Abstract
The degradable film can solve the problem that the traditional plastic film is difficult to recycle and heavy pollution for a long time. The effects of degraded film mulching on microbial diversity are significant. However, the responses of relevant microbial communities to degraded film mulching in different ecological niches (e.g., bulk soil, rhizosphere and endosphere) of sunflower roots are poorly understood. This study analyzed the effects of plastics film mulching on bacterial and fungal α-diversities (Shannon index), community assembly process, key dominant species of sunflower different ecological niches in roots. The results showed that degradable film mulching significantly increased the α-diversity (Shannon index) of bulk soil and rhizosphere soil bacteria and decreased the α-diversity of fungi (Shannon index), and the mulching treatment promoted the gradual shift of the rhizosphere microbial community assembly process to a deterministic process. Degradation film mulching increased the connectivity and complexity of bacterial networks and decreased the complexity of fungal networks. Plastic film mulching improves soil nutrients, temperature and moisture, enhances the positive correlation among microorganisms. At the same time, core species such as Amycolatopsis, Rhizobiaceae, and Sphingomonas that recruit beneficial microorganisms and accelerate the degradation of plastic film are significantly enriched. Degradable film covering promoted soil nutrient cycling, increased urease, alkaline phosphatase, sucrase, and thus increased sunflower yield. A comprehensive analysis of random forest and structural equations showed that the main driving microbial factors of yield were bulk soil bacterial diversity and endosphere fungal diversity. This study provides new ideas for the analysis of soil microbial mutual feedback mechanisms between degraded film mulch and rhizosphere ecosystems.
Similar content being viewed by others
Introduction
Sunflower (Helianthus annuus L.) is the fourth largest oil crop worldwide and one of China’s most important oil crops. Globally, the sunflower planting area is primarily concentrated in Europe, followed by Asia1. In 2023, China’s sunflower sown area was 597,000 hm2. The Inner Mongolia Autonomous Region is China’s largest sunflower sown area, accounting for > 68% of the country’s total sown area2. Because of the arid climate in the main sunflower-growing area of Inner Mongolia, seed yields are relatively low. thus, it is necessary to improve these yields to develop China’s sunflower industry. Long-term field studies have found that mulching increases yield and income and is an indispensable technology for dryland crop production3,4. Long-term use of ordinary mulch improves crop yield, but the long-term accumulation of residues in soil, coupled with a long degradation process, further leads to the serious pollution of farmland from residual film5. Degradable film mulching has similar thermal insulation and moisture retention effects to traditional film mulching and effectively reduces the pollution of mulching film residue in the soil environment and the risk of crop yield reduction6. Compared with bare soil, mulching reduces water evaporation, increases soil temperature, improves the soil microenvironment7, and promotes an increase in crop yield8,9. Soil enzyme activity can reflect the activity of soil microorganisms and biochemical reactions, as well as the status of nutrient circulation, and it is crucial in material circulation and energy transformation of the soil ecosystem10. Biodegradable mulching film is a type of degradable material that utilizes biodegradable polymers as its primary raw material. Microorganisms produce enzymes that break down the polymer into smaller molecular components, ultimately yielding water, carbon dioxide, and other harmless byproducts. These degradation products are environmentally benign and do not adversely affect natural ecosystems11. Degraded plastic film can be directly used as a carbon source for soil microorganisms, affect the microbial community, and alter enzyme activity by changing the microclimate12,13.
Microbial community composition directly affects soil nutrient cycling, crop growth status, and overall soil quality and indirectly determines the adaptability and stability of agroecosystems under environmental stress14,15. The assembly of microbial communities plays a critical role in shaping the formation and succession of their structures. Both deterministic and stochastic processes jointly regulate this assembly, ultimately influencing whether microbiome-mediated soil nutrient cycling is enhanced or suppressed16. Film mulching can affect the soil microbial community by changing the soil’s physical structure, microclimate, and nutrient status, regulating the quantity and activity of soil microorganisms and promoting the healthy development of the soil ecosystem17. Films mulching can significantly increase microbial diversity and richness18,19. The relative abundance of specific microorganisms (Acidobacteria) significantly increases20 and affects the assembly of microbial communities, transforming it into a determinate process21. Soil microbial composition is affected by abiotic and biological factors. Numerous studies on mulching have focused on soil physicochemical properties, microbial diversity, and crop yield. However, there are few reports on the effects of degradable films on the assembly process and co-occurrence network of microbial communities in different ecological niches in sunflower roots.
In this study, degradable and common films were selected to analyze the effects of degradable film mulching on microbial diversity, community assembly process, and sunflower yield in different ecological niches in sunflower roots. The primary objectives of this study were to assess (1) the effects of plastics film mulching on bacterial and fungal diversities in different ecological niches of root space, (2) the effects of plastics film mulching on the microbial co-occurrence network and community assembly process of different ecological niches in root space, and (3) the microbial regulation mechanism of sunflower yield increase under plastics film mulching.
Materials and methods
Testing condition
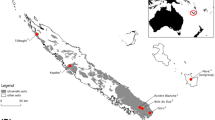
This experiment was conducted in 2023 in Shulinzhao Town (40°29′6.09″N, 109°52′24.9″E), Dalat Banner, Ordos City, Inner Mongolia Autonomous Region, China. The climate of this region is dry with low rainfall, typical of a temperate continental monsoon climate. The average annual sunshine is ~ 3000 h, the average annual temperature is 6.1–7.1 °C, the frost-free period is 135–150 days, and the average annual precipitation is 240–360 mm, mainly from July to September. The altitude is 1042 m, and the main soil type is tidal soil with moderate fertility (Table S1). In 2023, the precipitation was 215.9 mm, and the average annual temperature was 7.71 °C (Fig. 1).
Experimental design
The variety tested was Sanrui No. 10 (Sanrui Agricultural Technology Co., Ltd.). Three following treatments were set up: degraded film treatment (DF) (From Shanghai Hongrui Biotechnology Co., Ltd.), ordinary film treatment (CF) (From Qinfeng Agricultural Trade Limited Liability Company in Baotou City.), and no film treatment (CK). The plastic films in each treatment were 700 mm wide and 0.08 mm thick. Both conventional mulch film and degraded film are black in color, each treatment was repeated four repetitions. The previous crop was corn. The area of each plot was 465 m2. The seeding density was 22,500 plants·ha-1, the irrigation was carried out during mid-May, the irrigation volume was 150 m3, and the seeding date was June 2, 2023. A mechanized wide-narrow row planting method was adopted for fertilization, mulching, seeding, soil covering, and suppression, with a narrow row of 40 cm and a wide row of 90 cm. At the same time, 375 kg·ha-1 mixed Hefei (N-P2O5-K2O 26-10-12) and 225 kg·ha-1 urea were applied. The obtained sequences were submitted to the NCBI Sequence Read Archive (SRA) with BioProject number PRJNA1141434.
Microbial diversity assessment
All sampling tools were sterilized before sample collection, and sampling was performed in an area of uniform growth between two sunflower rows. It is to remove impurities such as stones and straw from 0 to 5 cm topsoil, and further mix with the underlying soil as soil samples. Impurities were removed and passed through a 1 mm sieve as a bulk soil sample. The loose soil attached to the sunflower roots was removed, the soil within 1 mm of the root surface was collected using a sterile brush, and the collected soil sample was filtered through a 1 mm sieve to obtain rhizosphere soil. The sunflower roots were washed with sterile water to remove all impurities, and the roots were cut evenly. The roots were treated with an ultrasonic cleaning apparatus and phosphate-buffered saline solution for 3 min. The root samples were stored in a sterile centrifuge tube. After liquid nitrogen was rapidly frozen, the samples were stored in the ultra-low temperature refrigerator at − 80 °C. The samples were used for subsequent 16 S rRNA and internal transcribed spacer (ITS) sequencing analysis. The primer information and polymerase chain reaction amplification methods are shown in Table S2.
Determination of soil properties and yield
Soil samples were collected during flowering. Each treatment plot was sampled using a five-point sampling method and repeated thrice, and the samples were fully mixed and divided into four replicates. Soil water content (SWC) was measured by volumetric weighing, soil temperature was measured using a curvature geothermometer, soil pH was measured using a pH meter, soil organic matter (SOM) was measured by potassium dichromate titration, and soil total nitrogen (TN) was measured by the semi-micro-Kjeldahl method. The activities of soil catalase (CAT), sucrase (SC), urease (UE), and alkaline phosphatase (AKP) were measured by the microplate method. The kit was obtained from Suzhou Michy Biomedical Technology Co., Ltd., and iD5 (Molecular Devices) was used for the enzyme-labeling apparatus. After the sunflowers reached maturity, 20 consecutive representative sunflowers with the same growth were selected from each plot, and the grain weight was measured by removing the flower heads.
Microbial co-occurrence network analysis
The amplicon sequence variant (ASV) relative abundance table was divided into three groups (bulk soil, rhizosphere, and endosphere), and ASVs with an average relative abundance > 0.01% were retained in each group, which was used to construct co-occurrence networks of bulk soil, rhizosphere, and endosphere bacterial and fungal communities under different degradation films. The co-occurrence network was constructed by calculating the Spearman correlation matrix using “hmisc,” “psych,” and “igraph” packages in R software. The P values of the correlation matrix were adjusted using the Benjamini–Hochberg method. The network was constructed using significant correlations (P < 0.05, R > 0.5) for pairs of ASVs and visualized using Gephi version 0.10.2. The intramodule connectivity (Zi) and intermodule connectivity (Pi) indexes were obtained using the “microeco” package in R to evaluate the key nodes in the network and obtain the core species. The Zi and Pi values of network nodes were calculated using the R “igraph” package, and nodes were divided into the following four types according to the topological characteristics of nodes: (1) module hubs, centers of modules, points with high connectivity within modules, Zi > 2.5 and Pi < 0.62; connectors, intermodule connection points, points with high connectivity between two modules, Zi < 2.5 and Pi > 0.62; (3) network hubs, points with high connectivity in the whole network, Zi > 2.5 and Pi > 0.62; and (4) peripherals, points that do not have high connectivity within or between modules, Zi < 2.5 and Pi < 0.62 [19]. Module hubs, connectors, and network hubs are generally considered key nodes, i.e., core species that are crucial for maintaining the stability of the network structure22.
Microbial community assembly process analysis
The β-mean nearest classification distance (β-NTI) value indicating the ecological assembly process of a bacterial community was calculated using the zero-modeling method using the “picante” package in R software23. |βNTI| > 2 indicated that the difference in community composition resulted from deterministic processes (βNTI < − 2: homogeneous selection; βNTI > 2: variable selection), and |βNTI| < 2 indicated a random process. The samples were divided into three groups: bulk soil, rhizosphere, and root plane. The bacterial communities were characterized by five assembly processes: homogeneous selection, variable selection, homogeneous dispersion, dispersion limitation, and drift24. The βNTI index, as described above, was calculated using the “iCAMP” package in R. A neutral community model (NCM) was established using the “Hmisc” package in R software. The Chi-square test is used to compare the observed species abundance or distribution with the predicted results of the zero model to check whether the difference between the two is significant.
Data analysis
Data were processed using Excel 2021 (Microsoft, USA) and presented as mean and standard deviation. Univariate analysis of variance (ANOVA) and Pearson correlation analysis were performed using SPSS version 22.0 (IBM, USA). Bioinformatic analysis of the soil microbiota was carried out using the Majorbio Cloud platform (https://cloud.majorbio.com). Cutadapt software was used to remove barcodes and primers from each sample sequence. Dada2 software on the QIIME2 (version 2024.10) platform was used to cluster sequences with 99% similarity to ASVs. The classification information of ASVs was annotated using the 16 S rRNA and ITS databases (silva/v138.1). Using the “vegan” package(version 2024.10) in R software (version 4.4.0) the ASV table was obtained and simplified to analyze the α-diversity of bacterial and fungal communities. Based on the ASVs information, rarefaction curves and alpha diversity indices including observed ASVs, Chao1 richness, Shannon index and Good’s coverage were calculated with Mothur (v1.30.1)25. Principal coordinate analysis (PCoA) β-diversity based on bray_curtis, permutational multivariate ANOVA (PERMANOVA) was used to analyze the effects of ecological niche (bulk soil, rhizosphere, and endosphere) and degradation membrane coverage on microbial community diversity and composition. The “vegan” package in R (version 2.6-8) was used to conduct ANOVA (Adonis). The key driving factors of yield were analyzed using the “random Forest” (RF) package(version 4.7–1.2) in R software (version 4.4.0), and a structural equation model partial least-squares path model (PLM-PM) was adopted. PLS-PM analyzed the effect of microbial diversity on yield in root ecosystems covered with different degradation membranes. The path coefficient represents the direction and strength of the linear relationship between potential variables, and R2 represents the percentage of variables explained by other variables. PLS-PM was constructed using the “plspm” package (version 0.5.1) in R26 (version 4.4.0).
Results
Effect of degradable films on soil properties and yield
UE, CAT, AKP, SC, SWC, ST, pH, and yield were significantly influenced by degradable film mulch (P < 0.01), whereas TN and organic matter content showed no significant differences among the different treatments (Table 1). The sunflower yield under film mulch was higher than that under CK treatment, and the yield magnitude was DF > CF > CK. Specifically, DF increased the yield by 19.02% and 33.49%, respectively, compared with those of CF and CK, and the difference was significant compared with ordinary mulching (P < 0.05). The DF increased the yield by 12.15%, compared with CF. Compared with CK, film mulching significantly increased soil temperature and water content, resulting in heat preservation and moisture retention. Meanwhile, film mulching increased UE, CAT, AKP, SC, SWC, ST, TN, and SOM and decreased soil pH. The Degradable film mulching treatment resulted in respective increases of 39.34%, 44.82%, 65.62%, 27.34%, 9.42%, 3.01%, 6.93%, 2.96%, and 12.30% for UE, CAT, AKP, SC, SWC, pH, ST, SOM, and TN compared to CK. Degradable film mulching regulated the underground soil microenvironment, provided a sufficient carbon source for microorganisms, and enhanced enzyme activity in the soil, promoting the increase in sunflower yield.
Microbial diversity responses to degraded film mulch at different root ecological niches
Analysis of α-diversity of bacterial (Fig. S1) and fungal communities (Fig. S2) in bulk soil and rhizosphere-related ecological niche (e.g., rhizosphere and endosphere) showed that the Shannon and Chao indices of bacterial and fungal communities in bulk and rhizosphere soil were significantly higher than those in the endosphere (Fig. S1 and S2). The degradation of film coverage significantly enhanced the diversity of bacterial microorganisms compared to CK (P < 0.05). In contrast, common film mulching reduced bacterial microbial diversity in both bulk soil and rhizosphere soil, while increasing it specifically in the rhizosphere. Plastic film mulching decreased the diversity of fungal microorganisms, and there was no significant difference among the different treatments, indicating that the effect of film covering on bacteria was higher than that of fungi. Linear regression also found that bacterial and fungal diversities were significantly correlated with the degradation of film mulching treatment in rhizosphere and endosphere, whereas the correlation of fungi was higher than that of bacteria (Fig. 2).
Microbial community responses to degradable film mulch at different root ecological niche
The dominant bacterial phyla in different ecological niches are Proteobacteria, Actinomyces, and Firmicutes (Fig. S3a–c), and the dominant fungal phyla are Ascomycota, Olpidiomycota, Basidiomycota, and Mortierellomycota (Fig. S3d–f). The main factors affecting the dominant phyla of bacteria and fungi were SWC, SC, UE, AKP, and CAT (Fig. S2), indicating that degradation membrane treatment was beneficial for water retention and microbial reproduction. PERMANOVA confirmed that ecological niche variation (bulk soil, rhizosphere, and rhizosphere) was the main factor influencing the diversity of bacterial and fungal communities (interpretive variation > 60%, P < 0.001), and the effect on bacteria was higher than that on fungi, whereas degradation film mulch had no significant effect on bacteria but had a significant effect on fungi. However, PERMANOVA of different ecological niches showed that degradable film mulch significantly affected bacterial and fungal endosphere community diversity (P < 0.001) (Table 2).
PCoA was used to analyze the effects of plastic film mulch on bacterial (Fig. 3a–d) and fungal (Fig. 3e–h) β-diversities in different ecological niches. There were significant differences in β-diversity between bulk soil and rhizosphere communities, whereas there were no significant differences between rhizosphere and endosphere (P < 0.05; Fig. 3). Significant differences in bacterial and fungal communities under different degradation membrane treatments indicated that degradable film mulch would significantly affect the changes in soil microbial community structure.
The random forest was used to identify bacterial and fungal biomarkers and environmental drivers covered by degradable film mulch (Fig. 4). The top 20 ASVs of bacteria (Fig. 4a, Table S3) and fungi (Fig. 4d, Table S3) were analyzed. ASV2121 (Amycolatopsis) and ASV2102 (unclassified_Bacteria) had significant differences in bacterial communities and were significantly enriched under degradable film mulch. ASV2121 (Amycolatopsis) was positively correlated with soil characteristics. In particular, there was a significant positive correlation with UE, CAT, and AKP (Fig. 4c). ASV6 (Schizothecium) exhibited significant differences in fungal communities and is significantly concentrated under CF. Through random forest analysis, the contribution of the top 20 ASVs of bacteria was > 50% (Fig. 4b) and that of the top 20 ASVs of fungi was > 60% (Fig. 4e). ASV556 (Fusarium) showed a significant negative correlation with UE, CAT, AKP, SC, SWC, ST, SOM, and TN (Fig. 4f), and pH was the key driving factor affecting the abundance of the top 20 ASVs in bacteria and fungi.
Bubble charts of the top 20 key microorganisms (ASV level) in bacteria (a) and fungi (d), and analysis of the importance of key microorganisms and their correlation with environmental factors in random forest analysis of bacteria (b) and fungi (e). Heat map of correlation between related physicochemical factors and ASV (c and f).
The assembly process of root microbial communities with different ecological niches
The whole community zero model analysis showed that the ecological assembly of bacterial and fungal communities under plastic film mulch was composed of selection and neutral processes, whereas neutral processes such as diffusion restriction and drift dominated bacterial and fungal community structures under each treatment (Fig. 5a and d). Compared to CK, the assembly process of bacterial communities in the laminating treatment increased the heterogeneous selection process and reduced the homogeneous diffusion process, whereas CK significantly increased the diffusion restriction process of fungi (Fig. 5b). By calculating the βNTI value based on the ASV abundance matrix to evaluate the ecological assembly process of the microbial community, the proportion of |βNTI| > 2 of the bacterial community under DF was also higher than that under the no film mulch, indicating that the composite film treatment would promote the transformation of the microbial community assembly process to a deterministic process (Fig. 5b and e). For different ecological niches, the proportion of rhizosphere |βNTI| > 2 was higher than that of bulk soil and endosphere (Fig. 5c and f), indicating that rhizosphere ecological assembly gradually changed from a random process to a deterministic process.
The NCM was used to predict the relationship between occurrence frequency and relative abundance of ASV in subcommunities of three different ecological niches in bulk soil, rhizosphere, and endosphere and in all root datasets (Fig. 5g–n). Results showed that the NCM successfully estimated most relationships between the occurrence frequency of ASV and its relative abundance change, with high interpretation rates (R2) in bulk soil, rhizosphere, endosphere, and sum, indicating that random processes are important for the formation of microbial community assembly in different ecological niches. Moreover, the Nm values of bacteria and fungi in bulk soil (Nm = 1074.81 and 371.14) were higher than those in the rhizosphere (Nm = 1022.74 and 491.49) than in endosphere (Nm = 678.13 and 224.26), indicating that the species diffusion of microorganisms in bulk soil was significantly higher than that in rhizosphere and endosphere.
Ecological assembly process of microbial communities and neutral community model fitting of ASV in different niche subcommunities. Zero-model analysis of bacterial and fungal (a) communities under degradation membrane (d), βNTI indexes of bacterial and fungal communities in bulk soil, rhizosphere, and root surface and under degradation membrane (b, c, e, and f). Neutral model fitting of bacterial (g-j) and fungal communities (k-n) in different ecological niches and root ecosystems. NRS represents bulk soil; RS represents rhizosphere; RI represents endosphere.
Microbial co-occurrence network changes at different root Spatial locations
Symbiotic networks of bacterial and fungal communities in bulk soil, rhizosphere, and endosphere were constructed under the degradable film mulch (Fig. 6). There were more nodes and edges of the bacterial community network, rhizosphere (nodes 197 and edges 4157) and endosphere (nodes 200 and edges 4181) than bulk soil (nodes 200 and edges 2908), and more nodes and edges of the fungal community network, rhizosphere (nodes 199 and edges 2984) than bulk soil (nodes 200 and edges 1727) and endosphere (nodes 118 and edges 788). It was shown that the complexity of the rhizosphere soil bacterial and fungal network was higher than that of bulk soil and endosphere (Table S4). The modular degree of bacterial and fungal community networks was the largest in the endosphere, and the positive correlation edges of fungi were significantly more than those of bacteria, indicating that fungi had a more obvious positive influence on the microbial network. Bacillus, Anaeromyxobacter, and Sphingomonas belong to the dominant genus of bulk soil bacteria. Pseudoxanthomonas, Rhodonellum, and Amycolatopsis belong to the rhizosphere dominant bacteria, and Aquiflexum, Aminobacter, and Aromatoleum belong to the endosphere core bacteria. Olpidium, Spizellomyces, Lecythophora, and Dichotomopilus belong to the dominant genus of bulk soil fungi. Wardomyces, Olpidium, and Schizothecium belong to the dominant rhizosphere fungi, whereas Sarocladium, Madurella, and Striaticonidium belong to the core endosphere fungi (Table S5).
The subordinate level analysis reveals the changes in the bacterial network graph (Fig. 4) after the degradable film cover was applied. The degradable film mulching significantly increased the number of edges in the bacterial network, decreased the modularity index, increased the average degree, and increased the network connectivity. The changing trend of a fungal network diagram differed from that of bacteria. Degradation film treatment can reduce the number of fungal network diagram edges, increase the proportion of degradable film mulching positive correlation edges, and positively regulate the change of microbial network. Moreover, degradation film treatment can significantly increase the average network diameter and reduce the modularity index and average degree of the network. It was suggested that degradation membrane covering reduced the network complexity of fungi (Fig. S4; Table S6).
According to ZiPi most ASVs in these co-occurring networks are classified as peripheral nodes. Module hubs, connectors, and networks were defined. The total number of key bacterial dominant species in hubs was 273 (e.g., Helianthus, Marmoricola, Chryseolinea, Massilia, Rhizobiaceae, Microbulbifer, Rhizobiaceae, Amycolatopsis, Sphingomonas, and Bacillus) (Table S6), The total number of fungal dominant species was 141 (e.g., Coprinopsis, Gibberella, Pseudeurotium, Golovinomyces, Kernia, Septoglomus, Olpidium, and Pyxidiophora) (Table S7).
Effects of different ecological niches in roots on increasing yield driven by microorganisms
Structural equation and random forest synthesis (Fig. 7) were used to analyze the effects of degradable film mulching on soil physicochemical properties, enzyme activity, and microbial diversity of different ecological niches of bacteria and fungi on yield. The results demonstrated that soil water content exerted a significant influence on soil temperature fluctuations. Soil temperature, in turn, modulated the diversity of rhizosphere microorganisms, including bacteria and fungi, by coordinating changes in soil chemical properties and enzyme activities, ultimately contributing to increased crop yield. The random forest further verified that the main microbial driving factors affecting yield were bacterial bulk soil diversity and fungal endosphere diversity, and the main soil driving factors were UE, AKP, CAT, SC, pH, and ST. Analysis of the direct, indirect, and total effects of yield contribution also verified that the main influencing factors of yield were soil water content and Soil temperature and soil enzyme activity.
Structural equation model showing the potential direct and indirect effects of soil variables and bacterial and fungal diversities on sunflower yield. Correlation between yield and each indicator (a): red represents positive correlation, blue represents negative correlation, and the width of the arrow is proportional to the strength of the path coefficient; random forest increment represents the main driver of yield increase (b), blue represents the correlation reaching a significant level, and gray represents the difference not reaching a significant level; direct, indirect, and total effects of yield contribution (c). *P < 0.05.
Discussion
Effects of degradation membranes on microbial diversity in different ecological niches
Microbial community composition in bulk soil plays a crucial role in shaping the rhizosphere microbial community. Compared with bulk soil, the rhizosphere microdomain exhibits higher microbial cell density, more complex interactions among microorganisms, and predominantly mutually reinforcing relationships. These characteristics suggest that rhizosphere microorganisms possess greater potential for mutually beneficial symbiosis, thereby enhancing the connectivity between soil and microorganisms27. In this study, compared to nonmulch and common film mulch treatments, degradable film mulch increased bacterial diversity in bulk soil rhizosphere and endosphere. As film mulch improved soil structure, compared to CK and ordinary mulch treatment, DF significantly increased soil temperature and SWC and optimized the growth environment of plant roots. Under appropriate water and temperature conditions, plant root exudates increase28, providing more nutrient sources for root microorganisms, thus increasing the bacterial Shannon index. The decrease in microbial diversity in the bulk and rhizospheres of fungi may be due to residual film and microplastics produced after partial fragmentation of plastic films, providing a unique habitat for microbial communities in soil. only specific microbial communities are enriched on plastic residual film, reducing microbial community diversity29,30. Changes in root-related niche microbial communities are mainly caused by plant host selectivity31. Compared to nonmulching treatment, film mulching impacts the soil microbial community by changing the soil’s physical structure, microclimate, and nutrient status32. The relative abundance of Proteobacteria in the rhizosphere was increased by the degradable film mulching. This may be because increased microplastic content can provide adsorption sites for microorganisms and alter the bacterial community structure33,34. At the same time, degradation film mulching significantly changed the composition of fungal communities in different root niches compared with the nonmulching treatment (Fig. S3).
Microbial assembly under degradation film treatment was mainly a random process
Neutral processes play a key role in constructing the microbial biogeography of entire biomes35. These processes are mainly generated by random ecological drift or diffusion36. The assembly process of bacterial and fungal communities under degradation film treatment was dominated by random processes, and the assembly process of microbial communities gradually shifted to deterministic processes due to the influence of rhizosphere effects (Fig. 5). In agroecosystems, environmental change is slower and the impact of environmental filtration is lower37, explaining that random processes are the main factors affecting the microbial community assembly. The percentage of random processes in bacterial and fungal community assembly in degradable film mulching soils was lower than that in nonmulching soils (Fig. 5). Other studies have also found that degradable film covering soil increases the random process of bacterial community assembly38. It may be due to the physical barrier layer of degradation film covering treatment, which creates a stable microenvironment and increases the available microbial resources39. Similar changes have also been observed in fungal communities due to the unique regional characteristics and limited dispersal ability of fungi40. In soil treated with biodegradable films, the bacterial and fungal community structure is also affected by soil characteristics. Therefore, degraded film mulching affects the aggregation process of microbial communities by changing soil nutrient availability and microenvironment.
Effect of degradation membrane on core microorganisms of the co-occurrence network of microbial community
Microorganisms in soil do not exist in isolation but are interrelated through a series of direct and indirect ecological processes, such as collaboration, competition, and antagonism, forming a complex microbial symbiosis network41,42. Different ecological niches also affect the network complexity of bacterial and fungal communities. In the bacterial network diagram, the complexity and modularity of rhizosphere networks are lower than those of bulk soil and rhizosphere, indicating that in most cases the network complexity gradually decreases along the bulk soil, and endosphere43. The rhizosphere network of fungi has higher complexity and connectivity, but the modularity coefficient of fungi is the lowest, and the modularity coefficient of fungi is the highest. Degradable film mulching increased fungal network connectivity and decreased modularity levels, possibly due to the increased availability of soil nutrients and plant growth promotion44. Therefore, the microbiome of different root-related niches can construct different complex correlation networks in response to environmental stress. Endosphere fungi increased the positive associations in fungal association networks. The positive association indicated that the biodegradable film covering reduced the competition of the fungal community, with most fungi being in a cooperative relationship, consistent with the findings of a previous study20. The increase in negative association indicates that the degradation film coverage enhances intraboundary competition, whereas increase in positive association proves that community competition has reduced45. In this study, degradation film treatment reduces the negative association of bacteria and increases the positive association of the fungal network, indicating that it will reduce the competition between bacteria and bacteria and that between fungi and fungi.
According to the joint analysis of ZiPi and network key species, Nocardiopsis, the core bacterium in the nonrhizotrophic bacterial network, has a strong salt tolerance46, and Anaeromyxobacter plays an important role in the process of nitrate dissimilation to ammonium47. Sphingomonas plays a positive role in repairing environmental pollution and promoting the degradation of compounds and plastics and biotransformation48. Pseudoxanthomonas and Amycolatopsis in the rhizosphere can promote the degradation of pollutants and positively affect mulching film degradation, facilitating the decomposition of microplastic debris49,50. Aminobacter also degrades pollutants in endosphere51. Olpidium, as a core species in the rhizosphere and bulk soil, plays a key role in the reconstruction of fungal flagellar loss52, whereas Schizothecium53 mainly promotes nutrient cycling and litter decomposition in the soil in the rhizosphere. These dominant bacterial genera play an important role in the collinear network of bacteria or fungi and are significantly enriched, which can strengthen the interrelationship between microorganisms, promote nutrient cycling, accelerate plastic film degradation, enhance soil resilience, provide a core hub for the microbiome and the plant worlds, it further improve sunflower yield, and also provide a new direction for further analysis of the degradation mechanism of microplastics.
Conclusion
This study showed that degradable film mulching increased soil temperature and water content, promoted the enrichment of beneficial bacteria (Amycolatopsis, Anaeromyxobacter, and Sphingomonas) in soil, secreted metabolites (enzyme activity) to promote nutrient circulation, and enhanced soil resilience. At the same time, bacterial and fungal diversities and community changes in large soil and sunflower root-related ecological niches were significantly affected. Bacterial and fungal communities under the degradable film mulching were driven by random processes, but the rhizosphere was more sensitive to external factors and gradually changed to a deterministic process, which promoted the association among beneficial microorganisms, reduced the competition among bacteria, and improved the complexity of bacterial networks in different ecological niches in sunflower roots while reducing the complexity of fungal networks. The positive correlation between microorganisms increased, and the coordinated interaction between microorganisms, soil, and sunflowers increased the yield. These findings provide new insights into degradable film mulching for soil microbiome assembly and sunflower yield stabilization.
Data availability
Sequence data that support the findings of this study have been deposited in the NCBI Sequence Read Archive (SRA) with the primary accession code PRJNA1141434.
References
Delen, Y. et al. Dissecting the genetic architecture of morphological traits in sunflower (Helianthus annuus L). Genes 15 (7), 950 (2024).
Guo, S. C. et al. Analysis of the overall development of sunflower industry in the world and China. China Seed Ind. 7, 10–13 (2021).
Lamont, W. J. Plastics.Modifying the microclimate for the production of vegetable crops. HortTechnology. 15, 477–481 (2005).
Feng, Y. et al. Infiltration and water use efficiency of maize fields with drip irrigation and biodegradable mulches in the West Liaohe plain, China. Plants (Basel Switzerland). 12 (5), 975 (2023).
Mansoor, Z. et al. Polymers Use as Mulch Films in Agriculture-A Review of History, Problems and Current Trends. Polymers. 14(23), 5062 (2022).
Wang, K. et al. Green production of biodegradable mulch films for effective weed control. ACS Omega. 6 (47), 32327–32333 (2021).
Zhang, P. et al. Plastic-Film mulching for enhanced Water-Use efficiency and economic returns from maize fields in semiarid China. Front. Plant Sci. 8 (512), 2017 (2017).
Dou, Y. et al. Effects of straw and plastic film mulching on microbial functional genes involved in soil nitrogen cycling. Front. Microbiol. 14, 1205088 (2023).
Li, S., Wang, Z., Li, S. & Gao, Y. Effect of nitrogen fertilization under plastic mulched and non-plastic mulched conditions on water use by maize plants in dryland areas of China. Agric. Water Manage. 162, 15–32 (2015).
Alkorta, I. et al. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health. 18 (1), 65–73 (2003).
Moreno, M. M. & Moreno, A. Effect of different biodegradable and polyethylene mulches on soil properties and production in a tomato crop. Sci. Hort. 116 (3), 256–263 (2008).
Bandopadhyay, S. et al. Biodegradable plastic mulch films: impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 9, 819 (2018).
Ding, F. et al. Does long-term use of biodegradable plastic mulch affect soil carbon stock? Resources Conserv. Recycling. 175, 105895(2021).
Ownley, B. H., Gwinn, K. D. & Vega, F. E. Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl. 55, 113–128 (2010).
Burke, D. J. et al. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a Northern hardwood forest. Soil Biol. Biochem. 43, 795–803 (2011).
Valyi, K., Mardhiah, U., Rillig, M. C. & Hempel, S. Community assembly and coexistence in communities of arbuscular mycorrhizal fungi. ISME J. 10, 2341–2351 (2016).
Zhang, M. et al. Effect of Long-Term biodegradable film mulch on soil physicochemical and microbial properties. Toxics. 10 (3), 129 (2022).
Dong, W. et al. Influence of film mulching on soil microbial community in a rainfed region of Northeastern China. Sci. Rep. 7 (1), 8468 (2017).
Li, Y. et al. Buried straw layer and plastic mulching increase microflora diversity in salinized soil. J. Integr. Agric. 15, 1602–1611 (2016).
Li, M. X. et al. Effects of biodegradable plastic film mulching on soil microbial abundance, activity, and community structure. J. Agro-Environment Sci. 41 (8), 1758–1767 (2022).
Zhang, H. et al. Biodegradable film mulching increases soil microbial network complexity and decreases nitrogen-cycling gene abundance. Sci. Total Environ. 933, 172874 (2024).
Olesen, J. M. et al. The modularity of pollination networks. Proc. Natl. Acad. Sci. U.S.A. 104 (50), 19891–19896 (2007).
Deng, Y. et al. Molecular ecological network analyses. BMC Bioinform. 13, 113–113 (2012).
Xun, W. et al. Specialized metabolic functions of keystone taxa sustain soil Microbiome stability. Microbiome. 9 (1), 1–15 (2021).
Schloss, P. D. et al. Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75 (23), 7537–7541 (2009).
Choudhary, K. & Sangwan, K. S. Green supply chain management pressures, practices and performance: a critical literature review. Benchmarking: Int. J. 3 (2), 226–244 (2021).
Zhang, R., Vivanco, J. M. & Shen, Q. The unseen rhizosphere root–soil–microbe interactions for crop production. Curr. Opin. Microbiol. 37, 8–14 (2017).
Osburn, E. D., Aylward, F. O. & Barrett, J. E. Historical land use has long-term effects on microbial community assembly processes in forest soils. ISME Commun. 1 (1), 1–4 (2021).
Siwer, P., Domagala-Swatkiewicz, I. & Kalisz, A. The influence of degradable polymer mulches on soil properties and cucumber yeld. Agrochimica. 59(2), 108–123 (2015).
Zhang, M. et al. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 688, 470–478 (2019).
Miao, L. et al. Microbial carbon metabolic functions of biofilms on plastic debris influenced by the substrate types and environmental factors. Environ. Int. 143, 106007 (2020).
Huang, Y. H. et al. Maize root-associated niches determine the response variation in bacterial community assembly and function to phthalate pollution. J. Hazard. Mater. 429, 128280 (2022).
Deng, Q. et al. Soil microbial community and its interaction with soil carbon and nitrogen dynamics following afforestation in central China. Sci. Total Environ. 541, 230–237 (2016).
Farmer, J. et al. Long-term effect of plastic film mulching and fertilization on bacterial communities in a brown soil revealed by high through-put sequencing. Arch. Agron. Soil. Sci. 63, 230–241 (2017).
Huang, F. et al. Effects of different long-term farmland mulching practices on the loessial soil fungal community in a semiarid region of China. Appl. Soil Ecology. 137, 111–119 (2019).
Holman, L. E. et al. Animals, protists and bacteria share marine biogeographic patterns. Nat. Ecol. Evol. 5 (6), 738–746 (2021).
Santorelli Junior, S. et al. Neutral processes and reduced dispersal across Amazonian rivers May explainhow Riversmaintain speciesdiver sity after secondary contact. Perspect. Ecol. Conserv. 20, 151–158 (2022).
Rabelo, P. C. M. et al. Tropical forest type inffuences community assembly processes in arbuscular mycorrhizal fungi. J. Biogeogr. 47, 434–444 (2020).
Guo, Y. et al. Community assembly patterns and processes of microbiome responses to habitats and Mytilopsis sallei invasion in the tidal zones of the Pearl River Estuary. Sci. Total Environ. 857(Pt 3), 159675 (2023).
Zhang, H. et al. Agronomic performances of biodegradable and non-biodegradable plastic Fflm mulching on a maize cropping system in the semi-arid loess plateau. Pedosphere. 34 (1), 88–96 (2024).
Peay, K., Kennedy, P. & Talbot, J. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 14, 434–447 (2016).
Wei, S. et al. Dynamic changes of soil microorganisms in rotation farmland at the Western foot of the greater Khingan range. Front. Bioeng. Biotechnol. 11, 1191240 (2023).
Gui, H. et al. Continental-scale insights into the soil microbial co-occurrence networks of Australia and their environmental drivers. Soil Biology Biochemistry. 186, 109177(2023).
Li, K. et al. The plastisphere of biodegradable and conventional microplastics from residues exhibit distinct microbial structure, network and function in plastic-mulching farmland. J. Hazard. Mater. 442, 130011 (2022).
Wang, Q. et al. Effects of microplastics and carbon nanotubes on soil geochemical properties and bacterial communities. J. Hazard. Mater. 433, 128826 (2022).
Bennur, T., Ravi Kumar, A., Zinjarde, S. S., & Javdekar, V. (2016). Nocardiopsis species: a potential source of bioactive compounds. J. Appl. Micro. 120(1), 1–16(2016).
Nelson, M. B., Martiny, A. C. & Martiny, J. B. Global biogeography of microbial nitrogen-cycling traits in soil. Proc. Natl. Acad. Sci. 113, 8033–8040 (2016).
Asaf, S., Numan, M., Khan, A. L. & Al-Harrasi, A. Sphingomonas: from diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 40 (2), 138–152 (2020).
Abdul, M. et al. Chapter 18 - Plant growth-promoting rhizobacteria as bioremediators of polluted agricultural soils: challenges and prospects, Academic Press. 265–275 (2022).
Song, Z. et al. Secondary metabolites of the genus Amycolatopsis: structures, bioactivities and biosynthesis. Molecules. 26, 1884 (2021).
McDonald, I. R. et al. Aminobacter ciceronei Sp. Nov. And Aminobacter lissarensis Sp. Nov., isolated from various terrestrial environments. Int. J. Syst. Evol. MicroBiol. 55 (Pt 5), 1827–1832 (2005).
Chang, Y. et al. Genome-scale phylogenetic analyses confirm Olpidium as the closest living zoosporic fungus to the non-flagellated, terrestrial fungi. Sci. Rep. 11 (1), 3217 (2021).
Lindahl, B. D. et al. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol. 173 (3), 611–620 (2007).
Funding
This study was supported by Integrated demonstration of key technologies and modes of mulch reduction and efficient recycling Ordos City “Open bidding for selecting the best candidates” project (JBGS-2021-001); Responses of greenhouse gas emissions and carbon, nitrogen and water footprint to agricultural practices in the northern agro-pastoral ecotone (2022ZY0216); Inner Mongolia Grassland Talents Science and Technology Program; and Inner Mongolia Leading Talent Team Project (2022LJRC0010).
Author information
Authors and Affiliations
Contributions
L.Z.Y., Z.X.Q. and Z.D.J conceived the ideas.C. X.Y ., W.W.N.,L. J.M.andZ. J.W., Z.M.and Z.X.Y. collected the soils and measured the physicochemical properties and potential nitrification rates. M.T.T. and H.T. B. conducted the microbiological analysis and created the graphs. M.T.T. wrote the paper. L.Z.Y., Z.X.Q. and Z.D.J. reviewed the paper, all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meng, T., Bu, H., Zhang, X. et al. Degradable film mulching recruited beneficial microbiota and increased rhizosphere bacterial diversity in sunflower. Sci Rep 15, 18522 (2025). https://doi.org/10.1038/s41598-025-03213-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03213-2