Abstract
The Cardiometabolic Index (CMI) is a new indicator of visceral fat distribution and dysfunction that can be calculated using lipid parameters, waist circumference, and height. The relationship between CMI and Obstructive Sleep Apnea (OSA) remains unclear. This cross-sectional study explored the association between CMI and OSA using nationally representative data from The National Health and Nutrition Examination Survey database, spanning the periods from 2005 to 2008 and 2015 to 2018. OSA was self-reported based on questionnaire responses. Weighted logistic regression and restricted cubic splines were used to investigate the relationship between CMI and OSA. In addition, subgroup analyses and interaction tests were performed to study the robustness of the association across different populations. Finally, a receiver operating characteristic curve (ROC) was used to determine the predictive power of the index for the OSA. A total of 8460 participants were enrolled in the study. In the fully adjusted model, multivariate regression revealed a significant relationship between CMI and OSA (OR = 1.75, 95% CI 1.46–2.10). Interaction tests, revealed no significant effects of age, sex, body mass index, and race on the relationship between CMI and OSA. The area under the curve (AUC) of the ROC curve was 0.605, indicating that CMI has a predictive value for the OSA. This study provides evidence that CMI is associated with OSA in the adult population of the United States. CMI may be an important predictor of OSA, which provides a new perspective for the future assessment and treatment of individuals with odds of OSA.
Similar content being viewed by others
Introduction
Obstructive Sleep Apnea (OSA) is a prevalent sleep disorder characterized by the recurrent partial or complete collapse of the upper airway during sleep, leading to intermittent hypoxemia (IH) and sleep fragmentation1. The prevalence of OSA in the general population is estimated to be 17% and 34% among female and male, respectively, and increases with age and obesity2. OSA is associated with an increased incidence of hypertension, type-2 diabetes mellitus, atrial fibrillation, heart failure, coronary heart disease, stroke and mortality3,4,5. Undiagnosed and untreated OSA also plays an important role in reducing quality of life and the occurrence and development of depressive disorder6. More importantly, OSA has been linked to cognitive impairment, dementia, depression, and motor vehicle accidents due to reduced daytime alertness7,8 and, as such, is a critical health issue affecting human well-being.
In recent years, the relationship between metabolic syndrome and OSA has attracted considerable attention. The Sleep Heart Health Study conducted a study, and observed that serum total cholesterol (TC) and triglycerides (TG) were directly or indirectly associated with high-density lipoprotein cholesterol (HDL-C) levels and OSA severity, respectively9. Studies have shown the prevalence of hyperlipidemia is significantly increased in patients with severe OSA10,11. A meta-analysis including 64 studies showed that serum levels of TC, low-density lipoprotein cholesterol (LDL-C), and TG were elevated and HDL-C levels were decreased in patients with OSA12. These studies suggest a close link between lipid metabolism disorders and OSA, which makes lipid profiling as an accessible indicator and potentially effective biomarker of OSA.
The Cardiometabolic Index (CMI) was first introduced in 2015 as a new indicator of visceral fat distribution and dysfunction, and can be calculated using lipid parameters, waist circumference, and height13. Several studies have demonstrated that the CMI is a potential indicator of health conditions, including type 2 diabetes, insulin resistance, cardiovascular disease, non-alcoholic fatty liver disease, and stroke13,14,15,16.
The clinical features of OSA are not typical, and the symptoms do not always parallel disease severity. Polysomnography is the gold standard of OSA diagnosis17, but it is time-consuming, expensive, professionally demanding, and requires a long waiting time for examination. Therefore, it is important to pay attention to the patient’s clinical symptoms and to enhance early diagnosis and treatment of OSA. Considering that the CMI is a composite indicator that combines obesity and lipid metabolism indices, it may be used as a simple and effective clinical tool to predict odds of OSA. Accordingly, the primary aim of the present study was to explore the correlation between CMI and OSA using nationally representative data sourced from the National Health and Nutrition Examination Survey (NHANES) database.
Methods
Study design and population
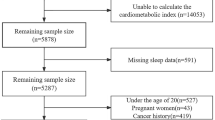
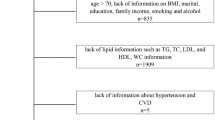
The NHANES is a nationally representative cross-sectional survey in the United States that uses a multi-stage, stratified, cluster-randomized sampling study design. In addition to standardized household interviews, the survey involves various aspects of health screening and laboratory testing, and all participants are required to provide written informed consent. Detailed statistics are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/). Due to limitations in the sleep questionnaire, participants were recruited from the NHANES database for four cycles: 2005–2008 and 2015–2018. A total of 39,722 participants were initially included in the study. First, we excluded 14,559 participants who had missing OSA questionnaire data. Next, we excluded 15,019 participants unable to calculate CMI due to missing data on height, waist circumference, TG, and HDL-C. Then, we excluded 1165 participants under 20 years old. Finally, we excluded 519 participants with missing data of Sampling weights for fasting subsamples (WTSAF2YR). Ultimately, a total of 8460 participants met the eligibility criteria and were included in the study. The selection process and search strategy are illustrated in detail in Fig. 1, which provides a flowchart of the study.
CMI calculation
The CMI was calculated using the following equations:
Waist-to-Height Ratio (WHtR) = WC (cm) / height (cm);
CMI = [TG (mmol/L) / HDL-C (mmol/L)] × WHtR.
CMI was regarded as a continuous variable, and group analysis was based on quartiles. The quartiles were defined as follows: Quartile 1 (CMI < 0.28), Quartile 2 (CMI 0.28–0.51), Quartile 3 (CMI 0.51–0.91), and Quartile 4 (CMI > 0.91).
Assessment of OSA
According to previous studies18,19, OSA was defined based on answers to three dichotomous questions, include (1) Snoring 3 or more nights per week; (2) snorting, gasping, or stopping breathing 3 or more nights per week; or (3) Feeling excessively sleepy during the day 16–30 times per month despite sleeping around 7 or more hours per night on weekdays or work nights. Participants positive for symptoms of OSA answered yes to at least one of the preceding 3 questions.
Covariables
Based on previous studies and clinical experience18,20, relevant and appropriate covariates were considered. Covariates included age, sex, race, education level, poverty-to-income ratio (PIR), smoking status, alcohol consumption, hypertension, diabetes, body mass index (BMI), WHtR, HDL-C, LDL-C, TC, and TG. Smoking21 was categorized as never smoker (< 100 cigarettes in a lifetime), former smoker (≥ 100 cigarettes in a lifetime, but not currently smoking), and current smoker (daily or occasional smoking at the time of the survey). BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2) and divided into 3 categories: ≤ 25 kg/m2; 25.0–29.9 kg/m2; and ≥ 30.0 kg/m2. Participants who fulfilled the following criteria were identified as diabetes cases: fasting blood glucose ≥ 7 mmol/L; glycated hemoglobin (HbA1c) ≥ 6.5%; and self-report questionnaire data including physician diagnosis of diabetes, current use of insulin or oral diabetes medications22. Blood pressure measurements were performed by a trained medical professional using a mercury sphygmomanometer, with a 5 min rest period during which the participant rested quietly. The mean of 3 consecutive measurements from the right arm (except in special circumstances) obtained with participants in a sitting position was calculated. The presence of hypertension was defined according to blood pressure measurements (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) or self-reported questionnaire data, including physician diagnosis of hypertension or taking blood pressure medications23.
Statistical analysis
Given the complexity of the NHANES sampling design, sample weights, clustering, and stratification were considered. According to the NHANES analysis guidelines, sample weights were calculated using dividing the weights for the 2-year cycle by 4. Continuous variables were described by sample-weighted means and standard error (SE) and categorical variables were expressed as weighted percentages (%). The CMI quartile groupings were compared using either the Chi-squared test or Kruskal-Wallis test. The association between CMI and OSA was determined using multivariate logistic regression models. Three models were used in this study. Model 1 was unadjusted for covariates; Model 2 was adjusted for age, sex, and race; and Model 3 was adjusted for age, sex, race, education level, marital status, smoking status, alcohol status, diabetes status, hypertension status, LDL-C, and PIR. Variance inflation factors were calculated, and Spearman’s correlation analysis was employed to examine collinearity among variables. A restricted cubic splines (RCS) model was used to identify potential nonlinear associations between CMI and OSA. CMI was introduced as a continuous variable and set up with 4 knots. In addition, the insertion of interaction terms helped assess potential differences in the relationships between subgroups. The results are presented as forest plots. A receiver operating characteristic (ROC) curve was used to assess the predictive value of the CMI for OSA, and discriminatory power was assessed by area under the curve (AUC). The current analysis used the R programming language (version 4.3.2); All statistical tests were two-sided and differences with P < 0.05 were considered to be statistically significant.
Results
Baseline characteristics of participants
The weighted demographic and baseline characteristics of the participants included in this study are summarized in Table 1. The study comprised 8460 participants with a median age of 47.12 ± 16.81 years, with 48.46% male and 51.54% female participants. The prevalence of OSA in this study was 48.51%. Compared with the participants in the lower CMI group, the participants in the CMI Q4 group were more male, more former smoker, more people with lower education levels, more obesity or central obesity, more histories of hypertension and diabetes, and had higher levels of TC, Importantly, participants with high level s of CMI had higher prevalence of OSA. There were no statistically significant differences in alcohol consumption or marital status between the CMI groups. To further validate the results, multiple imputations were conducted, with the distribution of baseline characteristics illustrated in Supplementary Table 1.
Association between CMI and OSA
The results of multivariable logistic regression analyses using various models are summarized in Table 2. In terms of collinearity, the variance inflation factors for the variables are all below 5 (Supplementary Table 2), and the |r| (Spearman correlation analysis) is less than 0.8 (Supplementary Fig. 1), indicating the absence of severe collinearity. There may be a positive correlation between CMI and OSA. Notably, after adjusting for potential confounding variables (model 3), this positive correlation remained even after accounting for all relevant variables (OR = 1.75, 95% CI 1.46–2.10, P < 0.001), suggesting that for every 1-unit increase in CMI, was associated with a 75% increase in the odds of OSA. Subsequently, the CMI was transformed into categorical variables based on quartiles. This trend remained statistically significant when CMI was considered a categorical variable (quartiles), with a progressively higher odds of OSA in the quartile with higher CMI compared with the lowest quartile in all multivariate logistic regression models. In addition, the increasing trend shown in all models was statistically significant based on the observed P-trend. Using multiple imputation of missing datasets corroborated these findings (Supplementary Table 3). RCS regression was used to quantify the dose-response correlation between CMI and OSA using multivariable-adjusted associations. Using the median CMI (0.487) as a reference point, a nonlinear relationship between CMI and OSA was observed (Fig. 2).
Subgroup analysis and predictive performance
Subgroup analysis was used to assess the strength and reliability of the association between CMI and OSA. This study explored the potential effects of age, sex, race, and BMI on these interactions. However, the analyses did not reveal any statistically significant associations between age, sex, race, and BMI (P > 0.05 for all interactions) (Fig. 3). The results indicate that CMI has a predictive value for OSA, with an AUC (95% CI) of 0.605 (0.59, 0.62) (Fig. 4). The optimal cutoff value of CMI was 0.491, with a sensitivity of 59.7% and a specificity of 56.1%.
Discussion
A cross-sectional study involving 8460 participants was conducted using data from the NHANES database, we analyzed the association between CMI levels and self-reported OSA. Our results showed that higher levels of CMI were associated with OSA in both continuous and categorical analyses of CMI. The RCS curves demonstrated a nonlinear positive association between CMI levels and OSA. Subgroup analyses further validate the consistent association across different population characteristics. Furthermore, CMI demonstrated a predictive performance for OSA. These findings indicate that CMI has the potential to be used as a monitoring indicator of OSA.
Obesity may be the most important risk factor for OSA24,25,26. OSA is present in more than 30% of patients diagnosed with obesity and in up to 50–98% of those who are morbidly obese24. Cohort studies have shown that weight gain is associated with an increased risk for developing moderate-to-severe OSA and is associated with increased Apnea-Hypopnea Index (AHI). Conversely, weight loss reduced the severity of AHI and OSA27,28,29,30. BMI is a traditional measure of generalized obesity that is commonly used to predict OSA. Rezaie et al.31 found that a higher BMI was positively correlated with a higher AHI index in patients with OSA. Although BMI is often used as a standardized measure to define obesity, it does not accurately reflect the distribution of body fat. Anatomical fat distribution is considered to be important because it produces different metabolic effects. Therefore, special attention should be devoted to increases in visceral adipose tissue around the abdominal organs14,26.In obese patients, abdominal visceral fat deposits but not subcutaneous fat area was associated with OSA and was an independent risk factor for OSA32. The WHtR has been recognized as a reliable measure for the assessment of abdominal obesity. The WHtR was based on waist circumference; its sensitivity is not affected by height, is easy to calculate, and is consistent across different populations. WHtR is a more accurate measure of abdominal obesity than traditional measures, such as BMI33. A retrospective study showed that the combination of BMI and WHtR, as a central indicator of obesity, was significantly associated with OSA and short sleep duration34. Therefore, weight management may play a crucial role and weight loss has been shown to be an effective treatment for improving OSA35.
The relationship between lipid indices and OSA has been investigated. Some clinical cohorts reported a positive correlation between TG and AHI36,37. A previous study showed that serum triglyceride levels were significantly associated with OSA38. The Sleep Heart Health Study found a close correlation between HDL-C levels and severity of sleep-disordered breathing9. Previous studies have shown that patients with OSA exhibit a higher prevalence of elevated TC and TG levels and lower HDL-C levels39,40,41,42. In a retrospective cohort involving 328 patients with newly diagnosed OSA, Popadic and colleagues discerned a significant correlation of HDL with OSA severity, indicating that patients with lower HDL values have higher AHI42. A cross-sectional study demonstrated that the decrease of HDL-C and the increase of TG reduce erythrocyte filtration, leading to tissue hypoxia in patients. TG and HDL-C abnormalities, in addition to upper airway obstruction, are among the factors that aggravate chronic hypoxia in tissues in patients with obstructive sleep apnea hypopnea syndrome43. Notably, HDL 8–10 contributes to the development of atherosclerosis in patients with OSA44. These studies indirectly support the aforementioned findings, suggesting a complex interaction between lipid metabolism and OSA pathogenesis.
CMI reflects visceral fat tissue distribution and function, not only confirming its association with increased risk of new-onset diabetes, but also expanding its association with cardiovascular disease and other metabolic diseases13,45,46,47. Cai et al. found CMI levels were positively associated with the risk of new-onset cardiovascular disease in patients with hypertension and OSA48. One study involving 280 participants who underwent polysomnography, patients with mild-moderate and severe OSA comprised 41.4% and 45.7% of the total, with an average AHI of 14.4 (9.0–22.1) events per hour and 54.9 (39.0–71.1) events per hour, respectively. There were statistically significant differences in CMI levels between the groups, which increased progressively with the severity of OSA49. The results of our study are consistent with this finding, showing that CMI levels were associated with developing OSA. Furthermore, for the screening of OSA, the AUC values for CMI were found to be 0.808 (95% CI 0.733–0.883, P < 0.01)49. Meanwhile, in our study the AUC value of CMI is 0.608, which means that this index has a predictive value for OSA. The cause of the difference may be the patients with OSA in this study was based on the questionnaire data.
The mechanisms underlying the association between CMI and OSA are unclear. WHtR predicts abdominal obesity in individuals, and TG/HDL-C predicts metabolic syndrome and insulin resistance50. Anatomical abnormalities of upper airway anatomical abnormalities are crucial in the pathogenesis of OSA. Obesity contributes to OSA by accumulating adipose tissue around the pharyngeal airway, reducing airway lumen size, and increasing the tendency of airway collapse during sleep51,52. The dysfunction of adipose tissue is an important contributor to the metabolic consequences of OSA53. The study has demonstrated cellular hypertrophy and inflammatory cell infiltration in the adipose tissue of obese animals and patients and elevated serum inflammatory markers in the circulation, including C-reactive protein, interleukin-2, and interleukin-6, which affects the respiratory regulation function of the central nervous system and further increases the risk of OSA54,55. Complex interactions between obesity, dyslipidemia, and OSA. The basic pathophysiological change in OSA is IH, which results from repeated airway obstruction and reopening. IH upregulates the key hepatic transcription factor (Sterol Regulatory Element Binding Protein 1) and the regulatory enzyme stearoyl-CoA desaturase-1, which converts saturated to monounsaturated fatty acids56. This process enhances cholesteryl ester and TC biosynthesis, leading to reduced clearance due to the inhibition of lipase activity and increased movement of free fatty acids from adipose tissue57. Additionally, IH may disrupt lipid metabolism by impairing LDL clearance. OSA may disrupt the balance of appetite-regulating hormones, leading to increased hunger and weight gain, which in turn can lead to obesity29. Furthermore, increased daytime sleepiness and fatigue can lead to decreased physical activity, which can lead to weight gain and an increased risk for obesity-related comorbidities24. These findings provide insights from different perspectives. More investigations are needed to confirm the relationship and understand these biological mechanisms.
Our study had several strengths. First, data from the NHANES were collected cautiously and are widely accepted because of their scientific design. In addition, to estimate the correlations accurately, we assessed and adjusted for several potentially confounding covariates. Finally, we further analyzed the results using RCS and subgroup analyses to enhance their robustness.
However, our study also had several limitations in our study, the first of which was its cross-sectional design, thus precluding us from establishing causality; as such, further longitudinal studies are warranted. Second, due to the lack of polysomnographic data during the NHANES collection process, OSA was determined based on NHANES questionnaire responses, which have introduced recall or self-report bias, although the NHANES performed well. Finally, although some conventional variables were fully controlled in this study, other confounders were not considered.
Conclusions
In conclusion, our investigation utilizing a nationally representative cohort of US population revealed a robust positive correlation between CMI and OSA. Of course, to confirm these findings, more extensive prospective studies are needed to thoroughly validate the role of CMI in the development of OSA.
Data availability
This study utilized publicly available datasets for analysis. The data for this analysis were sourced from the NHANES database (https://www.cdc.gov/nchs/nhanes).
References
Gottlieb, D. J. & Punjabi, N. M. Diagnosis and management of obstructive sleep apnea: A review. Jama 323, 1389–1400. https://doi.org/10.1001/jama.2020.3514 (2020).
Yeghiazarians, Y. et al. Obstructive sleep apnea and cardiovascular disease: A scientific statement from the American heart association. Circulation 144, e56–e67. https://doi.org/10.1161/cir.0000000000000988 (2021).
Dempsey, J. A., Veasey, S. C. & Morgan, B. J. O’Donnell, C. P. Pathophysiology of sleep apnea. Physiol. Rev. 90, 47–112. https://doi.org/10.1152/physrev.00043.2008 (2010).
Somers, V. et al. (ed, K.) Sleep apnea and cardiovascular disease: an American heart association/american college of cardiology foundation scientific statement from the American heart association Council for high blood pressure research professional education committee, Council on clinical cardiology, stroke Council, and Council on cardiovascular nursing. In collaboration with the National heart, lung, and blood Institute National center on sleep disorders research (National institutes of Health). Circulation 118 1080–1111 https://doi.org/10.1161/circulationaha.107.189375 (2008).
Javaheri, S. et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J. Am. Coll. Cardiol. 69, 841–858. https://doi.org/10.1016/j.jacc.2016.11.069 (2017).
Lyons, M. M., Bhatt, N. Y., Pack, A. I. & Magalang, U. J. Global burden of sleep-disordered breathing and its implications. Respirol. (Carlton Vic). 25, 690–702. https://doi.org/10.1111/resp.13838 (2020).
Lee, J. J. & Sundar, K. M. Evaluation and management of adults with obstructive sleep apnea syndrome. Lung 199, 87–101. https://doi.org/10.1007/s00408-021-00426-w (2021).
Marchi, N. A. et al. Obstructive sleep Apnoea and 5-year cognitive decline in the elderly. Eur. Respir. J. 61 https://doi.org/10.1183/13993003.01621-2022 (2023).
Newman, A. B. et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the sleep heart health study. Am. J. Epidemiol. 154, 50–59. https://doi.org/10.1093/aje/154.1.50 (2001).
Gunduz, C. et al. Hyperlipidaemia prevalence and cholesterol control in obstructive sleep apnoea: data from the European sleep apnea database (ESADA). J. Intern. Med. 286, 676–688. https://doi.org/10.1111/joim.12952 (2019).
Roche, F. et al. Obstructive sleep apnoea/hypopnea influences high-density lipoprotein cholesterol in the elderly. Sleep Med. 10, 882–886. https://doi.org/10.1016/j.sleep.2008.07.017 (2009).
Nadeem, R. et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J. Clin. Sleep. Medicine: JCSM : Official Publication Am. Acad. Sleep. Med. 10, 475–489. https://doi.org/10.5664/jcsm.3690 (2014).
Wakabayashi, I. & Daimon, T. The cardiometabolic index as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin. Chim. Acta. 438, 274–278. https://doi.org/10.1016/j.cca.2014.08.042 (2015).
Liu, Y. & Wang, W. Sex-specific contribution of lipid accumulation product and cardiometabolic index in the identification of nonalcoholic fatty liver disease among Chinese adults. Lipids Health Dis. 21 https://doi.org/10.1186/s12944-021-01617-3 (2022).
Wang, H. et al. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad. Med. 130, 325–333. https://doi.org/10.1080/00325481.2018.1444901 (2018).
Li, F. E. et al. Association between cardiometabolic index and stroke: A Population- based Cross-sectional study. Curr. Neurovasc. Res. 18, 324–332. https://doi.org/10.2174/1567202618666211013123557 (2021).
Chang, J. L. et al. International consensus statement on obstructive sleep apnea. Int. Forum Allergy Rhinology. 13, 1061–1482. https://doi.org/10.1002/alr.23079 (2023).
Cavallino, V. et al. Antimony and sleep health outcomes: NHANES 2009–2016. Sleep. Health. 8, 373–379. https://doi.org/10.1016/j.sleh.2022.05.005 (2022).
Gu, X., Tang, D., Xuan, Y., Shen, Y. & Lu, L. Q. Association between obstructive sleep apnea symptoms and gout in US population, a cross-sectional study. Sci. Rep. 13, 10192. https://doi.org/10.1038/s41598-023-36755-4 (2023).
Pan, X. et al. Association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and obstructive sleep apnea: a cross-sectional study from NHANES. Lipids Health Dis. 23, 209. https://doi.org/10.1186/s12944-024-02195-w (2024).
Li, Y. et al. Trends in Self-Reported Adherence to Healthy Lifestyle Behaviors Among US Adults, 1999 to March 2020. JAMA network open 6, e2323584, https://doi.org/10.1001/jamanetworkopen.2023.23584 (2023).
2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes care 44, S15-s33, (2021). https://doi.org/10.2337/dc21-S002
Whelton, P. K. & ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention., Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138, e426-e483, (2017). https://doi.org/10.1161/cir.0000000000000597 (2018).
Pillar, G. & Shehadeh, N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care. 31 (Suppl 2), 303–309. https://doi.org/10.2337/dc08-s272 (2008).
Veasey, S. C. & Rosen, I. M. Obstructive sleep apnea in adults. N. Engl. J. Med. 380, 1442–1449. https://doi.org/10.1056/NEJMcp1816152 (2019).
Weber, D. R., Leonard, M. B., Shults, J. & Zemel, B. S. A comparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. J. Clin. Endocrinol. Metab. 99, 3208–3216. https://doi.org/10.1210/jc.2014-1684 (2014).
Peppard, P. E., Young, T., Palta, M., Dempsey, J. & Skatrud, J. Longitudinal study of moderate weight change and sleep-disordered breathing. Jama 284, 3015–3021. https://doi.org/10.1001/jama.284.23.3015 (2000).
Tishler, P. V., Larkin, E. K., Schluchter, M. D. & Redline, S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. Jama 289, 2230–2237. https://doi.org/10.1001/jama.289.17.2230 (2003).
Newman, A. B. et al. Progression and regression of sleep-disordered breathing with changes in weight: the sleep heart health study. Arch. Intern. Med. 165, 2408–2413. https://doi.org/10.1001/archinte.165.20.2408 (2005).
Ong, C. W., O’Driscoll, D. M., Truby, H., Naughton, M. T. & Hamilton, G. S. The reciprocal interaction between obesity and obstructive sleep Apnoea. Sleep Med. Rev. 17, 123–131. https://doi.org/10.1016/j.smrv.2012.05.002 (2013).
Rezaie, L. et al. Compared to individuals with mild to moderate obstructive sleep apnea (OSA), individuals with severe OSA had higher BMI and Respiratory-Disturbance scores. Life (Basel Switzerland). 11 https://doi.org/10.3390/life11050368 (2021).
Ma, B. et al. Association between abdominal adipose tissue distribution and obstructive sleep apnea in Chinese obese patients. Front. Endocrinol. 13, 847324. https://doi.org/10.3389/fendo.2022.847324 (2022).
Ross, R. et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat. Rev. Endocrinol. 16, 177–189. https://doi.org/10.1038/s41574-019-0310-7 (2020).
Li, Y., Lu, Y., Zhao, Y. & Lyu, Z. Association of short sleep duration and obstructive sleep apnea with central obesity: A retrospective study utilizing anthropometric measures. Nat. Sci. Sleep. 16, 1545–1556. https://doi.org/10.2147/nss.S483984 (2024).
Xanthopoulos, M. S., Berkowitz, R. I. & Tapia, I. E. Effects of obesity therapies on sleep disorders. Metab. Clin. Exp. 84, 109–117. https://doi.org/10.1016/j.metabol.2018.01.022 (2018).
Kim, D. H., Kim, B., Han, K. & Kim, S. W. The relationship between metabolic syndrome and obstructive sleep apnea syndrome: a nationwide population-based study. Sci. Rep. 11, 8751. https://doi.org/10.1038/s41598-021-88233-4 (2021).
Silva, W. A. et al. Obstructive sleep apnea is associated with lower adiponectin and higher cholesterol levels independently of traditional factors and other sleep disorders in middle-aged adults: the ELSA-Brasil cohort. Sleep. Breath. = Schlaf Atmung. 25, 1935–1944. https://doi.org/10.1007/s11325-021-02290-7 (2021).
Shinoda, M. et al. Endocr. Practice: Official J. Am. Coll. Endocrinol. Am. Association Clin. Endocrinologists 25, 170–177, doi:https://doi.org/10.4158/ep-2018-0200 (2019).
Karadeniz, Y. et al. Determinants of obstructive sleep apnea syndrome: Pro-inflammatory state and dysfunction of high-density lipoprotein. Nutr. (Burbank Los Angeles Cty. Calif). 43–44, 54–60. https://doi.org/10.1016/j.nut.2017.06.021 (2017).
Sertogullarindan, B. et al. Betatrophin association with serum triglyceride levels in obstructive sleep apnea patients. Annals Thorac. Med. 14, 63–68. https://doi.org/10.4103/atm.ATM_52_18 (2019).
Zheng, M. et al. Association between glycolipids and risk of obstructive sleep apnea: A population-based study. Front. Nutr. 10 https://doi.org/10.3389/fnut.2023.974801 (2023).
Popadic, V. et al. Correlation of dyslipidemia and inflammation with obstructive sleep apnea severity. Front. Pharmacol. 13, 897279. https://doi.org/10.3389/fphar.2022.897279 (2022).
Fan, Z. et al. Association of dyslipidemia and hypoxemia in patients with obstructive sleep apnea hypopnea syndrome: a cross-sectional study. Am. J. Translational Res. 14, 8263–8270 (2022).
Kollar, B. et al. The impact of sleep apnea syndrome on the altered lipid metabolism and the redox balance. Lipids Health Dis. 20 https://doi.org/10.1186/s12944-021-01604-8 (2021).
Wakabayashi, I., Sotoda, Y., Hirooka, S. & Orita, H. Association between cardiometabolic index and atherosclerotic progression in patients with peripheral arterial disease. Clin. Chim. Acta. 446, 231–236. https://doi.org/10.1016/j.cca.2015.04.020 (2015).
Wang, H., Chen, Y., Guo, X., Chang, Y. & Sun, Y. Usefulness of cardiometabolic index for the Estimation of ischemic stroke risk among general population in rural China. Postgrad. Med. 129, 834–841. https://doi.org/10.1080/00325481.2017.1375714 (2017).
Guo, T., Zhou, Y., Yang, G., Sheng, L. & Chai, X. Association between cardiometabolic index and hypertension among US adults from NHANES 1999–2020. Sci. Rep. 15, 4007. https://doi.org/10.1038/s41598-025-87029-0 (2025).
Cai, X. et al. Associations of the cardiometabolic index with the risk of cardiovascular disease in patients with hypertension and obstructive sleep apnea: results of a longitudinal cohort study. Oxidative Med. Cell. Longev. 2022 (4914791). https://doi.org/10.1155/2022/4914791 (2022).
Wang, D. et al. Application value of cardiometabolic index for the screening of obstructive sleep apnea with or without metabolic syndrome. Nat. Sci. Sleep. 16, 177–191. https://doi.org/10.2147/nss.S449862 (2024).
Gong, R. et al. Associations between TG/HDL ratio and insulin resistance in the US population: a cross-sectional study. Endocr. Connections. 10, 1502–1512. https://doi.org/10.1530/ec-21-0414 (2021).
Lv, R. et al. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal. Transduct. Target. Therapy. 8, 218. https://doi.org/10.1038/s41392-023-01496-3 (2023).
Lin, H. et al. Upper airway lengthening caused by weight increase in obstructive sleep apnea patients. Respir. Res. 21, 272. https://doi.org/10.1186/s12931-020-01532-8 (2020).
Ryan, S. et al. Adipose tissue as a key player in obstructive sleep Apnoea. Eur. Respiratory Review: Official J. Eur. Respiratory Soc. 28 https://doi.org/10.1183/16000617.0006-2019 (2019).
Kheirandish-Gozal, L. & Gozal, D. Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int. J. Mol. Sci. 20 https://doi.org/10.3390/ijms20030459 (2019).
Liu, X. et al. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J. Neuroinflamm. 17 https://doi.org/10.1186/s12974-020-01905-2 (2020).
Li, J., Nanayakkara, A., Jun, J., Savransky, V. & Polotsky, V. Y. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol. Genom. 31, 273–280. https://doi.org/10.1152/physiolgenomics.00082.2007 (2007).
Drager, L. F. et al. Obstructive sleep apnea and effects of continuous positive airway pressure on triglyceride-rich lipoprotein metabolism. J. Lipid Res. 59, 1027–1033. https://doi.org/10.1194/jlr.M083436 (2018).
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
Financial support from the National Natural Science Foundation of China (No. 82272993 to Xiaokun Gang), the Jilin Provincial Natural Science Foundation Freedom to Explore Key Project (No. YDZJ202401410ZYTS to Xiaokun Gang), and the Jilin Provincial Science and Technology Innovation Centre Project (No. YDZT202402042CXTD to Xiaokun Gang) are greatly acknowledged.
Author information
Authors and Affiliations
Contributions
Mingyue Cui completed the final data organization and drafted the manuscript. Statistical analysis was done by Mingyue Cui and Xianchao Xiao. The manuscript was reviewed by Xianchao Xiao, Zhao Xue, Xiaokun Gang and Guixia Wang. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cui, M., Xiao, X., Zhao, X. et al. Association between cardiometabolic index and self-reported symptoms of obstructive sleep apnea: a cross-sectional study. Sci Rep 15, 28655 (2025). https://doi.org/10.1038/s41598-025-03186-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03186-2