Abstract
The objective of this study was to evaluate the efficacy of transcatheter aortic valve replacement (TAVR) via the femoral approach in patients with pure aortic regurgitation (AR), with a focus on mortality, adverse event rates, cardiac function, and clinical symptom improvement. The study utilised a single-centre experience to provide insights and offer guidance for the management of TAVR in AR patients. Patients with aortic valve pathology who underwent TAVR at the Second Affiliated Hospital of Nanchang University, from January 2018 to March 2023 were enrolled. They were classified into two groups: pure AR and aortic stenosis (AS) based on preoperative transthoracic echocardiography. We compared baseline characteristics, imaging data, surgical outcomes, and follow-up conditions between them. We focused on the safety and efficacy of TAVR in patients with AR and evaluated these results using regression analysis. The study cohort comprised 87 patients, with 21 in the AR group and 63 in the AS group. We revealed that AR patients exhibited low mortality and adverse event rates following transfemoral TAVR, with notable improvements in postoperative cardiac function and substantial symptom relief. However, the rates of paravalvular leak (PVL), permanent pacemaker (PPM) implantation, and valve-in-valve procedures were relatively elevated. While these findings suggest that TAVR may represent a viable therapeutic option for patients with AR, the elevated rates of PVL, PPM implantation, and readmission underscore the need for further investigation, with larger cohorts and extended follow-up, to more robustly evaluate the long-term safety and efficacy of this approach.
Similar content being viewed by others
Introduction
Aortic regurgitation (AR) is a type of valvular heart disease (VHD) in which the aortic valve fails to close properly during diastole, resulting in the backflow of blood from the aorta to the left ventricle, and it accounts for the highest proportion of moderate-to-severe aortic valve disease cases in China, reaching 38.8%1. Its etiology may be congenital structural and functional abnormalities of the aortic valve and ascending aorta, or secondary to diseases such as infective endocarditis and rheumatic heart disease2. As society continues to age worldwide, degenerative disease has become a major etiologic factor, accounting for 60.9% of AR patients ≥ 60 years of age1,3,4. Owing to the increase in counterflow, the volume burden of the heart continues to rise, the left ventricle continuously enlarges, and the left ventricular ejection fraction (LVEF) persistently decreases, which ultimately leads to left ventricular remodeling and heart failure. Most patients experience symptoms such as chest tightness, shortness of breath, dyspnea, panic, angina pectoris, dizziness, and even syncope.
Transcatheter aortic valve replacement (TAVR) is the first-line standard treatment option for patients with aortic stenosis (AS), with the advantages of less trauma, faster postoperative recovery, and lower complication rates5,6. Since the world’s first TAVR was performed by Cribier in 2002 and China’s first TAVR was performed by Prof. Junbo Ge’s team at Zhongshan Hospital of Fudan University in 2010, the technology of TAVR has rapidly developed in the past two decades, and the indications for TAVR have been expanded to include the treatment of AR. Owing to the special anatomical structure of AR patients, such as the lack of valve calcification, the absence of a stable prosthetic valve anchorage area, the large annulus diameter, the dilated ascending aorta, and the tendency toward valve displacement after implantation, the incidence of postoperative valve-in-valve and perivalvular leakage is high, which has limited the application of TAVR in the treatment of AR7.
Clinical treatment requires weighing the pros and cons of various treatment options. Surgical risk is only one factor in determining a patient’s treatment options. Guidelines for the treatment of valvular disease emphasise the need for a thorough assessment, taking into account life expectancy, prosthesis durability and anatomical features to determine the best treatment option. Rigorous preoperative screening is essential5. Transthoracic echocardiography (TTE) remains the preferred imaging modality for assessing valve regurgitation, heart size, and LVEF6. Key parameters such as LVEF, aortic valve morphology, and transvalvular pressure gradients provide essential information for staging AR and monitoring disease progression and postoperative recovery. Aortic root CT angiography (CTA) is considered the “gold standard” for assessing aortic root anatomy and guiding surgical planning8.
The J-Valve (Suzhou Jiecheng Medical Technology Co., Ltd.) is the only prosthetic aortic valve for TAVR in China that has been approved for use in cases of AR. While studies have demonstrated that it is a surgical option that is acceptable, SAVR remains the preferred option, as recommended by major guidelines such as the ESC/EACTS and ACC/AHA9. The limitations of TAVR for AR in these guidelines are due to concerns regarding the durability and effectiveness of the valve. Trials such as PARTNER and CoreValve further underscore the necessity for meticulous patient selection, as evidenced by their exclusion of AR patients10,11. TAVR complications, including paravalvular leak (PVL) and permanent pacemaker (PPM) implantation, are of significance. PVL, characterised by a leak around the prosthetic valve, has the potential to adversely impact long-term outcomes. PPM is the implantation of a permanent pacemaker and can occur as a result of the following conduction system disorders, primarily severe atrioventricular block. The potential impact of PVL on long-term prognosis and the challenges faced by patients with PPM in achieving optimal hemodynamic outcomes.
Although transapical TAVR (TA-TAVR) is more invasive, transfemoral TAVR (TF-TAVR) is less invasive with fewer complications. However, TAVR for AR remains technically challenging with limited experience in China. The present study aims to evaluate the outcomes of TAVR using Venus-A self-expanding valves via transfemoral access in AR patients, focusing on mortality, adverse events, cardiac function, and clinical symptoms.
Methods
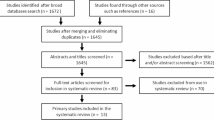
Subject: On the basis of the inclusion and exclusion criteria, a single-center retrospective study of 148 patients who received TAVR treatment in the Department of Cardiology at the Second Affiliated Hospital of Nanchang University from January 2018 to March 2023 was performed. The criteria for inclusion and exclusion were as follows: (1) age ≥ 60 years; (2) undergoing TF TAVR fro mod-sev AR with less than mild AS, and patients with severe AS. The exclusion criteria were as follows: (1) valve-in-valve TAVR; (2) Moderate-to-severe AR combined with moderate-to-severe AS; and (3) patients aged < 60 years. The patient screening process is shown in Fig. 1.
The AS group was selected as the reference point for comparison with the AR group in this study for several reasons. Primarily, although TAVR for AR and AS differ in terms of pathophysiology, anatomical considerations, and surgical approach, the two conditions share commonalities in terms of valve pathology and valve replacement requirements. Furthermore, the inclusion of the AS group was intended to provide a broad benchmark rather than a direct comparison of the two groups. All the subjects provided informed consent, and all the procedures and protocols were approved by the Clinical Research Ethics Committee of the Second Affiliated Hospital of Nanchang University and were performed in accordance with the Declaration of Helsinki.
Basic patient information
Preoperative demographic data including sex, age, body mass index (BMI), NYHA cardiac function classification, Society of Thoracic Surgeons (STS) score, smoking history and comorbidities such as hyperlipidaemia, hypertension, diabetes mellitus and coronary artery disease, imaging parameters, surgical information and postoperative follow-up results were collected from the hospital information system. TTE and aortic root CTA were performed in all patients to thoroughly assess valve anatomy and surgical risk.
Data from echocardiography and CTA
Preoperatively, the following data were collected from the TTE results: aortic valve annulus diameter (AVAD), ascending aortic diameter (AAD), left ventricular end-diastolic diameter (LVEDd), LVEF, aortic orifice flow velocity, aortic valve mean transvalvular pressure gradients, mitral regurgitation (MR), and tricuspid regurgitation (TR).
Moreover, we evaluated the aortic root via CTA and performed 3D reconstruction of the anatomical structures, etc. The following data were obtained: aortic valve calcification volume, aortic valve annulus diameter, annulus area, ascending aortic diameter, left ventricular outflow tract diameter (LVOTD), aortic angle, and coronary artery height, and the results were analyzed via 3-mensio Structural Heart software. Calculation of implanted valve oversize rate: oversize rate = (diameter of prosthetic valve/diameter of annulus measured by CTA − 1) *100%.
Postoperative follow-up and study endpoints
Patients were followed for six months after surgery with a combination of telephone consultations and outpatient visits. Echocardiograms were performed before discharge and at one and six months after surgery to assess parameters of prosthetic aortic valve function, LVEF, and the incidence of complications. These included permanent pacemaker implantation, stage 2–3 acute kidney injury, stroke, major vascular complications and the need for secondary valve-in-valve implantation. Data on mortality, readmission rates and an assessment of prosthetic valve morphology and function were also carefully collected. The primary endpoint of the study was all-cause death during the follow-up period, and the specific assessment time was postoperative, 1 month and 6 months after TAVR. Secondary endpoints encompassed the occurrence of surgery-related complications over the same period.Surgical success was determined according to the standardised criteria established by the Valve Academic Research Consortium (VARC-2), which include the absence of intraoperative mortality, correct positioning of the prosthetic valve, a mean pressure gradient across the aortic valve of less than 20 mmHg, and the absence of moderate-to-severe PVL12.
Operation procedure
After rigorous assessment and surgical planning by a multidisciplinary cardiac team, TAVR was performed in the cardiac interventional catheterisation laboratory. After the patient was anaesthetised, a femoral artery approach was selected for puncture and placement of an arterial sheath, and a pigtail catheter was advanced to the aortic root for imaging. After temporary pacing at 180 beats per minute to lower systolic blood pressure to less than 60 mmHg, an appropriately sized Venus-A prosthetic aortic valve was selected and delivered to the aortic annulus via a Lunderquist guidewire, precisely positioned according to intraoperative transesophageal ultrasound (TEE) and aortic root angiography, and the valve was released. TEE and aortic root angiography were repeated to assess the morphology and function of the implanted prosthetic valve and the condition of the aorta, and if the results showed moderate or greater perivalvular leakage of the prosthetic valve, post-balloon dilatation or valve-in-valve implantation was considered. The valve delivery system was removed at the end of the procedure, the femoral artery puncture site was sutured, and temporary pacing leads were left in place until 24 h after surgery. All patients were monitored in the intensive care unit for at least 8 h. Long-term antiplatelet therapy was given postoperatively, long-term oral anticoagulants were given to those with indications for anticoagulation, and echocardiograms and electrocardiograms were reviewed before discharge6,13. The procedure is shown in Fig. 2.
Illustration of the surgical procedure. (A,B) Preoperative TTE suggesting severe aortic regurgitation; (C,D) Preoperative CTA of the aortic root. (E) Intraoperative aortic root angiography for TAVR showing massive aortic regurgitation. (F) Repeat angiography after valve release showing no significant regurgitation.
Statistical analyses
Data were analysed using SPSS 26.0. Normality test and chi-square test were performed on all data. Normally distributed data were expressed as mean ± standard deviation (x ̅ ± s) and skewed data were expressed as median M (Q1, Q3). The paired-samples t-test was used to compare baseline and postoperative related indices in the same group of patients, and the independent-samples t-test or Mann–Whitney U-test was used to compare data between the two groups. Count data were expressed as the number of cases (%), and the χ2 test or Fisher’s exact probability method was used to compare data between the two groups. Kaplan–Meier curves and Cox proportional risk regression were used to assess the cumulative survival of the two groups at 6 months after surgery, and the log-rank test was used to compare the two groups. All tests were two-tailed and P < 0.05 was considered statistically significant.
Results
Comparison of clinical baseline characteristics between the two groups
A total of 84 patients were included in this study: 21 patients in the aortic regurgitation group and 63 patients in the aortic stenosis group, with a mean age of 71.6 ± 7.0 years, of whom 51 (60.7%) were male. STS score (8.2 ± 3.6%), NYHA class I-II, 8 patients (9.5%), class III, 58 patients (69.0%) and class IV, 18 patients (21.4%) were included. Compared with the AS group, the AR group had a lower percentage of bileaflet aortic valves (9.5% vs 69.8%; P < 0.001), and there were no statistically significant differences in age, sex, cardiac function class, STS score, or comorbidities including smoking, hypertension, hyperlipidaemia, diabetes mellitus, coronary artery disease, or atrial fibrillation (P > 0.05). Baseline clinical characteristics of the two groups are detailed in Table 1.
Comparison of preoperative echocardiography and CTA between the two groups of patients
Compared with those in the AS group, patients in the AR group had a larger left ventricular end-diastolic diameter on preoperative TTE (57.4 ± 6.9 vs 49.1 ± 8.2; P < 0.001), and the differences were not statistically significant in terms of AVAD, AAD, LVEF, or combined moderate to severe mitral or tricuspid regurgitation (P > 0.05). In addition, the volume of aortic valve calcification was significantly lower in the AR group than in the stenosis group (3.4 ± 9.1 vs. 557.1 ± 356.6; P < 0.001), of which only three patients had mild aortic valve calcification. The height of the left coronary artery was slightly greater in the AR group than in the AS group (14.7 ± 5.3 vs 14.4 ± 3.1; P = 0.017). The differences between the two groups in terms of annular diameter, annular area, AAD, LVOTD and aortic coarctation angle were not statistically significant (P > 0.05). In addition, annular internal diameter, ascending aortic internal diameter and annular area were greater in the AR group than in the AS group, although the differences were not statistically significant (P > 0.05). A comparison of the preoperative echocardiograms and CTAs between the two groups is detailed in Table 2.
Comparison of operations in the two groups of patients
The surgical success rate was high in both groups, with 1 postoperative death due to cardiogenic shock in the AR group, 2 intraoperative deaths due to cardiogenic shock, 3 postoperative deaths in the AS group, and 1 reoperation due to massive regurgitation observed by esophageal ultrasound after valve implantation during surgery; the difference was not statistically significant (95.2% vs. 90.5%; P > 0.05). Compared with the AS group, the implanted valve size (29.0 vs. 26.0; P < 0.001) and the valve oversize rate (17.1 ± 7.4 vs. 5.5 ± 8.2; P < 0.001) were greater in the AR group than in the stenosis group, and the rates of balloon predilatation (14.3% vs. 87.3%; P < 0.001) and postdilatation (4.8% vs. 38.1%; P = 0.004) were significantly lower in the AR group. And the rate of intraoperative valve-in- valve implantation was significantly higher (47.6% vs 14.3%; P = 0.002). A comparison of the surgical status of the two groups of patients is detailed in Table 3.
Comparison of preoperative and postoperative echocardiograms in patients with AR
A total of 20 patients in the AR group participated in the follow-up, and the transthoracic echocardiogram was reviewed at 1 month and 6 months after surgery. As shown in Table 4, the patients’ left ventricular end-diastolic diameters decreased to varying degrees, the left ventricular ejection fraction increased compared with the preoperative period, and the transvalvular pressure across the aortic valve also decreased significantly after surgery (P < 0.05).
Comparison of the occurrence of complications between the two groups
A total of 77 patients were followed up for 6 months in this study. A total of 6 patients underwent PPM for postoperative atrioventricular block of degree III, including 3 patients (14.3%) in the AR group and 3 patients (4.8%) in the AS group. One patient (4.8%) in the AR group developed a new cerebral infarction after surgery, but after cerebral protection and improvement in cerebrovascular circulation, the patient did not experience any special discomfort. There were no new deaths during the follow-up period, and a total of 4 patients were readmitted to the hospital, including 3 patients (15.0%) in the AR group, 1 patient who returned to the hospital due to heart failure induced by lung infection, 1 patient who returned to the hospital for review because of chest tightness and discomfort, and the other patient who returned to the hospital for simple review.
In the AS group, one patient (1.8%) was admitted to the hospital with chest pain, was diagnosed with coronary atherosclerotic heart disease via coronary angiography, and was discharged from the hospital with improved symptoms after secondary prevention of coronary artery disease such as antiplatelet and lipid modulation.At the end of the follow-up period, there were 3 cases of moderate-to-severe perivalvular leakage, including 2 cases (10.0%) in the AR group and 1 case (1.8%) in the AS group, and no patients had secondary midvalve implantation. The results of the analysis revealed that, compared with those in the AS group, patients in the AR group had no statistically significant differences in terms of complications between the two groups, except for a higher readmission rate (P = 0.02). The patients’ complications are shown in Table 5. The Kaplan–Meier survival curves (Fig. 3) at 6 months after procedure in both groups revealed that the difference in the cumulative survival rate was not statistically significant. In addition, we used Cox proportional hazards regression for a more robust analysis of differences in survival. The results are shown in Table 6, although the hazard ratio = 1.6445 indicates that the risk of death for patients in the AS group was 1.64 times higher than for patients in the AR group. However, this result was not statistically significant as the P value = 0.6498, indicating that the difference in risk of death between the two groups of patients was not significant. The reason for this may be due to our small sample size and short follow-up period, which led to weak explanatory power of the model, and more event data may be needed for further validation in the future.
Discussion
AR is one of the most common forms of VHD. A community-based population survey of patients with moderate or severe valvular heart disease in the United States showed that the prevalence of AR was much lower than the prevalence of AS: 0.6% and 1.4% in those aged 65–74 years, and 1.7% and 4.6% in those aged ≥ 75 years. In the 2017 European Valvular Heart Disease Observational Research Program II survey, severe pure AR accounted for 5.3% of severe VHD14. In China, echocardiographic databases show a higher detection rate of AR than AS15,16. In addition, as a degenerative disease, patients with AR often have serious complications such as heart failure, and the mortality rate is not low. Therefore, there is a need to update safer and more effective treatments for AR patients.
Current status of TAVR treatment for patients with AR
TAVR in patients with AR presents significant technical challenges compared to patients with AS. one of the major obstacles in patients with AR is the near absence of calcification in the aortic valve. whereas the valves in patients with AS are often severely calcified and stenotic, the aortic valves in patients with AR are usually uncalcified or only slightly calcified. the lack of calcification can complicate valve fixation in patients with AR. Lack of calcification can complicate valve fixation because calcified tissue provides a stable anchor for prosthetic valves. The lack of significant calcification in patients with AR increases the risk of valve migration, paravalvular leak and improper valve implantation. To minimise these risks, embolic protection devices are increasingly being used in TAVR to capture any debris or embolic material that may be displaced during valve implantation.
The anatomical characteristics of AR limit the development of TAVR surgery. First: AR patients lack the necessary conditions for valve implantation and autologous valve calcification, which makes prosthetic valve implantation prone to displacement and is prone to inducing complications. Second, in AR, oversized valves are often required to ensure a proper fit and prevent paravalvular leakage. Chronic regurgitation in AR often leads to dilation of the aortic annulus, requiring the use of larger valves to achieve an effective seal. However, oversizing carries additional risks, such as potential damage to surrounding tissue or increased risk of valve migration. Oversized valves also carry the risk of coronary artery obstruction or aortic root damage, which can lead to serious complications. AR patients have a significantly greater incidence of postoperative PVL and mortality during follow-up due to long-term blood regurgitation, which enlarges the aortic annulus17,18. Currently, the proportion of patients with pure AR treated with TAVR is very small in China, and most patients with chronic AR who are elderly, have severe underlying diseases and many comorbidities, and who choose conservative treatment due to the high risk of surgery, suffer from a mortality rate that can reach 20%19. In recent years, with the advancements in surgical methods and valve instrumentation, there have been new advances in the treatment of AR via TAVR, but SAVR is still the treatment of choice for simple severe AR in the current domestic and international guidelines and expert consensus. In fact, for AR patients with contraindications to conventional surgery or those at intermediate to high risk and with appropriate aortic valve anatomy, TAVR is not a viable treatment option, and many centers have applied TAVR to treat AR with good surgical efficacy20,21,22. The oversized valve annulus should be considered a limitation of the current device, and future improvements in bioprosthetic valve design to better accommodate AR patients are needed to minimise the need for such adjustments in future devices. In addition, future improvements in the accuracy of imaging and surgical techniques, such as accurate valve positioning and deployment, should be enhanced and play a key role in minimising complications associated with valve migration or embolization. Pre-operative imaging of the aortic annulus and root is also helpful in determining optimal valve size and placement technique, which is particularly important in patients with AR.
The 2020 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the management of valvular disease state that patients with mild asymptomatic or excessively high surgical risk AR may be treated with pharmacologic therapy, that aortic valve surgery should be performed for symptomatic severe AR regardless of left ventricular systolic function, and that surgical intervention is recommended for asymptomatic patients presenting with impaired left ventricular function (LVEF ≤ 55%)5. The 2021 European Society of Cardiology Guidelines for the Management of Valvular Disease similarly recommend surgery for symptomatic severe AR, whereas surgery is recommended for asymptomatic severe AR that presents with an LV end-systolic internal diameter of > 50 mm or an LVEF of ≤ 50%6. The latest 2023 Chinese Expert Consensus on Transfemoral Aortic Valve Replacement for AR states that patients with moderate-to-severe AR with anatomically appropriate contraindications to surgery or at high risk have an indication for surgical intervention via self-expanding valves with TF-TAVR, which needs to be performed in centers with a well-established team experienced in the field, with an efficacy that is noninferior to surgical intervention7. Our study revealed that TF-TAVR with self-expanding prosthetic aortic valves is feasible for treating symptomatic severe AR via TF-TAVR with self-expanding prosthetic aortic valves because of the low incidence of adverse events after TAVR, with an operative mortality rate of 1/21 (4.8%), and an incidence of severe perivalvular leakage at midterm postoperative follow-up of 2/20 (10.0%).
Procedural approach and selection of transcatheter prosthetic aortic valves
With respect to surgical devices, most prosthetic valves were initially designed for aortic valve calcification in patients with AS and were divided into two major categories: self-expanding valves and balloon-expandable valves. However, owing to the specificity of the anatomical structure of AR patients, which leads to a much greater incidence of postoperative complications such as dislocation due to insufficient anchoring of the prosthetic valve as well as postoperative complications such as rupture of the annulus, valve-in-valve implantation, and PVL, special improvements are needed23. In recent years, a new generation of valve systems designed for AR targeting has emerged, from the original CoreValve stent developed by Medtronic, to the Edwars valve and the JenaValve® system valve, with numerous studies demonstrating good surgical efficacy, with device success rates reaching more than 95%24,25. Structurally, the new generation of prosthetic valves has been specifically reinforced with valve anchors to prevent valve migration26. The transapical J-Valve is a guideline-recommended prosthetic aortic valve for the treatment of TAVR in AR patients; it is designed with a trident buckle and operats in a smooth transapical direction to minimize prosthetic valve dislocation during implantation, and its safety and efficacy have been verified by a large multicenter study at West China Hospital21. However, the transapical approach tends to face greater surgical risk and longer postoperative recovery time with greater myocardial damage to the patient; and thus, the transfemoral approach is currently the most widely used procedural route4. With less myocardial damage, a shorter mechanical ventilation time, and lower postoperative complications and mortality, TF-TAVR is a better regimen, and its efficacy in AR is no worse than that of the transapical or transabdominal route27. In our center, we use the domestically developed Venus-A self-expanding valve, which is a second-generation transcatheter prosthetic valve system with a retrievable function and a significantly higher success rate than ball-expandable valves28. The design of the three positioning points at the bottom of the valve skirt improves the radial support of the valve and allows for repeated intraoperative fixation of the valve to achieve precise positioning of the valve and minimize the risk of complications. Initially, designed for patients with AS, this study demonstrated the feasibility of this valve for the treatment of AR29. Additionally, in patients with AR, the use of a valve that is 15%-20% (but not more than 20%) larger than the annulus diameter usually improves the radial support of the valve28,30,31. Available evidence indicates that self-expanding valves are effective in improving the radial support of the valve.
Postoperative complications of TAVR in AR patients
The high incidence of PVL and PPM is one of the most common and significant complications in AR patients undergoing TAVR. PVL is among the most common and important complications after TAVR in AR patients and is correlated with increased mortality in advanced-stage patients32,33. The high rate of PVL and valve-in-valve implantation in patients with AR is related to its anatomical peculiarities: the lack of valve calcification; the increased LVEDd; AVAD, and AAD; the tendency of the valve to migrate after implantation; and the need for a valve-in-valve strategy in the presence of perivalvular leaks of greater than moderate severity to minimize residual aortic regurgitation in the postoperative period. Therefore, selecting the correct valve size and avoiding implantation too deep is critical to minimising PVL. In addition, to improve valve stability, patients with AR often need a larger prosthetic valve size to better anchor the valve stent by increasing radial support. Careful preoperative evaluation, selection of the appropriate prosthetic valve, and precise positioning and depth of valve implantation are effective measures to minimize perivalvular leakage and prevent valve migration.
In terms of the occurrence of PPM, compression of the cardiac conduction system during TAVR in AR patients with larger valves may lead to postoperative atrioventricular block, increasing the risk of pacemaker implantation34,35. The available literature suggests that oversized valves and valve implantation too deep may be a cause of conduction system compression36,37. Therefore, the blind use of oversized valves during the procedure should be avoided, especially in patients with preoperative conduction block, and the selection of balloon-expandable valves may help to minimize adverse events.
In addition, vascular complications, stroke and acute kidney injury are also common complications after TAVR, and the incidence of these complications has been reduced to varying degrees with improvements in operator proficiency and updating of instrumentation and devices3,4. In the present study, only 1 case of postoperative death (4.8%), no secondary valve-in-valve implantation, 3 cases of postoperative PPM (14.3%), 1 case of new cerebral infarction (4.8%), and 2 cases of moderate-to-severe perivalvular leakage (10.0%) were included in the 21 AR patients included, with no ruptured annulus or significant displacement, resulting in a low incidence of adverse events. In addition to the higher readmission rate in the AR group than in the AS group, the rates of other common complications, including PPM acute kidney injury, and stroke, were not significantly different from those in the AS group, suggesting that TF-TAVR is technically feasible and safe for the treatment of AR. Besides, our results are consistent with the existing literature and suggest that accurate preoperative assessment, selection of the appropriate valve model, and precise localisation of the implantation position and depth can effectively reduce the incidence of PVL and PPM, thereby reducing the incidence of postoperative complications.
Recovery after TAVR in patients with AR
We followed the enrolled patients for 6 months, and the reviewed echocardiographic results revealed that the AR patients had varying degrees of improvement in cardiac structure and function after the procedure (Fig. 4). Although severe cardiac insufficiency and ventricular remodeling in AR patients were not completely reversed before surgery, the symptoms were reduced to different degrees. Current guidelines emphasize the assessment of patient surgical efficacy through improvements in postoperative life expectancy and quality of life as the primary metrics, and TAVR is the first choice for symptomatic patients of any age who are at high risk for or have contraindications to surgery if posttreatment survival is expected to be > 12 months with an acceptable quality of life38,39. TF-TAVR is safe and effective for the treatment of AR as assessed by a postoperative survival benefit to patients of > 25% (improvement of at least 1 grade in the NYHA cardiac function classification; improvement of at least 1 grade in the Canadian angina classification; and improvement in quality of life or prolongation of life expectancy) for the assessment of surgical efficacy.
Limitations
This study is a single-center retrospective study with a limited sample size and a short follow-up period. The small sample size may have impacted the statistical power of the study, potentially limiting the generalizability of the results. In addition, the findings provide preliminary clinical experience in the use of the Venus-A self-expanding valve for TF-TAVR in patients with AR, which requires validation through larger, multicenter studies with longer follow-up periods. Such studies are essential to confirm the robustness of the observed trends and to further establish the efficacy of the Venus-A valve in this patient population. Furthermore, preoperative transthoracic echocardiography and CTA were performed by different physicians, introducing the possibility of measurement bias.
Conclusion
TF- TAVR with Venus-A self-expanding valves in patients with pure AR has demonstrated feasibility. However, challenges such as a high incidence of PVL and PPM rates highlight the technical difficulties associated with AR. These findings highlight the need for larger, prospective, multicentre studies with long-term follow-up to validate the results and refine procedural strategies to improve outcomes in patients with AR.
Data availability
The data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zhe, Li. et al. Characteristics, management and prognosis of patients with moderate and severe aortic valve disease in China: From China-VHD study. Chin. Circ. J. 4, 0322–0407. https://doi.org/10.3969/j.issn.1000-3614.2022.04.002 (2022).
Iung, B. et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 24(13), 1231–1243. https://doi.org/10.1016/S0195-668x(03)00201-X (2003).
Akinseye, O. A., Pathak, A. & Ibebuogu, U. N. Aortic valve regurgitation: A comprehensive review. Curr. Probl. Cardiol. 43(8), 315–334. https://doi.org/10.1016/j.cpcardiol.2017.10.004 (2018).
Généreux, P. et al. Clinical outcomes after transcatheter aortic valve replacement using valve Academic Research Consortium definitions: A weighted meta-analysis of 3,519 patients from 16 studies. J. Am. Coll. Cardiol. 59(25), 2317–2326. https://doi.org/10.1016/j.jacc.2012.02.022 (2012).
Otto, C. M. et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease. J. Thorac. Cardiovasc. Surg. 162(2), E183–E353. https://doi.org/10.1016/j.jtcvs.2021.04.002 (2021).
Vahanian, A. et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 43(7), 561–632. https://doi.org/10.1093/eurheartj/ehab395 (2022).
Chinese Physicians Association of Cardiovascular Physicians Branch. Chinese expert consensus on transfemoral aortic valve replacement for simple aortic regurgitation 2023. Chin. J. Interv. Cardiol. 31(11), 801–809. https://doi.org/10.3969/j.issn.1004-8812.2023.11.001 (2023).
Blanke, P. et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 13(1), 1–20. https://doi.org/10.1016/j.jcct.2018.11.008 (2019).
Coisne, A., Lancellotti, P. & Habib, G. et al. ACC/AHA and ESC/EACTS guidelines for the management of valvular heart diseases: JACC Guideline Comparison. J. Am. Coll. Cardiol. 82(8), 721–34 (2023). https://doi.org/10.1016/j.jacc.2023.05.061
Kirtane, A. J. & Leon, M. B. The placement of aortic transcatheter valve (PARTNER) trial. Circulation 125(25), 3229–3232. https://doi.org/10.1161/CIRCULATIONAHA.112.093070 (2012).
Gleason Thomas, G. et al. 5-year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. JACC. 72(22), 2687–2696. https://doi.org/10.1016/j.jacc.2018.08.2146 (2018).
Kappetein, A. P. et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 60(15), 1438–1454. https://doi.org/10.1016/j.jacc.2012.09.001 (2012).
ten Berg, J. et al. Management of antithrombotic therapy in patients undergoing transcatheter aortic valve implantation: a consensus document of the ESC Working Group on Thrombosis and the European Association of Percutaneous Cardiovascular Interventions (EAPCI), in collaboration with the ESC Council on Valvular Heart Disease. Eur. Heart J. 42(23), 2265–2269. https://doi.org/10.1093/eurheartj/ehab196 (2021).
Iung, B. et al. Contemporary presentation and management of valvular heart disease: The EURObservational research programme valvular heart disease II survey. Circulation 140(14), 1156–1169. https://doi.org/10.1161/CIRCULATIONAHA.119.041080 (2019).
Nkomo, V. T. et al. Burden of valvular heart diseases: A population-based study. Lancet (London, England). 368(9540), 1005–1011. https://doi.org/10.1016/S0140-6736(06)69208-8 (2006).
Pan, W. Z., Zhou, D. X., Cheng, L. L. & Ge, J. B. Aortic regurgitation is more prevalent than aortic stenosis in Chinese elderly population: Implications for transcatheter aortic valve replacement. Int. J. Cardiol. 201(2), 547–548. https://doi.org/10.1016/j.ijcard.2014.10.069 (2015).
Heidari, B. et al. Transcatheter aortic valve replacement outcomes in mixed aortic valve disease compared to predominant aortic stenosis. Int. J. Cardiol. 299, 209–214. https://doi.org/10.1016/j.ijcard.2019.07.099 (2020).
Shoar, S., Naderan, M., Hosseini, F. & Shoar, N. Comment on “Transcatheter aortic valve replacement in patients with pure native aortic valve regurgitation: A systematic review and meta-analysis”. Clin. Cardiol. 42(1), 167–168. https://doi.org/10.1002/clc.23127 (2019).
Wernly, B. et al. Transcatheter aortic valve replacement for pure aortic valve regurgitation: “on-label” versus “off-label” use of TAVR devices. Clin. Res. Cardiol. 108(8), 921–930. https://doi.org/10.1007/s00392-019-01422-0 (2019).
Liu, L., Chen, S., Shi, J., Qin, C. & Guo, Y. Transcatheter aortic valve replacement in aortic regurgitation. Ann. Thorac. Surg. 110(6), 1959–1965. https://doi.org/10.1016/j.athoracsur.2020.03.112 (2020).
Shi, J. et al. Transcatheter aortic valve implantation with j-valve: 2-year outcomes from a multicenter study. Ann. Thorac. Surg. 111(5), 1530–1536. https://doi.org/10.1016/j.athoracsur.2020.06.139 (2021).
Elbadawi, A., Gilani, S. & Jneid, H. Transcatheter aortic valve replacement for native aortic regurgitation: Are we there yet?. JACC Cardiovasc. Interv. 16(16), 1961–1964. https://doi.org/10.1016/j.jcin.2023.07.018 (2023).
El-Gamel, A. Transcatheter aortic valve replacement in pure native aortic valve regurgitation: Challenging pathology awaiting specialized devices. Aorta (Stamford). 9(2), 56–59. https://doi.org/10.1055/s-0041-1725122 (2021).
Baumbach, A. et al. A heart valve dedicated for aortic regurgitation: Review of technology and early clinical experience with the transfemoral Trilogy system. Catheter. Cardiovasc. Interv. 102(4), 766–771. https://doi.org/10.1002/ccd.30795 (2023).
Arias, E. A., Bhan, A., Lim, Z. Y. & Mullen, M. TAVI for pure native aortic regurgitation: Are we there yet?. Interv. Cardiol. Rev. Res. Resour. 14(1), 26–30. https://doi.org/10.15420/icr.2018.37.1 (2019).
Yoon, S. H. et al. Transcatheter aortic valve replacement in pure native aortic valve regurgitation. J. Am. Coll. Cardiol. 70(22), 2752–2763 (2017).
Arai, T. et al. Direct comparison of feasibility and safety of transfemoral versus transaortic versus transapical transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 9(22), 2320–2325. https://doi.org/10.1016/j.jcin.2016.08.009 (2016).
Yin, W. H. et al. Outcomes of transcatheter aortic valve replacement for pure native aortic regurgitation with the use of newer- vs. early-generation devices. Ann. Transl. Med. 10(1), 24. https://doi.org/10.21037/atm-21-6936 (2022).
Werner, N. & Sinning, J. M. Aortic regurgitation after transcatheter aortic valve replacement—Nothing to worry about anymore?. Circ. J. 78(4), 811–818. https://doi.org/10.1253/circj.CJ-14-0113 (2014).
Vahl, T. P. et al. Transcatheter aortic valve implantation in patients with high-risk symptomatic native aortic regurgitation (ALIGN-AR): A prospective, multicentre, single-arm study. Lancet (London, England). 403(10435), 1451–1459. https://doi.org/10.1016/s0140-6736(23)02806-4 (2024).
Willson, A. B. et al. Computed tomography-based sizing recommendations for transcatheter aortic valve replacement with balloon-expandable valves: Comparison with transesophageal echocardiography and rationale for implementation in a prospective trial. J. Cardiovasc. Comput. Tomogr. 6(6), 406–414. https://doi.org/10.1016/j.jcct.2012.10.002 (2012).
Takagi, H., Umemoto, T. & Grp, A. Impact of paravalvular aortic regurgitation after transcatheter aortic valve implantation on survival. Int. J. Cardiol. 221, 46–51. https://doi.org/10.1016/j.ijcard.2016.07.006 (2016).
Moreno-Samos, J. C. & Cruz-Gonzalez, I. Paravalvular leak after transcatheter aortic valve replacement: Smoke on the horizon?. JACC Case Rep. 1(5), 703–704. https://doi.org/10.1016/j.jaccas.2019.10.012 (2019).
Siontis, G. C. et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J. Am. Coll. Cardiol. 64(2), 129–140. https://doi.org/10.1016/j.jacc.2014.04.033 (2014).
El-Sabawi, B. et al. Temporal Incidence and predictors of high-grade atrioventricular block after transcatheter aortic valve replacement. J. Am. Heart Assoc. 10(10), e020033. https://doi.org/10.1161/JAHA.120.020033 (2021).
Lenders, G. D. et al. Depth of valve implantation, conduction disturbances and pacemaker implantation with CoreValve and CoreValve Accutrak system for transcatheter aortic valve implantation, a multi-center study. Int. J. Cardiol. 176(3), 771–775. https://doi.org/10.1016/j.ijcard.2014.07.092 (2014).
Pagnesi, M. et al. Incidence, predictors, and prognostic impact of new permanent pacemaker implantation after TAVR with self-expanding valves. JACC Cardiovasc. Interv. 16(16), 2004–2017. https://doi.org/10.1016/j.jcin.2023.05.020 (2023).
Prendergast, B. & Vahanian, A. The 2021 ESC/EACTS guidelines for the management of valvular heart disease: A new template for Heart Teams and their patients. Cardiovasc. Res. 118(1), E11–E13. https://doi.org/10.1093/cvr/cvab362 (2022).
Inanc, I. H. et al. Comparison of American and European guidelines for the management of patients with valvular heart disease. Cardiovasc. Revasc. Med. 47, 76–85. https://doi.org/10.1016/j.carrev.2022.10.005 (2023).
Acknowledgements
We express our sincere thanks to the volunteers who participated in the study.
Funding
This work was supported by the Jiangxi Provincial Health Commission Science and Technology Innovation Key Project (2024ZD007).
Author information
Authors and Affiliations
Contributions
Jie Feng & Haixia Wei: Data curation, Investigation, Methodology, Writing; Shiyuan Zhang: Interpretation of imaging data; Yao Li & Hangyu Liu: Data analysis, graphing; Yanqing Wu: Funding acquisition, Project administration, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All participants provided informed consent, and all procedures and protocols were approved by the Clinical Research Ethics Committee of the Second Affiliated Hospital of Nanchang University (approval 2022.No.07) and implemented in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feng, J., Wei, H., Zhang, S. et al. Efficacy and safety of transfemoral TAVR in pure aortic regurgitation patients: a single center study. Sci Rep 15, 17951 (2025). https://doi.org/10.1038/s41598-025-03214-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03214-1