Abstract
Bone healing after internal fixation of intertrochanteric hip fractures is difficult to monitor with radiography, particularly with sliding hip screws (SHS). In this proof-of-concept study, we develop an ovine hip fracture model and use it to evaluate a novel sliding hip screw system with a sensor that can non-invasively detect bending of the screw implant during healing. An existing SHS implant was modified and implanted in a Suffolk Ewe sheep. A straight, radiodense 1 mm tungsten rod was attached to the screw in the cannula to enable quantification of screw bending by radiographically observing relative position of the rod compared to the screw tip. After surgery, weekly loaded and unloaded radiographs were taken of the femur. Once fracture stabilization had been indicated for at least three weeks, the femur was extracted and potted and radiographs were taken under simulated axial loading from 5 to 400 N. By week 3, full in vivo weight bearing over the fractured femur was achieved. In response, implant loading was clearly observed with the novel sensor. This observed implant loading continued until week 8, wherein it ceased entirely. Upon extraction, the femur showed a fully developed and very stable callus surrounding the fracture site. Subsequent mechanical tests confirmed no measurable implant loading under axial loads of up to 400 N. This test indicates that the proposed ovine model produces sufficient force over an SHS implant in an induced fracture. This allowed our sensor to indicate fracture healing through radiography in load scenarios greater than 25 kg.
Similar content being viewed by others
Introduction
Intertrochanteric (IT) hip fractures are a common fracture type among elderly patients, with over 330,000 occurrences annually in the United States alone1. The sliding hip screw (SHS) implant has been considered a standard treatment for such fractures since its implementation in the early 20th century, but postoperative implant failure can occur if fracture non-union is not identified within a timely manner2,3,4,5.
Previous benchtop and cadaveric tests of an X-ray visualized implanted sensor for bending of sensing SHS (X-VIS-SHS) have shown that when under axial load similar to weight bearing, an unstable fracture will not stress-shield a X-VIS-SHS implant and cause the lag screw to undergo measurable bending6,7. Conversely, a fully healed fracture callus will stress shield a X-VIS-SHS implant, showing no indicator movement. Further testing of such an approach necessitates an in-vivo animal model, but due to the longstanding clinical history of the SHS implant as a treatment for IT fractures, there is a lack of literature concerning SHS-based in-vivo animal models. Therefore, to further explore the uses for implant bending sensing in IT fracture treatment, we must first select a viable in-vivo animal model.
Ovine animal models have been used for various orthopaedic fracture studies, often due to the hardy nature of sheep regarding fracture healing and similar anatomical structure8,9,10,11. Specific for our application, an ovine femur shares many similar anatomical features with a human femur, such as distinguishable greater and lesser trochanters and a pronounced femoral head and neck. These features enhance the clinical efficacy of using a sliding hip screw to treat an ovine intertrochanteric fracture, which would otherwise not be possible in a smaller animal model. Provided that the animal is large enough, sufficient hip joint loading can be generated either via body weight or external loading to induce measurable levels of lag screw bending in the implant with research showing that sheep often exert about half the force of a walking human across their hips. In summary, the sheep could be a suitable animal model for intertrochanteric fracture fixation studies due to its extensive use in the literature for similar applications, comparable anatomy, and the similar bone metabolism. This proof-of-concept study introduces an ovine model for the evaluation of IT fractures with the SHS implant and determines if this model is appropriate for further exploring the relationships between implant bending and bone healing.
Materials and methods
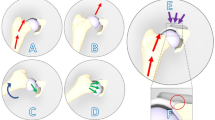
The X-VIS-SHS was recently developed and tested in biomechanically equivalent Sawbones® and human cadavers to determine if fracture movement under loading could be identified through plain radiograph. The X-VIS-SHS design consists of a typical screw housing a rigid, radio-dense tungsten rod as a highly visible indicator (Fig. 1). As the screw deforms under load, the end of the indicator rod will move relative to the end of the lag screw. This motion can be seen under plain radiography and enables clinicians to directly compare loaded and unloaded IT fracture radiographs and determine fracture stability based on indicator movement. As the fracture heals, the callus increasingly shares load and the implant bends less. Our group has previously developed X-ray visualized sensors for plate bending12,13, synovial fluid pH14,15 and CO216, and optical sensors to measure screw tension17,18. Herein, the SHS screw was applied to the ovine femur and thus the length and diameter of the SHS were both reduced to allow for equivalent rod deflection given the in-vivo models reduced axial load and shorter femoral neck, while also considering the ultimate strength of the implant.
(A) Graphical rendering of a Narang sliding hip screw with sensor under a modeled loading cap from a load testing machine (B) Graphical rendering of a radiograph of the sliding hip screw with sensor (C) Comparison of sensor position within lag screw at low (i) and high (ii) loads. (Images produced by the authors using SolidWorks Version 2020, https://mysolidworks.com)
Suffolk sheep (Ovis aries) are a British breed of domestic sheep known for their large frame, hardiness, and mild temperament. The average adult Suffolk ewe stands at a height of 74 cm, weighs about 88 kg, and is primarily raised for its meat. Due to these traits, the Suffolk ewe is one of the most abundant breeds of sheep worldwide and are commonly available for research purposes.
One Suffolk Ewe was acquired from the Clemson University Animal Research Farm. The animal was housed at Clemson University’s Godley-Snell Research Center and maintained in accordance with the Guide for the Care and Use of Laboratory Animals. The facility is accredited by AAALAC International, and animal use was humanely conducted and approved by the Clemson University’s Institutional Animal Care and Use Committee. All methods were performed in accordance with the relevant guidelines and regulations. The experiment also complied with the ARRIVE guidelines. The sheep underwent a complete physical examination including a radiographic assessment to ensure the femur can accommodate the hip screw. The sheep was acclimated for 10 days during which they were trained on the measurement technique (standing radiographs taken on scale or pressure mat). The sheep was weighed on the day of surgery to establish baseline measurements and once per week thereafter. The sheep was monitored for normal activity, food and water consumption, and fecal and urine output.
Prior to surgery, the following drugs were administered: Atropine (0.2 mg/kg SC), acepromazine (0.5 mg/kg SC), flunixin (2 mg/kg, IV q24), buprenorphine (0.005 mg/kg SC, q12 hr). Anesthesia was induced via IM injection of 40 mg/kg ketamine and maintained with 1–4% isoflurane during surgery.
A classical Watson-Jones (anterolateral) approach to the right hip was followed involving incision over the lateral aspect of the femur. Dissection was carried down to the greater trochanter and the hip joint capsule opened to expose the femoral neck. The greater trochanter was severed with a chisel to create a model fracture (medium neck type, Garden III type, fracture gap 0.5 mm). The femur was implanted with a 135° sliding hip screw plate and modified lag screw (Narang Medical Ltd.) with integrated sensor. The incision was closed in routine fashion with sutures and/or staples and glued.
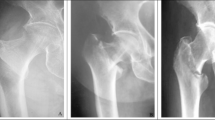
Post-surgery, standing radiographic images were taken weekly for 10 weeks. Each weekly session consisted of taking radiographs of low and high load states over the implanted leg with load bearing recorded for each image. Image analysis was conducted through ImageJ to quantify any sensor movement between the low and high load images from each session. Radiographs were taken using a POSKOM Vet-20BT portable radiographic imaging system. If the animal was hesitant to bear weight and cocked the index leg, a staff member lifted the contralateral leg. If necessary, the animal was imaged under light anesthesia via subcutaneous administration of Buprenorphine. After 10 weeks, the sheep was euthanized with 0.025 mg/kg Xylazine followed by 20 ml of Vet One Euthanasia solution and a terminal blood sample obtained up to 10 ml. The blood was analyzed for inflammatory markers (e.g., erythrocyte sedimentation rate ESR, C-reactive protein CRP, and leukocyte count) in the unlikely event of an infection and to provide a baseline for possible future studies on infection18. A period of 10 weeks was selected because prior research indicates that the bone has significantly healed by that time and bone mineral density has essentially normalized19. However, an extension to 12 or 14 weeks would have been allowed if the bone is not deemed to be sufficiently healed. Figure 2A shows the surgery; Fig. 2B shows a photo of the procedure for imaging with the sheep applying weight on a scale during radiography for loaded condition or minimal weight for unloaded condition; Fig. 2C shows a typical radiograph of the hip, implant, and indicating pin in the implant.
(A) Sheep in surgery at the Godley-Snell Research Center. (B) Post-surgical weight monitoring and testing, note a veterinary staff member held the sheep during radiography, digitally removed for anonymity per journal guidelines. Note also, the X-ray was taken with a diagonal view through the hip. (C) Radiograph of a modified sliding hip screw implant in a sheep one week after surgery.
After sacrifice, the fractured femur was extracted, and a CT scan was taken of the femur to document the position of the implant and fracture callus development. The femur was potted in liquid Fast-Cast cement and secured via vice grip under an ESM303 axial load testing machine. The femur was axially loaded at 5 N and 400 N with radiographs being taken at each load. As previously conducted during the in vivo portion of this study, image analysis was conducted through ImageJ to quantify any sensor movement.
Results
Once the sheep began bearing significant loads over the fracture leg at Week 2, n = 32 measurable radiographs were taken across the 8 subsequent weeks until sacrifice (2–5 each week). The weight that the sheep applied to the scale was noted with the radiograph number, and the indicator rod position was measured and compared to the applied load. At week 2, significant sensor rod movement was observed at 29 kg, and this continued until week 8. At week 8, a noticeable decrease in sensor movement was observed at all loads, and after further observation at weeks 9 and 10, the sheep was sacrificed. Figure 3 shows example radiographs for loaded and unloaded conditions at Week 4 when most displacement was apparent and Week 8 when motion had essentially stopped. The quality of the radiograph was imperfect because the animal could move, the source and detector were hand-held, and no antiscatter grids could be used due to variable positioning of source and detector. However, the indicator rod position was apparent in the canulation and the addition of boxes in Image J allowed for the measurement of rod position.
Results
Figure 4 shows the slope of the load-displacement curve (mm/kg) versus week number. It was evident that there was significant fracture healing and increase in load sharing by Week 8. These trends were further verified via F-tests for sample variance and the appropriate T-tests, which demonstrated a significant difference between the loaded and unloaded measurements within the week 1–7 period and a lack of significant difference between the loaded and unloaded measurements within the week 8–10 period. The error bars show the uncertainty in slope at each time point (no error bars were given if only two points were measured). The average stiffness uncertainty was 0.003 ± 0.001 mm/kg, and the y-axis (indicator displacement) residual off the regression curve for pooled data from weeks 1–6 was 70 μm. This corresponds to about 1 pixel at magnifications used and is in the range of uncertainties we have seen for similar X-ray visualized displacement sensors (e.g., 60 μm in cadaveric femur screw bending model6,7, 50–80 μm in a fluidic level tibial-plate bending sensor12, and 80 μm in an implanted peritoneal fluid hydrogel-actuated pH sensor in a rat model14). Figure S1 and Table S1 in Electronic Supporting Information show a representative selection of radiographs and sensor displacement measurements.
The animal was sacrificed at week 10, after three weeks of radiographs showed negligible motion under load, and the femur specimen was harvested to confirm whether the lack of in vivo motion corresponded to fracture healing. Inspection of the femur revealed that a fracture callus had fully formed and solidified around the fracture site. No other anomalies were noted in the necropsy. CT scans at Greenville Memorial Medical Center confirmed that a fracture callus had fully developed and that the sensor and implant were properly aligned within the femur.
With confirmation that a fracture callus had indeed fully formed, the extracted femur was taken back to GSRC for axial testing. The femur was potted in Fast-Cast cement and subjected to axial loaded as outlined previously. At 400 N of load, the sensor rod did not show detectable movement using ImageJ analysis (Fig. 5).
Discussion
Though sheep are sometimes uncooperative to physical handling and completion of tasks, it was possible to acquire 32 clear radiographs of various load states throughout the ten-week healing process (see Figure S1 in Supporting Information). Though there was little fine control over load application, it was still possible to identify precisely when the fracture callus had stabilized and hardened through sensor readings alone. In an arguably unfavorable environment, the sensor provided statistically significant readings that were confirmed through more traditional mechanical tests. Sensor movement through the first 8 weeks indicates that the sheep femur healed at that time and was confirmed by verifying fracture stability.
The mechanics of a traditional SHS implant are not the subject of most modern animal models, but this application could be useful for testing innovative approaches to IT fracture care. Jukkala-Partio et. al’s test of a poly-L-lactide screw in a sheep IT model indicates that innovative implant design testing may have use for a strain sensor when implant strain may be a concern20.
While an ovine model has many merits as an in-vivo candidate, it is not without challenges. The femoral anatomy of the sheep necessitated shortening of the lag screw and plate design. To compensate for the increased stiffness of a shorter lag screw, the screw outer diameter was decreased from 7.8 to 6.0 mm and the barrel length was cut from 38 to 20 mm so that bending could be measured, and the construct retained an acceptable ultimate strength.
In addition, the sheep itself was far from an ideal clinical patient. Its four-legged nature made a traditional AP radiograph impossible instead prompting a diagonal approach and viewing angle through the side of the hip in order to see the implant. The sheep was occasionally uncooperative making it difficult to capture clear radiographic footage for fine-level measurement. Due to these factors, there was often little to no control over the force application across the femur. There was some level of “low,” “medium,” and “high” control when it came to encouraging weight bearing, but there was no guaranteed method for achieving a consistent threshold of measurement on a given session. Even when higher levels of weight bearing could be achieved, the maximum amount of measured weight over the fracture leg (> 25 kg) is roughly equal to a quarter of the maximum force from previous tests5,6.
While this proof-of-concept study shows promising results towards development of a further in vivo model, considerations must be made regarding measurement repeatability. The current methodology of manually defining sensor position relative to the screw wall allows for potential observer bias. This highlights the opportunity for future exploration toward a robust and impartial measurement system, potentially an algorithm-based analytic tool capable of repeatedly measuring sensor position relative to defined markers along the screw. The precision could also be improved with better X-ray techniques (e.g., fixed source and detector as well as antiscatter grids). Additionally, the device can be modified to show motion more clearly by using a mechanical gain mechanism in the screw to amplify the relatively small displacements observed during screw bending into large displacements of the indicator gauge (e.g., we reported a fluidic gain mechanism to observe displacements in plate bending12, and a wedge to amplify screw extension during tightening in an optical screw tightening sensor17).
This study indicates that the proposed ovine model produces sufficient force over an SHS implant in an induced intertrochanteric fracture. As such, this allowed a prototype sensor to indicate fracture healing through radiography in load scenarios greater than 25 kg across the fracture. Low-load radiographs established a consistent baseline for diagnostic measurements, and high-load radiographs offered a real-time insight into fracture stability. Despite limitations in size and animal behavior, this ovine model offers the potential to further explore the in-vivo mechanical behavior of hip fracture fixation implants.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Johnell, O. & Kanis, J. A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 17, 1726–1733 (2006).
Colón-Emeric, C. S. Postoperative management of hip fractures: interventions associated with improved outcomes. Bonekey Rep. https://doi.org/10.1038/bonekey.2012.241 (2012).
Babcock, S. & Kellam, J. F. Hip fracture nonunions: diagnosis, treatment, and special considerations in elderly patients. Adv. Orthop. https://doi.org/10.1155/2018/1912762 (2018).
Whittaker, D. E., McMillan, T. E. & Stevenson, I. M. Management of surgical complications in fracture fixation of the hip. Orthop. Trauma. 34(2), 73–79. https://doi.org/10.1016/j.mporth.2020.01.003 (2020).
Kates, S. L., Behrend, C., Mendelson, D. A., Cram, P. & Friedman, S. M. Hospital readmission after hip fracture. Arch Orthop. Trauma. Surg. 135(3), 329–337. https://doi.org/10.1007/s00402-014-2141-2 (2015). PMID: 25550095.
Carrington, N. T. et al. A novel load-sensing sliding hip screw to aid in the assessment of intertrochanteric fracture healing. J. Biomech. 179, https://doi.org/10.1016/j.jbiomech.2024.112481 (2025).
Carrington, N. T. Design and development of a strain-sensing sliding hip screw for the treatment of intertrochanteric hip fracture, Clemson University PhD dissertation, (2020).
Pearce, A. I., Richards, R. G., Milz, S., Schneider, E. & Pearce, S. G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 13, 1–10. https://doi.org/10.22203/eCM.v013a01 (2007).
Nunamaker, D. M. Experimental models of fracture repair. Clin. Orthop. Relat. Res. 1(3558), 856–865. https://doi.org/10.1097/00003086-199810001-00007 (1998).
Fountain, S. et al. Monitoring healing progression and characterizing the mechanical environment in preclinical models for bone tissue engineering. Tissue Eng. - Part. B. 22(1), 47–57. https://doi.org/10.1089/ten.teb.2015.0123 (2016).
Reichert, J. C. et al. The challenge of Establishing preclinical models for segmental bone defect research. Biomaterials 30(12), 2149–2163. https://doi.org/10.1016/j.biomaterials.2008.12.050 (2009).
Rajamanthrilage, A. C. et al. A method to track fracture healing: measuring tibial plate bending strain/bending under load with an attached fluidic sensor read via plain radiography with an integrated fluidic sensor. IEEE Trans. Biomed. Eng. https://doi.org/10.1109/TBME.2021.3092291 (2021).
Pelham, H. et al. Implantable strain sensor to monitor fracture healing with standard radiography. Nat. Sci. Rep. 7, 1489 (2017).
Kiridena, S. D. et al. X-Ray visualized sensors for peritoneal Dialysis catheter infection. Adv. Funct. Mater. 2204899 https://doi.org/10.1002/adfm.202204899 (2023).
Wijayaratna, U. N., Kiridena, S. D., Adams, J. D., Behrend, C. J. & Anker, J. N. Synovial fluid pH sensor for early detection of prosthetic hip infections. Adv. Funct. Mater. 2104124 https://doi.org/10.1002/adfm.202104124 (2021).
Kiridena, S. D., Wijayaratna, U. N., Wang, R. & Anker, J. N. X-Ray visualized synovial fluid carbon dioxide sensor for the early detection of prosthetic hip infections. Adv. Sens. Res. 2(11), https://doi.org/10.1002/adsr.202300001 (2023).
Ravikumar, N. et al. Development of an Optically-based Tension-Indicating Implanted Orthopedic Screw with a Luminescent Spectral Ruler, SPIE Proceedings 100810 N (2017).
Beagan, M. L. C. et al. : the potential of sheep in preclinical models for bone infection research – A systematic review. J. Orthop. Translation. 45, 120–131 (2024). ISSN 2214-031X.
Suckey, M. M., Benza, D. W., Arifuzzaman, M. & Millhouse, P. W. Luminescent spectral rulers for noninvasive displacement measurement through tissue. ACS Sens. 5, 711–718 (2020). Heath J.DesJardins J.D., and Anker J.N.
Jukkala-Partio, K. et al. Healing of subcapital femoral osteotomies fixed with self-reinforced poly-L-lactide screws: an experimental long-term study in sheep. Arch. Orthop. Trauma. Surg. 122(6), 360–364 (2002).
Acknowledgements
We acknowledge Josh Finkel for contributions to the manuscript.
Author information
Authors and Affiliations
Contributions
Nathan T. Carrington contributed to the methodology and investigation of this project in addition to the writing and visualization of the original paper. Savannah R. Forrester contributed to the writing review and editing of this paper. Josh Finkel assisted with visualization. Caleb J. Behrend assisted with the conceptualization of this project, and Paul W. Milhouse, Tom B. Pace, and Kyle Jeray contributed to the methodology and investigation of this project. Jeffrey N. Anker and John D. DesJardins contributed project conceptualization, administration, and supervision. They also acquired funding to support the project and contributed to the writing review and editing of this paper. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
CJB, JNA, and JDD are all co-inventors and cofounders of Aravis Biotech LLC, which has an exclusive license to related US patent 10667745B2, “Radiographic discernable sensors and orthopedic applications for same.” This work was financially supported by NIH NIGMS through grant NIH NIGMS 5P20GM103444 and NIH NIAMS through grant R43AR081748. NTC, SRF, PWM, TBP, and KJ do not have any competing interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Carrington, N.T., Forrester, S.R., Milhouse, P.W. et al. Proof of concept measurement of ovine intertrochanteric fracture stability with an X-ray visualized implantable screw bending sensor. Sci Rep 15, 19725 (2025). https://doi.org/10.1038/s41598-025-03216-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03216-z