these oxides into a nanocomposite structure can lead to synergistic effects that improve their overall performance and broaden their applicability. However, there remains a gap in understanding how the specific interactions between BaFeO2.67 and Fe2O3 can be optimized for enhanced functionality in targeted applications. In the existing literature, several works have focused on the individual properties of BaFeO2.67 and Fe2O3; however, limited research has been dedicated to exploring their combined effects in a nanocomposite format. For instance, while studies have demonstrated the magnetic properties of BaFeO2.67 and the photocatalytic capabilities of Fe2O3 separately, the synergistic benefits of their integration remain underexplored. This study aims to fill this gap by investigating the structural, spectral, morphological, and optical properties of the synthesized BaFeO2.67/Fe2O3 nanocomposite. By characterizing these properties, insights into the potential applications of this nanocomposite in various fields will be provided. The co-precipitation method is a well-established technique for synthesizing metal oxide nanocomposites due to its simplicity, cost-effectiveness, and ability to produce uniform particle sizes18,19,20,21. In this study, distilled water was employed as the sole solvent for the co-precipitation process, followed by a calcination step at 700 0C for five hours to ensure the formation of a stable nanocomposite structure. This method allows for better control over the morphology and crystallinity of the resulting material. The primary objective of this research is to investigate the structural, spectral, morphological, and optical properties of the synthesized BaFeO2.67/Fe2O3 nanocomposite. Additionally, the limitations of current methodologies will be highlighted, and exploration of how these findings can lead to improvements in material performance for specific applications will be conducted. Understanding the relationship between synthesis conditions and material properties will contribute to the development of advanced materials with tailored functionalities. This study represents a significant step toward exploring the capabilities of BaFeO2.67/Fe2O3 nanocomposites in modern technological applications and aims to establish a comprehensive understanding of their potential benefits in various fields.
Experimental procedure
Materials
The compounds utilized in the synthesis of the sample included barium nitrate (Ba(NO3)2), iron nitrate nonahydrate (Fe(NO3)3·9H2O), and sodium hydroxide (NaOH) as precursor materials. These reagents were procured from the commercial vendor, Sigma Aldrich, with a guaranteed analytical purity of 99.0%, and no additional purification steps were undertaken prior to their use. Distilled water was employed exclusively in all experimental procedures to maintain uniformity and consistency throughout the process.
Synthesis of BaFeO2.67/Fe2O3
To commence the experimental procedure, a solution of barium nitrate was meticulously prepared by dissolving the compound in distilled water with a concentration of 0.3 M in a beaker placed at ambient temperature. Continuous stirring of the solution was carried out for a quarter of an hour using a magnetic stirrer to guarantee thorough dissolution. Simultaneously, in a distinct step, an iron nitrate solution of the same molar concentration of 0.3 M was meticulously formulated in another beaker using an equal volume of distilled water and underwent the same stirring process until complete dissolution was achieved. Subsequent to the completion of the preparation steps for both solutions, they were amalgamated in a singular beaker and subjected to heating and continuous stirring at a temperature of 80 °C for a duration extending two hours. Throughout this amalgamation process, a sodium hydroxide solution was meticulously prepared by dissolving NaOH in 50 mL of distilled water to the desired concentration. Incrementally, this sodium hydroxide solution was introduced into the stirred amalgamation until adjustment of the pH level was achieved to 11. Post this pH adjustment, the amalgamated solution underwent filtration using specialized filter paper to effectively segregate any precipitate formed. The resultant precipitate was meticulously rinsed multiple times with a combination of distilled water and ethanol to effectively eliminate any residual impurities. The refined precipitate was meticulously gathered in a crucible and subjected to a drying process at a temperature of 200 °C until complete elimination of all moisture content was achieved. Upon achieving a state of complete dryness, the resultant solid material was meticulously ground to a fine powder consistency and subjected to calcination at a temperature of 700 °C over a duration of five hours. The ultimate product was securely stored in pristine containers, awaiting subsequent comprehensive analysis18. In addition, this is a schematic illustration of the possible chemical reactions that occur during the synthesis process.
Characterization
The optical characteristics were explored using a UV-3600 diffuse reflectance spectrometer (DRS) to ascertain the band gap energy. Fourier-transform infrared (FTIR) spectroscopy was conducted with a Nicolet iS10 FTIR spectrometer (Madison, WI, USA), spanning a wavenumber range of 400–4000 cm−1. Zeta potential analysis was carried out using a Malvern Zetasizer from the Nano series to assess the nanoparticles’ surface charge. The morphological attributes were analyzed through scanning electron microscopy (SEM) provided by Jeol Ltd., Tokyo, Japan. The purity and crystallite size of the samples were evaluated with a PANalytical X-ray diffractometer across a 2θ range of 10° to 80° with a scanning rate of 0.02 min− 1, employing Cu-Kα radiation with a wavelength of 1.5406Å.
Results and discussion
XRD analysis
The structural analysis of the BaFeO2.67/Fe2O3 nanocomposite was carried out using X-ray diffraction (XRD) to elucidate the crystalline structure and determine the crystallite size. The obtained XRD pattern exhibited sharp diffraction peaks at specific 2-theta angles, as illustrated in Fig. 1.
Comparing the experimental diffraction values with the standard data from JCPDS card No. 70-1321 and 16-065322 confirmed the hexagonal crystal structure of BaFeO2.67 and the monoclinic crystal structure of Fe2O3. This validation supports the successful synthesis of the BaFeO2.67/Fe2O3 nanocomposite. The crystallite size was calculated using the Debye-Scherrer equation: D = 0.9λ/βcosθ, where λ is the X-ray wavelength (0.154 nm), β is the full width at half maximum (FWHM) of the diffraction peak, and θ is the Bragg angle23,24,25,26. The average crystallite size was determined to be approximately 40.50 nm. Moreover, the average dislocation density (δ) and micro-strain(\(\:\epsilon\:\)) were computed using δ = 1/D2 and\(\:\:\epsilon\:={\upbeta\:}\text{c}\text{o}\text{s}{\uptheta\:}/4\), respectively27,28,29. In addition to, the micro-strain(\(\:\epsilon\:\)) was computed using the W-H formula :\(\:\:{\upbeta\:}\text{c}\text{o}\text{s}{\uptheta\:}=\frac{\text{K}{\uplambda\:}}{\text{D}}+4{\upepsilon\:}\:\text{s}\text{i}\text{n}\:{\uptheta\:}\)30.These results are summarized in Table 1. The lattice strain(\(\:\epsilon\:\)) values presented in Table 1 reveal that the BaFeO2.67/Fe2O3 nanocomposite experiences notable lattice strain. This strain suggests that intrinsic stress exists within the nanocomposite, likely arising from the diverse phases and their interfacial interactions. The presence of lattice strain can influence the material’s characteristics and functionality in both beneficial and detrimental ways. On one hand, increased lattice strain may lead to improvements in certain properties, like electrical or ionic conductivity, by generating additional active sites, which is advantageous for catalytic and energy storage applications. Conversely, excessive strain might undermine the structural integrity and reduce the mechanical stability of the material. Therefore, it is essential to comprehend the nature and degree of lattice strain in order to adjust the synthesis methods and enhance the specific properties of the nanocomposite for targeted applications. A thorough examination of lattice strain offers critical insights into the structural features of the nanocomposite, thereby deepening our understanding of its performance and potential uses29,30.
The lattice constants (a, b, and c), volume(Å3), and density(g/cm3) for the BaFeO2.67 and Fe2O3 nanocomposite were extracted from the XRD data and are presented in Table 2. These values closely match the corresponding entries in JCPDS card 70-1321 and 16–0653 for BaFeO2.67 and Fe2O3, respectively, reaffirming the structural coherence of the synthesized nanoparticles. The specific surface area (SSA) plays a crucial role in nanoparticles due to their high surface-to-volume ratio. The SSA, an intrinsic property, offers valuable insights into material characteristics, particularly in applications involving adsorption, catalysis, and surface reactions. The SSA can be calculated using the formula: SSA = 6 × 1000 / (D x ρ), where D represents the crystallite size and ρ is the density of the nanoparticles31.Crystallinity (%) = Acp./Ata, In this context, Acp. denotes the area beneath crystalline peaks, while Ata represents the entire area18,32.The computed SSA and crystallinity values are detailed in Table 2.
In conclusion, this comprehensive investigation not only validates the successful synthesis of the BaFeO2.67/Fe2O3 nanocomposite but also provides substantial data on their structural attributes and potential applications across diverse fields. These findings underscore the significance of utilizing XRD as a robust technique for characterizing nanomaterials and comprehending their distinctive features influenced by size and structure.
FTIR analysis
The Fourier Transform Infrared (FTIR) spectroscopy analysis provides crucial information about the molecular structure, functional groups, and chemical bonding present in the BaFeO2.67/Fe2O3 nanocomposite. As depicted in Fig. 2, the distinct absorption bands observed in the spectrum illustrate specific vibrational modes that correspond to the various functional groups and bonding environments of the compound. The broad absorption peaks at 3465.19 cm− 1 and 1623.07 cm− 1 are prominently associated with the stretching and bending vibrational modes of hydroxyl (OH) groups. This suggests the presence of adsorbed water or hydroxyl functional groups which may be vital for the stability and morphology of the nanocomposite33,34,35,36,37. The intensity of these peaks can indicate the extent of hydroxylation which could influence the material’s properties, such as reactivity and surface characteristics. The peak identified at 2935.90 cm− 1 is attributed to the stretching vibrations of N-H bonds. This implies that organic or nitrogen-containing species may be present in the synthesis process, possibly linked with the surfactants used for stabilization38. Understanding the role of such species is essential, as they could affect the aggregation and dispersion of nanoparticles during synthesis. The significant absorption feature at 1380.86 cm− 1 corresponds to the -C-O stretching vibrations, which are typically found in alkyl ethers. The presence of these vibrations could indicate the incorporation of organic compounds during synthesis34. This organic matter might play a crucial role in modifying the physical and chemical properties of the nanocomposite. Furthermore, the absorption peaks observed at 890.43, 837.78, 769.33, 641.45, and 490.26 cm− 1are attributed to Fe-O and Ba-O bonds, confirming the successful formation of the Fe2O3 and BaFeO2.67 phases within the composite. These peaks provide insights into the coordination environment of the metal ions and suggest that strong ionic interactions are present, which may significantly contribute to the structural integrity and stability of the nanocomposite39,40. The shifting of these peaks compared to their respective pure oxides could indicate variations in bond strengths and electronic environments due to the formation of the composite structure. Overall, the FTIR analysis provides substantial qualitative data regarding the interactions and configurations of functional groups within the BaFeO2.67/Fe2O3 nanocomposite.
Zeta potential determination
The surface charge characteristics of the synthesized nanoparticles were assessed using zeta potential (ζ) analysis conducted in triplicate to ensure the robustness of the results. Average values were calculated for each sample from the gathered data. The evaluation indicated that the BaFeO2.67/Fe2O3 nanocomposite displayed a net surface charge of − 20.85 ± 0.50 mV. According to established criteria, zeta potential values exceeding ± 30 mV typically indicate a stable and well-dispersed colloidal system, whereas values below ± 5 mV suggest a propensity for particle aggregation41. The results confirm that the nanoparticles generated exhibited zeta potential values above 5 mV, implying their stability and reduced likelihood of agglomeration.
SEM and EDX analysis
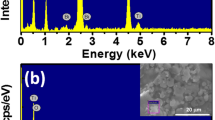
The morphological and surface properties of the prepared sample were characterized using Scanning Electron Microscopy (SEM), as illustrated in Fig. 3 at two different magnifications. The SEM images reveal a rough surface with irregularly shaped and sized nanoparticle agglomerates. Additionally, some of these agglomerates resemble nanosheets. The presence of increased agglomeration suggests a strong interaction between barium (Ba) and iron (Fe)42,43. Notably, the high degree of agglomeration makes it challenging to accurately determine the size and distribution of the nanoparticles. Energy Dispersive X-ray Spectroscopy (EDX) and elemental mapping analysis confirms the presence of barium (Ba), iron (Fe), and oxygen (O) in the synthesized compound BaFeO2.67/Fe2O3, (See Fig. 4). These confirmations are essential for validating the successful synthesis of the compound and ensuring its potential for various applications, such as catalysis or magnetic materials. The elemental analysis via SEM reveals distinct spatial patterns for barium (Ba) and iron (Fe) on the surface of the prepared sample. It is evident that Ba, Fe, and O are more uniformly distributed within the synthesized BaFeO2.67/Fe2O3 compound. Such homogeneous distributions indicate effective interactions among the elements, which enhance the physical and chemical properties of the material and reflect the success of the synthesis process.
UV-visible analysis
To thoroughly investigate the optical properties of the BaFeO2.67/Fe2O3 nanocomposite synthesized at 700℃, diffuse reflectance spectroscopy was utilized. The reflectance spectrum, shown in Fig. 5, spans the wavelength range of 200 to 900 nm, effectively illustrating the optical reflectance behavior of the sample. This method provides crucial insights into how the material interacts with light, which is pivotal for various applications, including photocatalysis and optoelectronics.
The Kubelka-Munk equation was employed to convert the reflectance data into absorbance values, formulated as F(R) = (1 - R)2/2R, where R is the diffuse reflectance percentage and F(R) represents the Kubelka-Munk function associated with absorbance44,45. This transformation allows for a more accurate analysis of the optical transitions within the material. To ascertain the bandgap energy (Eg) and understand the optical transitions between the valence band (VB) and conduction band (CB), a modified version of the Kubelka-Munk equation was used: (F(R)hv) = A(E - Eg)ⁿ46. In this equation, the value of n indicates the type of optical transition; a value of 1/2 denotes a direct allowed bandgap.The constant A signifies the transition probability, while E is defined as hv, with h representing Planck’s constant and v being the frequency of the incident light. By plotting (F(R)hν)2) and extrapolating the linear portion to determine where (F(R)hν)2 equals zero, the bandgap energy of the material was estimated.
This analysis, as presented in Fig. 6, has demonstrated that the BaFeO2.67/Fe2O3 nanocomposite exhibits a bandgap energy of 2.09 eV. This bandgap is indicative of a material suitable for various optical applications, aligning with findings reported in the literature, which underline the significance of bandgap tuning in nanocomposite materials. These enhancements are expected to contribute significantly to the depth and clarity of the discussion on the optical properties of the synthesized nanocomposite.
Conclusion
In conclusion, this study successfully synthesized BaFeO2.67/Fe2O3 using a chemical co-precipitation method devoid of capping agents, demonstrating the feasibility of producing complex oxide materials with tailored properties. Structural analysis via X-ray diffraction confirmed a stable hexagonal crystalline structure with an average crystallite size of 40.50 nm, indicating high purity. The identification of functional groups through Fourier-transform infrared spectroscopy further validated the compound’s structural integrity. Notably, surface potential measurements revealed a net surface charge of − 20.85 ± 0.50 mV, suggesting that BaFeO2.67/Fe2O3 possesses excellent electrostatic stability, which is crucial for its application in various fields. Morphological analysis using scanning electron microscopy highlighted rough surfaces and irregular agglomerates, indicative of strong interactions between barium and iron. These characteristics may enhance the material’s performance in catalytic and magnetic applications. Additionally, the optical analysis indicated a band gap energy of 2.09 eV, positioning BaFeO2.67/Fe2O3 as a promising candidate for optoelectronic devices. The findings from this research not only provide valuable insights into the synthesis and characterization of BaFeO2.67/Fe2O3 but also pave the way for future investigations aimed at optimizing its properties for specific applications. Future work should focus on exploring scalable production methods and assessing the material’s performance in real-world applications, particularly in catalysis, magnetic materials, and advanced optoelectronic systems. By translating these findings into practical applications, BaFeO2.67/Fe2O3 has the potential to contribute significantly to technological advancements in various industrial sectors.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Al-Asbahi, M. G. S. S., Al-Ofiri, B. A., Saad, F. A. A., Alnehia, A. & Hadi, M. Ag-Ag2O nanocomposite biosynthesis by mixed bacterial cultivation and effect of the pH on size and optical properties of the nanocomposite. J. Mater. Science: Mater. Med. Vol. 36, 19 (2025).
Guti´errez-Martín, D. et al. Unveiling the magnetic structure of BaFeO3 – y: shedding light on the elusive magnetic behavior. J. Alloys Compd. 1010, 177081 (2025).
Ansari, M. R. et al. Structural, optical, magnetic and anti-bacterial properties of green synthesized spinel zinc ferrite by microwave-assisted method. Mater. Chem. Phys. Vol. 301, 127641 (2023).
Gharbi, A. H. et al., Novel CuO–SiO2nanocomposites: Synthesis, kinetics, recyclability, high stability and photocatalytic eiciency for Rose Bengal dye removal.Transit. Metal Chem., (2024).
Zhao, J. et al., A novel silver-doped nickel oxide hole-selective contact for crystalline silicon heterojunction solar cells. Front Chem. Sci. Eng, 18, (2024).
Jiang, C. et al. Particle size dependence of the lithium storage capability and high rate performance of nanocrystalline anatase TiO2 electrode. J. Power Sources. 166, 239–243 (2007).
Naik, E. I., Naik, H. S. B., Sarvajith, M. S. & Pradeepa, E. Co-precipitation synthesis of Cobalt doped ZnO nanoparticles: characterization and their applications for biosensing and antibacterial studies. Inorg. Chem. Commun. Vol. 130, 108678 (2021).
Al-Shabib, N. A. et al. Biogenic synthesis of zinc oxide nanostructures from Nigella sativa seed: prospective role as food packaging material inhibiting broad-spectrum quorum sensing and biofilm. Sci. Rep. Vol. 6, 36761 (2016).
Somavia Ameen, R. et al. Investigation of structural, morphological, thermal, optical, and magnetic properties of graphene–embedded hematite and magnetite nanocomposites. Opt. Quant. Electron. 56, 1539 (2024).
Yazdi, N. A., Arefi, M. R., Mollaahmadi, E. & Nejand, B. A. To study the effect of adding Fe2O3 nanoparticles on the morphology properties and microstructure of cement mortar. Life Sci. J. 8, 550–554 (2011).
Havenga, D. et al. From Himba Indigenous knowledge to engineered Fe2O3 UV–blocking green nanocosmetics. Sci. Rep. 12, 2259 (2022).
Al–Hammadi, A. H., Alnehia, A., Al–Sharabi, A., Alnahari, H. & Al–Odayni, A. B. Synthesis of trimetallic oxide (Fe2O3–MgO–CuO) nanocomposites and evaluation of their structural and optical properties. Sci. Rep. Vol. 13, 12927 (2023).
Wollstadt, S. et al. Structural and magnetic properties of BaFeO2.5 synthesized by oxidizing BaFeO2.667 obtained via nebulized spray pyrolysis. Inorg. Chem. 60, 10923–10933 (2021).
Nuengmatcha, P., Porrawatkul, P., Chanthai, S., Sricharoen, P. & Limchoowong, N. Enhanced photocatalytic degradation of methylene blue using Fe2O3/graphene/CuO nanocomposites under visible light. J. Environ. Chem. Eng. 7, 103438 (2019).
Wang, F. et al. Synthesis, structure, magnetism and photocatalysis of a-Fe2O3 nanosnowflakes. RSC Adv. 9, 35372 (2019).
Hitam, C. N. C. & Jalil, A. A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manage. Vol. 258, 110050 (2020).
Saleem, S. et al. Analysis and characterization of opto-electronic properties of iron oxide (Fe2O3) with transition metals (Co, Ni) for the use in the photodetector application. J. Mater. Res. Technol. 15, 6150–6166 (2023).
Alnehia, A., Al-Sharabi, A., Alnahari, H. & Hadi, M. Synthesis and Characterization of MgO–CuO–Fe2O3 Nanocomposite: A study of its structural, optical, and antibacterial properties, J. Chem., vol. p. 10, 2024. (2024).
Pricilla, R. B. et al. Studies on the structural, optical and photocatalytic properties of CuO/MgO nanocomposite prepared by facile chemical co-precipitation, Mater. Today: Proc., 47, 837–842, (2021).
Urooj, A. et al. Morphological and optical investigation of 2D material-based ternary nanocomposite: Bi2O3/MgO/GO synthesized by a co-precipitationtechnique. RSC Adv. 12, 32986 (2022).
Kannan, K. et al. Facile fabrication of novel ceria-based nanocomposite (CYO-CSO) via co-precipitation: Electrochemical, photocatalytic and antibacterial performances. J. Mol. Struct. 1256, 132519 (2022).
Wei, D. et al. Mesoporous Fe2O3 nanomaterials from natural rust for lithium storage. J. Mater. Sci.: Mater. Electron. 28, 19098–19104 (2017).
Abdel–Basit Al–Odayni and, Naaser, A. Y. & Abduh Synthesis of magnesium-doped copper oxide nanocomposites using Tangerine Peel extract: Structural, optical, and bioactivity studies. Biomass Conv Bioref, (2024).
Hadi, M. Co3O4-CuO-ZrO2 nanocomposite: Optical, spectral, morphological,structural and antibacterial studies. Results Chem. 7, 101311 (2024).
Iman Kir, H. A. et al. Plant extract–mediated synthesis of CuO nanoparticles from lemon peel extract and their modiication with polyethylene glycol for enhancing photocatalytic and antioxidant activities. J. Polymers Environ., 32, 718–734, (2024).
Ansari, M. R., Agrohi, P. & Arya, Y. Doping effect of alkali Earth metal on structural, morphological, optical and magnetic properties of green synthesized ZnFe2O4 Nano-particles. Next Res. 2, 100175 (2025).
Saravanakkumar, D. et al. Synthesis and characterization of ZnO–CuO nanocomposites powder by modified perfume spray pyrolysis method and its antimicrobial investigation. J. Semicond. 39, 1–7 (2018).
Saleem, S. et al. A comparative analysis of optical and electrical properties of pure CuO and Zn doped CuO nanoparticles for optoelectronic device applications. J. Solgel Sci. Technol. 113, 213–224 (2025).
Saleem, S. et al. A band gap engineering for the modification in electrical properties of Fe3O4by Cu2+doping for electronic and optoelectronic devices applications. J. Solgel Sci. Technol. 109, 471–482 (2024).
Alnehia, A., Alnahari, H. & Al-Sharabi, A. Characterization and antibacterial activity of MgO/CuO/Cu2MgO3 nanocomposite synthesized by sol-gel technique. Results Chem. 8, 101620 (2024).
Haima Salmi, S. E., Laouini, S. & Meneceur and Hamdi Ali M. Biosynthesized MgO@SnO nanocomposite and their modiication with Polyvinylpyrrolidone. Eiciency for removal of heavy metals and contaminants from industrial petroleum wastewater. Clean Technol. Environ. Policy, (2024).
Ansari, M. R., Khaladkar, S., Kalekar, A., Pathi, P. & Peta, K. R. Effect of annealing temperature on structural and optical properties of BaFe2O4 nanocrystals: Energy storage applications. J. Energy Storage. 113, 115650 (2025).
Revathi, V. & Karthik, K. Microwave assisted CdO–ZnO–MgO nanocomposite and its photocatalytic and antibacterial studies. J. Mater. Sci.: Mater. Electron. 29, 18519–18530 (2018).
Amina Tabet, S. et al. One post biosynthesis of novel ternary nanocomposite ZnO/CuO/Cu2MgO3 for enhancing photocatalytic degradation of bromocresol green in wastewate. J. Cluster Sci. Vol. 35, 765–777 (2024).
Rahman, A. et al. Structural, optical and photocatalytic studies of trimetallic oxides nanostructures prepared via wet chemical approach. Synth. Met. 259, 116228 (2020).
Selvi, K. T., Mangai, K. A., Priya, M. & Sagadevan, S. Enhanced electrical and magnetic properties of CuO/MgO nanocomposites. Chem. Phys. Lett. Vol. 765, 138320 (2021).
Kumar, N. et al. NiFe2O4 nanoparticles as highly efficient catalyst for oxygen reduction reaction and energy storage in supercapacitor. Mater. Chem. Phys. Vol. 316, 129072 (2024).
Alali, K. T. et al. Fabrication of electrospun Co3O4/CuO p-p heterojunctions nanotubes functionalized with HFIP for detecting chemical nerve agent under visible light irradiation. Sens. Actuators: B Chem. Vol. 134, 128076 (2020).
Abbas, N. et al. Synthesis and characterization of Fe-substituting BaO nanoparticles by sol-gel method. Digest J. Nanomater. Biostruct.. 18, 1327–1338 (2023).
Mandizadeh, S., Sadri, M. & Salavati-Niasari, M. Sol-gel auto combustion synthesis of BaFe18O27 nanostructures for adsorptive desulfurization of liquid fuels. Int. J. Hydrog. Energy. 42, 12320–12326 (2017).
Khan, M. M. et al. Antibacterial studies of ZnO and Cu-doped ZnO nanoparticles synthesized using aqueous leaf extractof stachytarpheta jamaicensis BioNanoScience, 10, 1037–1048, (2020).
Khodadadi, A. & Talebtash, M. R. Investigation and synthesis of Fe doped Al2O3Nanoparticles by Co-Precipitation and Sol gel method. Asian J. Nanosci. Mater. 5, 36–47 (2022).
Yan, B., Wang, B., Wang, L. & Jiang, T. Ce-Doped mesoporous alumina supported Fe-Based catalyst with high activity for oxidative dehydrogenation of 1-Butene using CO2 as soft oxidant. J. Porous Mater. 26, 1269–1277 (2019).
Ansari, M. R., Agrohi, P. & Peta, K. R. Effect of citric acid on structural, optical, morphological properties of ZnO and the bactericidal applications against human pathogenic bacteria E. coli DH5α Materials Today: Proc. (2024).
Ansari, M. R., Khaladkar, S., Kalekar, A., Kim, M. D. & Peta, K. R. Effect of annealing temperature on structural, optical and magnetic properties of green synthesized ZFO nanoparticles for electrochemical energy storage applications. J. Energy Storage Vol. 74, 109494 (2023).
Jain, P., Ansari, M. R., Shankar, S., Thakur, O. P. & Peta, K. R. Al³⁺-substituted magnesium ferrites (MgAlxFe2 – xO4) for advanced hydroelectric cells and pseudocapacitors: Insights into the structural, morphological, and electrical studies. Mater. Res. Bull. 183, 113196 (2025).
Acknowledgements
The authors would like to thank Mr.Adel Taleb for his support and cooperation in providing chemicals in Sana’a, Yemen. Also, The author Muhammad Hadi extends their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through large groups Project under grant number R.G.P.2/511/46.
Funding
No fund received.
Author information
Authors and Affiliations
Contributions
Adnan Alnehia and Annas Al-Sharabi wrote and discussed the manuscript. Muhammad Hadi prepared the sample and characterized it. M A Ahlam writing-review and editing.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alnehia, A., Al-Sharabi, A., Hadi, M. et al. Preparation and analysis of structural, morphological and optical properties of BaFeO2.67/Fe2O3 nanocomposite. Sci Rep 15, 21822 (2025). https://doi.org/10.1038/s41598-025-02908-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02908-w