Abstract
To identify the factors affecting adverse reproductive outcomes in TPOAb-positive patients and to establish a predictive model based on these factors to assess the risk of adverse reproductive outcomes in patients. A retrospective cohort study was conducted, including 326 TPOAb-positive female patients who visited the reproductive medicine clinic of our hospital from January 2020 to December 2022. Patients were divided into groups with adverse reproductive outcomes and without adverse reproductive outcomes based on clinical outcomes. Data analysis was performed using SPSS software version 26.0 and R software, and independent risk factors for adverse reproductive outcomes were identified through univariate and multivariate logistic regression analysis, followed by the construction of a nomogram predictive model. The predictive performance of the model was assessed using the ROC curve. Additionally, a subgroup analysis was conducted within the adverse reproductive outcomes group. Logistic regression analyses were performed for the three subgroups: recurrent miscarriage, repeated implantation failure, and no usable embryos, to explore specific risk factors for each subgroup and compare the performance of predictive models for each subgroup. Univariate analysis showed that age, AMH levels, TPOAb concentration, TSH levels, and endometriosis are significant factors affecting adverse reproductive outcomes (P < 0.05). Multivariate logistic regression analysis further confirmed these factors as independent risk factors for adverse reproductive outcomes. The established nomogram predictive model showed good predictive performance in both the training set (AUC = 0.901) and the validation set (AUC = 0.858). Subgroup analysis showed that TSH levels, TPOAb concentration, age, AMH levels, and endometriosis were common risk factors for the three groups, but their weights differed. The nomogram model demonstrated the best predictive performance in the RIF group (AUC = 0.926), while its predictive performance was relatively lower in the RPL group (AUC = 0.869). This study successfully established a nomogram predictive model for adverse reproductive outcomes in TPOAb-positive patients. Through subgroup analysis, we identified the specific risk factors and predictive performance for subgroups of recurrent miscarriage, repeated implantation failure, and unavailable embryos, providing a reference for precise clinical assessment and intervention.
Similar content being viewed by others
Introduction
Thyroid peroxidase (TPO) is a key enzyme in the synthesis of thyroid hormones, with its mRNA and protein being expressed not only in the thyroid but also in follicular fluid, endometrial lining, and placenta, among other sites 1,2. Studies have shown that the positive status of TPOAb may increase the risk of various adverse reproductive outcomes through immune imbalance (such as disruption of the Th1/Th2 balance) and thyroid dysfunction. These adverse outcomes include recurrent miscarriage, repeated embryo implantation failure, and the presence of non-viable embryos. These effects may be mediated through their impact on embryo quality, embryo implantation, and endometrial receptivity3,4. However, it is not yet clear whether there is a dose–response relationship between the concentration of TPO antibodies (TPOAb) and the risk of thyroid dysfunction or adverse reproductive outcomes. The discrepancies in existing research findings may be attributed to the inclusion of varying concentrations of TPOAb in the studies, or to the influence of potential risk factors such as age, thyroid-stimulating hormone (TSH), and anti-Müllerian hormone (AMH). These factors may be responsible for the differences in pregnancy outcomes among TPOAb-positive women. This study aims to fill this knowledge gap by comprehensively considering potential risk factors such as age, TSH levels, AMH, and TPOAb concentrations to accurately assess the risk of adverse reproductive outcomes in TPOAb-positive patients. To this end, we conducted a retrospective cohort study and collected data from TPOAb-positive female patients. Through univariate and multivariate Logistic regression analysis, we identified independent risk factors affecting adverse reproductive outcomes and constructed a novel nomogram model to analyze the differentiated risks among subgroups such as recurrent miscarriage, repeated embryo implantation failure, and non-viable embryos. This model visually presents the key risk factors at different TPOAb concentrations, providing clinicians with a practical tool for individualized risk assessment and intervention.
Materials and methods
Data sources
All female patients (n = 786) with positive TPOAb who visited the reproductive and gynecological outpatient departments from January 2020 to December 2022, aged between 18 and 45 years old. The study was approved by the Ethics Committee of the Zigong Maternal and Child Health Hospital.
Research methods
Study design
This study is a retrospective cohort study. A total of 786 TPOAb-positive patients were enrolled, and 326 patients were finally included based on the inclusion and exclusion criteria. Participants were divided into two groups based on clinical outcomes: the adverse reproductive outcome group (n = 160) and the non-adverse reproductive outcome group (n = 166). See Fig. 1
Inclusion criteria: TPOAb ≥ 9 IU/ml, no history of other thyroid diseases, serum FT3 (1.8–4.2 pg/ml), FT4 (0.87–1.85 ng/dL), and TSH (0.35–4 mIU/L) levels are all within the normal range.
Exclusion criteria: ① Autoimmune diseases: Antiphospholipid syndrome, systemic lupus erythematosus, rheumatoid arthritis, Sjögren’s syndrome, systemic sclerosis, etc.; ② Polycystic ovary syndrome; Hyperprolactinemia; ③ Significant hydrosalpinx; ④ Pre-ovulatory or pre-transfer ultrasound indicating endometrial thickness less than 7 mm; ⑤ Abnormal uterine cavity morphology: uterine adhesions, septate uterus, endometrial polyps, etc.; ⑥ Chromosomal abnormalities in both partners; ⑦ Positive for antisperm antibodies, anti-endometrial antibodies, anti-cardiolipin antibodies, anti-ovarian antibodies; ⑧ Abnormal coagulation function, hyperhomocysteinemia; ⑨ Internal diseases such as hypertension, diabetes, kidney disease, etc.
Diagnostic criteria
-
(1)
An adverse reproductive outcome is defined as the occurrence of any of the following conditions: recurrent pregnancy loss, recurrent implantation failure, or no viable embryos.
-
Recurrent pregnancy loss (RPL): Refers to the loss of two or more pregnancies. In 2018, the European Society of Human Reproduction and Embryology (ESHRE) recommended using the term RPL instead of RSA (Recurrent spontaneous abortion), which includes miscarriages that do not result in a visible embryo, i.e., chemical pregnancies 5.
-
Recurrent implantation failure (RIF): Defined as failure to achieve a clinical pregnancy after two consecutive IVF, frozen-thawed embryo transfer (FET), or ICSI cycles, with at least two blastocysts transferred or at least four high-quality cleavage-stage embryos. High-quality embryos here refer to day 3 embryos (with ≥ 8cells, even blastomere size, and fragmentation rate of less than 10%) and blastocysts (with a grade of ≥ 3BB) 6.
-
No viable embryos: This encompasses two scenarios: discarded embryos and low-quality blastocysts 7: Discarded embryos: Refers to embryos rated as grade IV on day 2/3. Low-quality blastocysts: Include blastocysts rated as stage 3 or higher, with a grade of CC, on day 5/6, or blastocysts with an inner cell mass (ICM) grade of D.
-
-
(2)
The non-adverse reproductive outcome group is defined as: women of reproductive age with normal thyroid function, with no history of recurrent miscarriage, repeated implantation failure, or no viable embryos, who have had a history of normal pregnancy or are currently past the 12th week of gestation.
-
(3)
Metabolic abnormalities are defined as a series of pathological states related to energy balance and nutrient metabolism, including obesity, hyperlipidemia, insulin resistance, and dysglycemia. Here are the specific definitions and assessment criteria for these conditions:
-
Obesity 8: Assessed by the Body Mass Index (BMI), calculated as BMI = weight (kg)/height squared (m2). Based on the BMI values, we categorize weight status into the following classes:
Underweight: BMI < 18.5 kg/m2
Normal: 18.5 kg/m2 ≤ BMI < 24.0 kg/m2
Overweight: 24.0 kg/m2 ≤ BMI < 28.0 kg/m2
Obesity: BMI ≥ 28.0 kg/m2
-
Insulin Resistance (IR)9: Assessed by the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), calculated as HOMA-IR = fasting blood glucose (mmol/L) × fasting insulin (mIU/L)/22.5. Clinically, we consider fasting insulin (INS) > 15 mIU/mL and/or 2-h insulin (2hINS) > 80 mIU/mL to indicate the presence of insulin resistance.
-
Dysglycemia refers to impaired glucose regulation 10
Impaired glucose regulation includes Impaired Fasting Glucose (IFG) and Impaired Glucose Tolerance (IGT).
IFG: 6.1 mmol/L ≤ fasting blood glucose < 7.0 mmol/L, and 2-h blood glucose after a glucose load < 7.8 mmol/L
IGT: Fasting blood glucose < 7.0 mmol/L, and 7.8 mmol/L ≤ 2-h blood glucose after a glucose load < 11.1 mmol/L
-
Hyperlipidemia11: Hyperlipidemia is present when serum triglycerides (TG) are > 1.7 mmol/L (150 mg/dl) or high-density lipoprotein cholesterol (HDL-C) is less than 1.03 mmol/L in males and less than 1.29 mmol/L in females.
Metabolic abnormalities are categorized based on the number of positive factors among the four mentioned. If two or more factors are present, the subject is classified into the metabolic abnormality group; if only one factor is positive or all four factors are negative, the subject is classified into the group without metabolic abnormalities.
-
-
(4)
Endometriosis (EMs)12: Referring to the 8th edition of "Gynecology and Obstetrics," the diagnosis mainly relies on histological examination or sonographic evidence, in addition to clinical symptoms and signs.
Data collection
All retrospective data were derived from the electronic medical record system. The indicators collected and analyzed in this study include the woman’s age, education level, occupation, frequency of sexual activity, nighttime sleep duration, smoking history, duration of infertility, number of miscarriages, AMH, TPOAb concentration, TSH levels, endometriosis, and metabolic abnormalities.
Statistical analysis
Data analysis was conducted using SPSS 26.0 and R software. Continuous data conforming to a normal distribution are presented as mean ± standard deviation (x̄ ± s) and compared using independent samples t-tests; data not conforming to a normal distribution are presented as median (interquartile range) [M(P25, P75)] and compared using the Mann–Whitney U test. Categorical data are represented as percentages or proportions, and group comparisons are made using chi-square tests. The subgroup analysis was limited to patients with adverse reproductive outcomes, stratified by recurrent miscarriage, repeated embryo implantation failure, and no available embryos. The impact of key variables (age, AMH, TSH, TPOAb concentration, and endometriosis) on different subgroups was analyzed using a logistic regression model, calculating the odds ratios (OR) and 95% confidence intervals, and assessing the predictive model performance using ROC curves. The differences in ROC curves between models were compared using the DeLong test to quantify the statistical significance of differences in predictive models across subgroups.
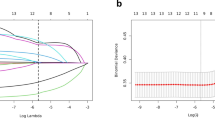
The patient population was randomly divided into two groups using R software, with 70% of patients in the training set and 30% in the validation set. In the construction of the Nomogram predictive model, univariate analysis and multivariate logistic regression were applied to the training set data to identify factors associated with adverse reproductive outcomes, with associations expressed as odds ratios (OR) and 95% confidence intervals (CI), where adverse reproductive outcomes are coded as Y = 1 and no adverse reproductive outcomes as Y = 0. The predictive model was established using the training set, and its accuracy was verified using the validation set.
The discriminative ability of the predictive model was evaluated using the area under the receiver operating characteristic curve (AUC), and the calibration of the predictive model was assessed using the Hosmer–Lemeshow goodness-of-fit test. The level of significance was set at 0.05, and all tests were two-tailed. A P-value of less than 0.05 was considered to indicate statistical significance.
Results
Comparison of general clinical data of patients
A total of 326 non-pregnant patients were ultimately included in the study. Patients were divided into an adverse outcome group (n = 160) and a non-adverse outcome group (n = 166) based on clinical outcomes. Differences between the two groups were analyzed using chi-square tests and independent samples t-tests, see Table 1.
There were no statistically significant differences between the two groups in terms of the women’s education level, occupation, smoking history, family harmony, duration of infertility, and number of miscarriages (P > 0.05). However, there were statistically significant differences between the two groups in age, AMH, TPOAb concentration, TSH levels, presence of endometriosis, comorbidity of metabolic abnormalities, nighttime sleep duration, and monthly frequency of sexual activity (P < 0.05).
Further evaluation of the statistical power of the variables involved in the t-test revealed that some variables (such as education level, occupation, smoking history, family relationship harmony, duration of infertility, and number of miscarriages) had low statistical power (ranging from 0.055 to 0.295), suggesting that the sample size may be insufficient to draw statistically significant conclusions. In contrast, variables with high statistical power (all > 0.8), such as age, AMH levels, TPO titers, TSH levels, endometriosis, and metabolic abnormalities, indicate that the sample size is adequate for statistical testing of these variables.
Establishment of the nomogram predictive model
The R software was used to randomly divide the 326 patients into two groups, with 70% in the training set and 30% in the validation set. Statistical tests showed that the distribution of all indicators was balanced between the two groups, with no statistical differences (See Table 2).
In multivariate logistic regression analysis, the risk of adverse reproductive outcomes was significantly higher in patients with advanced age [OR = 22.756, 95% CI (6.932–74.702), P < 0.05], low AMH [OR = 3.021, 95% CI (1.355–6.736), P < 0.05], TSH levels between 2.5 mIU/L and 4.0 mIU/L [OR = 6.965, 95% CI (2.990–16.233), P < 0.05], high concentration of TPOAb [OR = 3.486, 95% CI (1.413–8.599), P < 0.05], and comorbid endometriosis [OR = 3.820, 95% CI (1.691–8.632), P < 0.05]. Consequently, being aged 35 or above, having AMH ≤ 1.1 ng/ml, TSH levels between 2.5 and 4.0 mIU/L, TPOAb concentrations ≥ 500 U/ml, and having endometriosis are key factors influencing adverse reproductive outcomes (P < 0.05) (See Table 3).
A Nomogram is a method for quantifying and visualizing the results of logistic regression analysis. Based on the logistic regression analysis results from Table 3 (P < 0.05), predictive factors were selected to develop a Nomogram for the risk of adverse reproductive outcomes in TPOAb-positive patients. (See Table 4 and Fig. 2). At the same time, the statistical performance of the Logistic regression model was evaluated. The results indicated that the model’s efficacy was 0.9999, suggesting that the sample size provided sufficient statistical power for fitting the Logistic regression model. This further supports the reliability and robustness of the predictive model results.
Validation of the nomogram predictive model
To validate the model, ROC curves were plotted for both the training and validation cohorts, with AUCs of 0.901 (0.863–0.940) and 0.858 (0.732–0.935), respectively (Figs. 3 and 4). The Hosmer–Lemeshow goodness-of-fit test chi-square value was 4.737, with p > 0.05. Additionally, the maximum Youden index value from the ROC curve corresponded to a total score of 77.82, as calculated from the training set.
Analysis of three subgroups of adverse reproductive outcomes
Subgroup classification and case numbers
This study further categorizes adverse reproductive outcomes into three subgroups: recurrent miscarriage (RPL, 45 cases), repeated embryo implantation failure (RIF, 57 cases), and no available embryos (58 cases). The analysis of each subgroup aims to identify specific risk factors and evaluate the effectiveness of predictive models.
Comparison of demographic and clinical characteristics among the three subgroups
The differences in demographic characteristics (such as age, education level, etc.) and clinical characteristics (such as AMH levels, TSH levels, etc.) among the RPL group, RIF group, and no available embryos group did not reach statistical significance (P > 0.05). (See Table 5).
Subgroup analysis of key risk factors
Based on multivariable logistic regression analysis, the independent risk factors for each subgroup are as follows (see Table 6):
In the recurrent pregnancy loss (RPL) group, significant risk factors include TSH levels (OR = 7.584, 95% CI 2.818–24.035, P < 0.001) and TPO antibody titers (OR = 4.607, 95% CI 2.482–9.212, P < 0.001). Secondary risk factors include age (OR = 2.367, 95% CI 1.349–4.382, P = 0.004) and endometriosis (OR = 2.506, 95% CI 0.944–6.586, P = 0.062).
In the recurrent implantation failure (RIF) group, the primary risk factors are endometriosis (OR = 5.780, 95% CI 2.341–16.014, P < 0.001) and TSH levels (OR = 5.668, 95% CI 2.257–15.090, P < 0.001). Additionally, age (OR = 4.273, 95% CI 2.385–8.073, P < 0.001), AMH levels (OR = 2.455, 95% CI 1.084–5.549, P = 0.030), and TPO antibody titers (OR = 1.892, 95% CI 1.111–3.360, P = 0.023) also demonstrate certain risk effects.
In the group with no available embryos, significant risk factors include age (OR = 5.821, 95% CI 2.508–14.946, P < 0.001), AMH levels (OR = 5.295, 95% CI 2.172–13.646, P < 0.001), TSH levels (OR = 4.882, 95% CI 2.510–10.379, P < 0.001), TPO antibody titers (OR = 1.694, 95% CI 1.058–2.781, P = 0.031), and endometriosis (OR = 2.740, 95% CI 1.160–6.467, P = 0.021).
The subgroup analysis indicates that TSH levels, TPO antibody titers, age, AMH levels, and endometriosis are common risk factors across the three groups; however, the weight of different influencing factors varies among the groups.
Performance evaluation of the prediction model
The performance analysis results of the prediction model for the three subgroups are as follows (see Table 7).
The Nomogram prediction model demonstrated good performance across all subgroups, with AUC values as follows: RPL group: 0.869 (95% CI 0.809–0.929), indicating good predictive performance; RIF group: 0.926 (95% CI 0.889–0.963), showing the best predictive efficacy; and the group with no available embryos: 0.871 (95% CI 0.818–0.924), indicating strong predictive capability.
By comparing the ROC curves and AUC values of different subgroups, it was found that the Nomogram model had the highest predictive efficacy for the RIF group, followed by the group with no available embryos, while the predictive performance for the RPL group was relatively lower. This may be attributed to the smaller sample size in the RPL group or differences in the effect sizes among the variables. This suggests that the model is more suitable for predicting the risk of recurrent implantation failure, but there is still room for optimization in predicting recurrent pregnancy loss.
Discussion
Association between TPOAb concentration and the risk of adverse reproductive outcomes
This study identified TPOAb concentrations ≥ 500 IU/ml, age ≥ 35 years, AMH ≤ 1.1 ng/ml, TSH levels between 2.5 and 4.0 mIU/L, and endometriosis as independent risk factors for adverse reproductive outcomes through multifactorial Logistic regression analysis. Among the findings,TPOAb concentrations ≥ 500 IU/ml serve as an independent predictive marker for adverse reproductive outcomes, consistent with previous research findings 13,14,15,16. Prior research has indicated that TPOAb positivity is closely associated with an increased risk of recurrent miscarriage, decreased embryo quality, and poor outcomes in assisted reproductive technology (ART) pregnancies. One possible mechanism is that TPOAb may affect the immune function at the maternal–fetal interface through autoimmune imbalance (such as disruption of the Th1/Th2 balance), thereby increasing the risk of embryo implantation failure or pregnancy loss. Furthermore, TPOAb positivity may influence embryo development and placental function even in individuals with normal thyroid function, potentially through mild fluctuations in thyroid function or underlying thyroid inflammation 3,26. However, there is currently no consensus on a defined threshold for TPOAb concentration. Furthermore, this study further clarifies the critical value of TPOAb levels in predicting adverse reproductive outcomes as ≥ 500 IU/ml, providing a concrete threshold reference for clinical practice. In comparison with the study by Tan et al. 17, it was found that TPO-Ab levels exceeding 100 IU/mL did not affect the pregnancy rate in women with normal thyroid function. However, our study results reveal a significant association between higher TPOAb concentrations and an increased risk of adverse reproductive outcomes. This discrepancy may stem from the heterogeneity in study design, including different antibody screening criteria and concentration thresholds. Furthermore, we believe that the presence of other potential risk factors, such as age and TSH levels, may also account for the differences in outcomes among TPOAb-positive women.
Analysis of influencing factors in nomogram prediction model
This study validated the reliability of the T-test and Logistic regression through statistical power analysis. The high power of the Logistic regression indicates that the sample size sufficiently supports the robustness of the model. In contrast, some variables in the T-test (such as occupation and smoking history) exhibited lower power, necessitating cautious interpretation in light of the limitations of the sample size. This suggests that power analysis provides an important basis for model development and result interpretation.
In our study, a novel nomogram model was developed to provide personalized risk prediction for adverse reproductive outcomes for patients. According to the model, the critical total score is 77.82, and we use this to define risk levels: Patients with a total score below this threshold are considered to be at lower risk and may not require immediate intervention; While patients with a total score above this threshold face a higher risk of adverse reproductive outcomes, and it is recommended that they undertake preventive treatment measures. This model indicates that when multiple risk factors coexist, the risk of adverse reproductive outcomes increases, highlighting the importance of considering various factors in clinical practice to prevent adverse reproductive outcomes and avoid unnecessary treatments.
Tim et al.18 indicated that for women with high TPOAb concentrations, if their TSH levels are also ≥ 2.5 mU/L, they face a significantly increased risk of adverse pregnancy outcomes. Xie et al.19 also reached a consistent conclusion that women who are TPOAb positive and have TSH levels ≥ 2.5 mU/L are at an increased risk of miscarriage in early pregnancy. These research findings collectively emphasize the synergistic effect of TPOAb and TSH as predictors of pregnancy outcomes. Consistent with this, our study results also show that the risk of adverse reproductive outcomes significantly increases when TPOAb levels are ≥ 500 IU/ml and TSH levels are between 2.5 and 4.0 mU/L. This highlights the necessity of considering both TPOAb concentrations and TSH levels when assessing thyroid function and potential pregnancy risks, which has significant guiding implications for clinicians in monitoring and managing women with potentially high-risk pregnancies.
Endometriosis is associated with RPL and may lead to repeated implantation failure or poor embryo quality 20,21. Our study indicates that the coexistence of high concentrations of TPOAb and endometriosis significantly increases the risk of adverse reproductive outcomes. The proposed mechanism may be: TPOAb could be involved in the death of autologous immune cells through antibody-dependent cellular cytotoxicity (ADCC) of natural killer (NK) cells and complement-mediated cytotoxicity (CDC) of C3, activating the immune system at the maternal–fetal interface22. As an estrogen-dependent inflammatory disease, endometriosis’ local inflammatory response may affect oocyte quality and early embryonic development, exhibiting a pro-inflammatory state of the immune environment, including an increase in NK and T cells and a decrease in macrophages; this immune imbalance may further disrupt the immune function at the maternal–fetal interface 23,24. Therefore, TPOAb and endometriosis may interact through similar mechanisms, exacerbating oocyte damage, embryonic developmental disorders, and immune dysregulation at the maternal–fetal interface, increasing the risk of adverse reproductive outcomes.
Advanced maternal age is a well-recognized independent risk factor for miscarriage. Studies indicate that in patients over 35 years old, the live birth rate with the use of ART is usually below 30%25, and advanced age is a significant risk factor for adverse reproductive outcomes. Women who are TPOAb positive tend to be older and may have decreased ovarian reserve and poor embryo quality, with high concentrations of TPOAb being associated with a lower live birth rate 26. AMH levels, as an indicator of ovarian reserve, are typically lower in TPOAb-positive patients27, suggesting a potential relationship between TPOAb and ovarian reserve. Although another case–control study did not find a direct correlation between TPOAb and AMH in women with normal thyroid function28,29, the relationship between TPOAb and ovarian reserve remains a question worthy of further investigation. Our study results indicate that patients who are aged ≥ 35 years or have AMH ≤ 1.1 ng/ml and high TPOAb levels are at a greater risk of adverse reproductive outcomes. This suggests that age, AMH levels, and TPOAb positivity may have complex interactions that affect the success rate of pregnancy. Therefore, for this specific group, enhanced management and treatment are key to improving the success rate of pregnancy.
Subgroup analysis of adverse reproductive outcomes and differences in model efficacy
Building on the overall analysis, this study further elucidates the differential risk factors and their clinical significance among patients with recurrent pregnancy loss (RPL), recurrent implantation failure (RIF), and those with non-viable embryos through subgroup analysis. The primary risk factors in the RPL group were found to be TSH levels and TPO antibody titers, which further validate the critical roles of thyroid dysfunction and immune imbalance in recurrent miscarriage, potentially increasing the risk of miscarriage by affecting the immune function at the maternal–fetal interface. In the RIF group, endometriosis emerged as a significant risk factor, likely influencing embryo implantation through local inflammation. For the non-viable embryo group, the main risk factors identified were age and AMH levels, indicating the crucial roles of ovarian reserve and oocyte quality. Additionally, TSH levels and TPO antibody titers may also further reduce embryo quality through indirect mechanisms.
The nomogram prediction model demonstrated the best predictive efficacy in the RIF group, indicating its significant clinical value in identifying high-risk patients for recurrent implantation failure. The model also showed relatively high predictive efficacy in the non-viable embryo group, suggesting its potential application in predicting risks associated with decreased ovarian reserve. However, the predictive efficacy of the model in the RPL group was relatively low, highlighting the need to incorporate additional variables (such as immune markers) in the future to enhance predictive performance. Furthermore, increasing the sample size for the assessment of variables with lower statistical power could further improve the reliability of the predictions.
The subgroup analysis results are consistent with the overall conclusions drawn earlier, further revealing the heterogeneity of different types of adverse reproductive outcomes and the need for personalized treatment approaches. For patients with recurrent pregnancy loss (RPL), it is recommended to closely monitor and intervene in thyroid-stimulating hormone (TSH) levels and thyroid peroxidase (TPO) antibody titers to improve immune balance. In the case of patients with recurrent implantation failure (RIF), optimizing the endometrial environment is crucial, particularly in managing endometriosis, while also incorporating thyroid function monitoring to enhance embryo implantation rates. For patients with non-viable embryos, the focus should be on improving ovarian reserve and oocyte quality. Additionally, the Nomogram model developed in this study provides an effective tool for risk assessment across different subgroups, but its performance still needs optimization in specific subgroups (such as RPL) to enhance predictive capability.
Conclusion
This study identified key factors influencing adverse reproductive outcomes in TPOAb-positive patients through a retrospective cohort analysis. These factors include age, AMH levels, TPOAb concentration, TSH levels, and the presence of endometriosis. Based on these factors, an efficient nomogram prediction model was constructed. The model demonstrated good predictive performance in the overall population as well as in subgroups of recurrent miscarriage, repeated embryo implantation failure, and cases with no available embryos. The findings indicate that even with normal thyroid function, elevated TPOAb concentrations significantly increase the risk of adverse reproductive outcomes. This suggests that clinical assessments should incorporate TPOAb concentrations along with other relevant factors to achieve personalized risk evaluation and intervention.
However, this study has some limitations that need to be considered in future research. Firstly, the retrospective design of this study may limit our ability to infer causal relationships. Secondly, the study sample may have selection bias because our data comes from a single center, which may affect the generalizability of the results. Furthermore, although we have considered multiple potential confounding factors, there may be other unmeasured or unknown factors that have influenced the study results.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Due to privacy and ethical restrictions, the full dataset generated during the current study is not publicly available. However, a de-identified dataset that supports the study’s findings can be requested from the corresponding author upon reasonable request. The request must include a detailed proposal outlining the analysis plan, which will be reviewed by the research team for scientific merit and feasibility. Should the request be approved, data access will be granted under a data-sharing agreement. This agreement will specify the terms of use, including the maintenance of confidentiality and the designated purposes for data usage. The dataset includes de-identified participant information such as education level, occupation, smoking history, family harmony, duration of infertility, number of miscarriages, age, AMH levels, TPOAb concentration, TSH levels, endometriosis status, metabolic abnormalities, nighttime sleep duration, and monthly frequency of sexual activity. The data will be retained by the research team for future research and are not available for distribution at this time.
References
Rahnama, R. et al. Thyroid peroxidase in human endometrium and placenta: A potential target for anti-TPO antibodies. Clin. Exp. Med. https://doi.org/10.1007/s10238-020-00663-y (2021).
Yan, Z. & Yang, S. The impact of thyroid hormones and autoantibodies on female reproductive function. West. Med. https://doi.org/10.3969/j.issn.1672-3511.2021.10.032 (2021).
Dhillon-Smith, R. K. & Coomarasamy, A. TPO antibody positivity and adverse pregnancy outcomes. Best Pract. Res. Clin. Endocrinol. Metab. https://doi.org/10.1016/j.beem.2020.101433 (2020).
Zhou, P. et al. IVF/ICSI outcomes of euthyroid infertile women with thyroid autoimmunity: Does treatment with aspirin plus prednisone matter?. BMC Pregnancy Childbirth https://doi.org/10.1186/s12884-022-04532-2 (2022).
Zhang, Y. et al. NOD1 modulates decidual stromal cell function to maintain pregnancy in the early trimester. Cell Biochem. Funct. https://doi.org/10.1002/cbf.3417 (2019).
Zhang, G. et al. Research progress on the causes of repeated implantation failure in assisted reproduction and related countermeasures. Matern. Child Health Care China 33(8), 1908–1911 (2018).
Chinese Medical Doctor Association Reproductive Medicine Professional Committee. Chinese expert consensus on the morphological evaluation of human cleavage-stage embryos and blastocysts. Chin. J. Reprod. Contracept. https://doi.org/10.3760/cma.j.cn101441-20220619-00266 (2022).
World Health Organization. Obesity: preventing and managing the global epidemic. World Health Organ. Tech. Rep. Ser. 894: i-xii, 1–253 (2000).
Luo, J. Research progress on insulin resistance and cardiovascular diseases in type diabetes. Public Health Prevent. Med. 34(05), 125–128 (2023).
Chinese Diabetes Society. Guidelines for the prevention and treatment of type 2 diabetes in China (2020 edition) (Part I). Chin. J. Pract. Intern. Med. https://doi.org/10.19538/j.nk2021080106 (2021).
Ji, L. Interpretation of the IDF global consensus definition of the metabolic syndrome. Chin. J. Diabetes https://doi.org/10.3321/j.issn:1006-6187.2005.03.009 (2005).
Practical Obstetrics and Gynecology. (3rd Edition) published and distributed. Chin. Clin. Doctor (6): 73 (2014).
Bliddal, S. et al. Thyroid peroxidase antibodies and prospective live birth rate: A cohort study of women with recurrent pregnancy loss. Thyroid https://doi.org/10.1089/thy.2019.0077 (2019).
Zhang, Y. et al. Impaired embryo development potential associated with thyroid autoimmunity in euthyroid infertile women with diminished ovarian reserve. Front. Endocrinol. https://doi.org/10.3389/fendo.2024.1376179 (2024).
Bucci, I., Giuliani, C., Di Dalmazi, G., Formoso, G. & Napolitano, G. Thyroid autoimmunity in female infertility and assisted reproductive technology outcome. Front. Endocrinol. https://doi.org/10.3389/fendo.2022.768363 (2022).
Zhang, S. et al. High level of thyroid peroxidase antibodies as a detrimental risk of pregnancy outcomes in euthyroid women undergoing ART: A meta-analysis. Mol. Reprod. Dev. https://doi.org/10.1002/mrd.23677 (2023).
Tan, S., Dieterle, S., Pechlavanis, S., Janssen, O. E. & Fuhrer, D. Thyroid autoantibodies per se do not impair intracytoplasmic sperm injection outcome in euthyroid healthy women. Eur. J. Endocrinol. https://doi.org/10.1530/EJE-13-0790 (2014).
Korevaar, T. I. M. et al. Dose dependency and a functional cutoff for TPO-antibody positivity during pregnancy. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/jc.2017-01560 (2018).
Xie, J. et al. Effect of antithyroid antibodies on women with recurrent miscarriage: A meta-analysis. Am. J. Reprod. Immunol. https://doi.org/10.1111/aji.13238 (2020).
Pirtea, P., Cicinelli, E., De Nola, R., de Ziegler, D. & Ayoubi, J. M. Endometrial causes of recurrent pregnancy losses: Endometriosis, adenomyosis, and chronic endometritis. Fertil. Steril. https://doi.org/10.1016/j.fertnstert.2020.12.010 (2021).
Boucher, A. et al. implantation failure in endometriosis patients: Etiopathogenesis. J. Clin. Med. https://doi.org/10.3390/jcm11185366 (2022).
Nazarpour, S., Ramezani Tehrani, F., Rahmati, M. & Azizi, F. Prediction of preterm delivery based on thyroid peroxidase antibody levels and other identified risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. https://doi.org/10.1016/j.ejogrb.2023.03.025 (2023).
Wu, X. G. et al. Identification and validation of the signatures of infiltrating immune cells in the eutopic endometrium of women with endometriosis. Front. Immunol. https://doi.org/10.3389/fimmu.2021.671201 (2021).
Vallvé-Juanico, J., Houshdaran, S. & Giudice, L. C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update https://doi.org/10.1093/humupd/dmz018 (2019).
Leridon, H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum. Reprod. https://doi.org/10.1093/humrep/deh304 (2004).
Wei, S. X. et al. TPOAb positivity can impact ovarian reserve, embryo quality, and IVF/ICSI outcomes in euthyroid infertile women. Gynecol. Endocrinol. https://doi.org/10.1080/09513590.2023.2266504 (2023).
Metwalley, K. A. et al. Ovarian reserve in adolescent girls with autoimmune thyroiditis. Arch. Endocrinol. Metab. https://doi.org/10.20945/2359-3997000000597 (2023).
Li, Z. et al. Association between thyroid autoimmunity and the decline of ovarian reserve in euthyroid women. Reprod. Biomed. Online https://doi.org/10.1016/j.rbmo.2022.05.015 (2022).
Rao, M. et al. Subclinical hypothyroidism is associated with lower ovarian reserve in women aged 35 years or older. Thyroid https://doi.org/10.1089/thy.2019.0031 (2020).
Funding
This study was supported by the Zigong Science and Technology Bureau project (2023ZC26).
Author information
Authors and Affiliations
Contributions
Z. Qin contributed to Formal Analysis, Investigation, Resources, Writing—Review & Editing, Visualization, Supervision, and Project Administration. J. Lin was responsible for Data Curation, Formal Analysis, and Investigation. Y. Yang participated in Investigation, Resources, and Project Administration. L. Hao managed Data Curation and Resources. L. He performed Data Curation, Software, and Validation. Y. Qiu conceptualized the study, validated the findings, provided Supervision, Project Administration, and Funding Acquisition, and was involved in Data Curation, Methodology, Writing—Original Draft, Writing—Review & Editing, and Software preparation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study involves human participants and was conducted in accordance with the ethical standards of the Ethics Committee of Zigong Maternal and Child Health Hospital. The study was performed in compliance with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. Additional informed consent was not required for this study due to the retrospective nature of the data analysis, which involved the use of de-identified data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qin, Z., Qiu, Y., Lin, J. et al. Analysis of the influencing factors for adverse reproductive outcomes in patients with positive TPOAb and the establishment of a nomogram prediction model. Sci Rep 15, 19637 (2025). https://doi.org/10.1038/s41598-025-02990-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02990-0