Abstract
Colonization with extended-spectrum cephalosporin-resistant Enterobacterales (ESCrE) in communities may contribute to proliferation of resistance genes and drug-resistant community and hospital infections. Previous work in the Western Highlands of Guatemala found that approximately 46% of the population is colonized with these bacteria, setting the stage to identify factors that are associated with increased odds of ESCrE colonization. Stool samples and questionnaire data were collected from randomly selected participants in the catchment area of the third largest tertiary hospital in Guatemala. Logistic regression path analysis was used for this cross-sectional study to identify potential direct and indirect risk factors for colonization with ESCrE. Participants (N = 951) had a higher odds of ESCrE colonization if they had exposure to a healthcare facility within 30 days of enrollment (OR: 2.12, 95% CI = 1.19–3.77), if they resided in urban areas (OR: 1.93, 95% CI 1.09–3.42), if they did not have a service to remove household trash (OR: 1.99, 95% CI 1.11–3.58), and if the household reported drinking water from non-bottled sources (OR:1.53, 95% CI 1.0–2.33). Antibiotic self-medication was not significantly associated with the risk of colonization (OR: 1.16, 95% CI 0.65–2.06). Multiple transmission-related factors were associated with increased likelihood of ESCrE colonization, but the cross-sectional nature of this study does not distinguish factors that are correlated with an individual’s risk for colonization whence exposed. Assessing risk factors associated with colonization with antibiotic resistant bacteria may be useful for identifying mitigation strategies and evaluating the effectiveness of interventions against antibiotic resistance in community settings.
Similar content being viewed by others
Introduction
Antibiotic resistance poses a significant global public health threat1, and antibiotic use is an important cause for the emergence and proliferation of antibiotic-resistant bacteria2,3. At the community level, antibiotic use has been identified as a risk factor for colonization with antibiotic-resistant bacteria4, but it has long been recognized that other social and ecological factors may contribute to this problem5. For example, several studies from low- and middle-income countries found that antibiotic use per se is not predictive of colonization with antibiotic-resistant bacteria6,7, or it is predictive only when there is an interaction effect with sanitation and hygiene8. Several recent country-level papers also failed to consistently link the prevalence of such bacteria with antibiotic use, but instead found associations with limited sanitation, hygiene, governance, and public health investments9,10,11.
Colonization with antibiotic resistant bacteria is problematic for both individuals and the larger community. Individuals who are colonized with antibiotic resistant bacteria are at heightened risk for developing resistant infections12. They may also serve as reservoirs for these organisms, posing a risk for onward transmission to others, whether in the community or while seeking healthcare services13. Thus, it is important to understand the factors associated with community colonization to help identify strategies to reduce colonization and prevent the bi-directional spread of antibiotic resistance across the community-healthcare divide. Additionally, colonization could be used to assess effectiveness of community public health interventions, such as sanitation and hygiene interventions, at mitigating the spread of antibiotic resistant bacteria14.
Antibiotic Resistance in Communities and Hospitals (ARCH) studies have been initiated in six countries worldwide to generate estimates for the prevalence of colonization with ESCrE as well as carbapenem-resistant Enterobacterales in communities and associated hospitals15. ARCH studies found relatively high rates of ESCrE colonization in study communities from Kenya (34–52%)16, Guatemala (46%)17, Chile (29%)18, India (72%)19, Botswana (24–26%)20, and Bangladesh (78%)19. The current study considers factors that may be associated with ESCrE colonization from the ARCH study community located in the Western Highlands of Guatemala.
Methods
Study location
Details about methods used in ARCH studies and for Guatemala specifically are provided elsewhere15,17. Briefly, we carried out a cross-sectional study in the catchment area of the Quetzaltenango regional hospital in the Western Highlands of Guatemala. The study area was delimited by estimating 80% of the eligible case-patients from the facility-based surveillance platform for respiratory, diarrheal, and febrile illness, encompassing 16 municipalities in the region21. The catchment area was comprised of communities where 53% of the population is female, 51% and 49% of the population is Maya or mestizo ethnicities, respectively, and literacy rates for community members 15 years old is 84%22. Urban locations were defined per Guatemalan census guidelines as cities, towns, villages, or neighborhoods with > 2,000 inhabitants, provided that 51% or more of households have electricity and piped water 22. Rural locations included all areas not meeting the urban definition.
Community sampling and participant selection
Community sampling and enrollment was divided into three stages by partitioning the study site into geographic clusters based on population density, verifying presence of households by using satellite imagery and ground truthing, and completing a census of household members17. Selection of geographic clusters, households within the clusters, and participants within the households was random. Sample collection was restricted to one individual per household. Individuals were excluded if they had been living in the household < 6 months, or if they presented with fever, diarrhea, or cough at the time of interview or specimen collection. Those undergoing quarantine or isolation due to COVID-19 exposure or infection were also excluded as were households with members who tested positive for SARS-CoV-2 in the ten days prior to recruitment. After obtaining written consent, a structured questionnaire was used to collect information on demographics, possession of household assets, hygiene and sanitation conditions within the household, history of household participant’s antibiotic use, and clinic and hospital visits.
Sample collection and processing
Stool samples were collected in stool cups, transported to a local laboratory, and streaked onto CHROMagar™ ESBL as well as MacConkey agar (Hardy Diagnostics, CA) as a positive control for gram-negative bacteria, followed by overnight incubation at 37 °C. Every batch of CHROMagar™ media was tested with positive control strains (ATCC BAA-2469 and ATCC 700603) and negative control strains (ATCC 29212 and ATCC 25922). Up to three morphologically distinct colonies were recovered from chromogenic plates and later identified to species and characterized for antibiotic susceptibility using a VITEK® 2 instrument. Individuals were classified as colonized with ESCrE if at least one recovered isolate was resistant to ceftriaxone while being susceptible or intermediate to three carbapenems included in the assay (ertapenem, imipenem, and meropenem).17,23.
Statistical methods
Logistic regression path analysis was used to explore putative direct effects associated with the risk of bacterial colonization (Table 1) 17. This methodology is used to estimate hypothesized direct and indirect effects (odds ratios) on an outcome variable (ESCrE colonization) and can be depicted in a graphical format (Fig. 1). Direct effects are those that are expected to directly impact the likelihood of colonization, whereas indirect effects are hypothesized to operate through direct effects to influence the probability of colonization. For example, hospitalization may directly affect the probability of ESCrE colonization as a result of exposure to environments where antimicrobial resistant Gram-negative bacteria are more likely to be encountered. Therefore, activities such as vaccination might be associated with reduced visits to clinics and hospitals (Fig. 1) and would thus indirectly decrease risk of colonization with ESCrE. Some variables could have both a direct and indirect association, such as household sources of water (Fig. 1). Definitions of these variables and rationale for variable selection based on expert opinion can be found in Tables S1-S3.
Path model. Statistically significant relationships are shown with black lines and arrows, and nonsignificant relationships are shown in light gray. See Table 3 for odds ratios and 95% CI. Healthcare contact has both direct and indirect effects. Table S1 describes the rationale for the relationships tested in the model.
Prior to model development, the distribution of each variable (Table S4) was explored. Variables with sufficient variance (greater than 5% coefficient of variation) were included in model selection exercises supported by the step() function using backward selection to identify the model with the best fit (Wald statistic). Multicollinearity was documented for factors used in the models by using a variance inflation factor (VIF). We considered several antibiotic use variables, including antibiotic use for syndromic illnesses, and any antibiotic use including prescribed and self-medicated. Using the step() function, we determined that of the antibiotic use variables, antibiotic self-medication resulted in the largest Wald statistic, which is also consistent with previous descriptions that self-medication with antibiotics is relatively common in Guatemala25,26. Variables identified using the step() function were applied to construct the final path model.
We employed variable reduction strategies to reduce the dimensionality of the analysis, including construction of a composite variable through description of household assets (see Table 1 and Table S1). We adjusted for clustering due to our three-stage sampling design using the svyset command in Stata (ver. 1727). The post strata and post weight conditions in svyset were applied using the reference population from national census data to normalize weighted estimates according to distributions of age, sex, and urban/rural households and is described elsewhere22. Data cleaning was carried out with R28.
Ethical considerations
Written informed consent was obtained in Spanish or verbally translated into Mam for eligible adult (≥18 years) participants, and informed assent was obtained for children (7–17 years). Informed consent was obtained from guardians of participating children (≤17 years). The protocol was approved by the Universidad del Valle de Guatemala-Center for Health Studies Ethics committee and the Guatemalan Ministry of Health Ethics Committee (194–04-2019, 16–2019 respectively). All research was performed in accordance with the relevant guidelines and regulations of these institutions.
Results
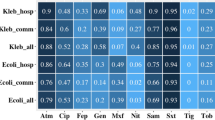
From a total of 919 enrolled participants, 915 individuals provided valid stool samples and did not have missing data for any dependent and outcome measures. Across the sample, the median age of adults was 41 years (range 18–93), and for children was 6 years (range 0–17). Sixty-eight percent of the population was female, and 61% indigenous, respectively, and 67% of the population was from rural households. The weighted prevalence of ESCrE colonization for community participants was published previously (46%17). Other descriptive statistics for dependent and independent variables can be found in Table 2 and are aggregated according to ESCrE colonization status.
Correlates with ESCrE colonization
Direct effects were specified between eight variables and the odds of ESCrE colonization was significantly correlated with four of these variables (Fig. 1 and Table 3). Participants visiting hospitals or clinics for an illness within 30 days of enrollment had two-fold increase in the odds of ESCrE colonization (OR: 2.12, 95% CI 1.19–3.77). A similar effect was documented for participants residing in urban areas with an increase in the odds of being colonized with ESCrE (OR: 1.93, 95% CI 1.09–3.42). Households that did not have a service to remove household trash exhibited a similar increased odds of ESCrE colonization (OR: 1.99, 95% CI 1.11–3.58), which was not mediated by urban/rural location. Finally, participants reporting that their household drinking water came from non-bottled sources, including piped water and from wells, had 0.53 increase in the odds of being colonized with ESCrE (OR: 1.53, 95% CI 1.00–2.33). All other independent variables, including age, self-administration of antibiotics, toilet facilities, and phase of data collection (i.e., pre/post COVID-19 lock downs) were not significantly associated with ESCrE colonization.
Significant indirect factors associated with ESCrE colonization included years of schooling and phase of data collection because of significant correlations with health-seeking practices. For every year increase in the number of years of schooling, the odds of a hospital or clinic visit increased by 1.06 (95% CI 1.00–1.13) and for participants enrolled after the pandemic, odds of clinic visits decreased by 0.55 (95% CI 0.32–1.27) (Fig. 1). No other hypothesized factors, including urban location, age, sex, household assets, non-bottled water sources, no garbage service and toilet status were significantly related to visiting hospitals or clinics and so were not indirectly associated with ESCrE colonization (Table 3).
Discussion
The prevalence of ESCrE colonization, most of which was E. coli, for presumptively healthy individuals residing in the western highlands of Guatemala (46%17) was similar to another ARCH site in Kenya (45–52%) 16,20 but higher than what was reported in the Botswana ARCH site (24–26%)20,29. Separate risk factor analyses found that contact with healthcare services was associated with an increased odds of ESCrE colonization in communities in Kenya (OR: 1.12, 95% CI 1.03 –1.21) and Botswana (OR: 1.37, 95% CI 1.08–1.73)29,30. For the current study, contact with healthcare during the previous 30 days was also associated with increased risk for colonization with ESCrE (OR: 2.12, P < 0.05). Another way to consider this finding is that 21.1% of the individuals who were colonized with ESCrE sought healthcare during the previous 30 days compared to 13.2% of the individuals who were not colonized with ESCrE (Table 2).
The cross-sectional nature of our study does not allow us to distinguish between correlation versus cause and effect, but one possible mechanism underlying the healthcare association is that exposure to a healthcare environment increases the risk of contact-dependent transmission of bacteria to patients. This might occur through contact with the healthcare environment (e.g., surfaces, devices, water, etc.) or through interaction with medical personnel31,32,33,34. Environmental exposure to factors increasing transmission risk may be reduced with appropriate infection prevention and control (IPC) and water, sanitation, and hygiene (WASH) programs, including water treatment 35,36.
While direct transmission from interactions with healthcare environments is a demonstrated mechanism for spreading resistant organisms, it is also possible that individuals seeking healthcare are predisposed to colonization with ESCrE. That is, underlying health issues responsible for the need to seek healthcare may be accompanied by physiological conditions that are favorable to the growth of ESCrE. For example, dysbiosis or disruption of the enteric microbiome due to inflammation, has been linked to colonization and overgrowth of enteric bacterial pathogens, potentially increasing the risk of colonization with bacteria like E. coli37, and earlier work showed that most ESCrE bacteria are E. coli in this community17. Chronic disease, malnutrition, autoimmune disease, chronic stress, or cancer may be contributing to healthcare seeking behavior, but may additionally result in conditions that are conducive to ESCrE colonization38. Additional work, including longitudinal studies, is needed to identify the causal mechanism underlying this association between ESCrE colonization and healthcare interaction.
Environmental transmission and WASH factors were significantly associated with ESCrE colonization, with households that did not have a garbage service and those using non-bottled water having higher odds of ESCrE colonization (OR: 1.99 and OR: 1.53, respectively). However, improved, improved-limited and non-improved toilets were not significantly associated with ESCrE colonization in this study. Additionally, exposure to animals, including chickens, and sources of food (including fruits, vegetables, and food-animal products) were not significantly related to colonization and consequently were not included in the model, although these were significant in Botswana and Kenya ARCH studies29,30. Hygiene-based factors that facilitate transmission of bacteria in communities have been reported in other community-based studies in the context of low- and middle-income countries. These include use of informal handwashing stations that are shared between households39, presence of backyard chickens, postulated exposure to animal feces through consumption-of or exploratory behavior-with soil40, living in crowded households, working in agricultural environments41, and household water sources shared with larger livestock herds and wildlife 6. The associations with WASH factors may not be as important in high-income countries and likely represents increased risk of bacterial transmission and is consistent with observations linking WASH variables to the occurrence of infectious disease42,43,44,45. Of particular interest in the context of the current study is that this association with WASH means that ESCrE colonization may serve as a measure of the efficacy of public health interventions to improve WASH. However, given mixed results from analyses of WASH intervention on health outcomes, more work is needed to understand what components of WASH are driving bacterial transmission and infectious disease within varying socioeconomic and geographic locations46,47,48,49.
The association that we found between urban environments and risk of colonization with ESCrE has been reported for other antibiotic-resistant bacteria5,50, including recent work using country-level data10,11,51. Urbanity, per se, is not a mechanism, but rather represents a set of interacting factors that could, for example, enhance the effects of poor sanitation and hygiene (e.g., higher population densities, access to healthcare facilities), while adding interacting socio-economic, migration, security, political, and governance issues6,52. The association between urban environments and antibiotic resistance suggests that community-level interventions targeting urban environments may require different intervention strategies compared with rural environments.
For the current study, non-bottled water was correlated with ESCrE colonization. Biofilms in water pipes encase E. coli populations and likely provide a source of these bacteria for transmission to and colonization of people53,54. Handling of water when it is not properly stored may result in contamination of water sources55. And while over half of the households in the main ARCH study reported that they treated drinking water, fewer than 0.5% of households used chlorine for treatment, leaving no residual protection from recontamination with hands or other hazards if water was stored in the home (Table S2). We did not collect information about storage practices, but if bottled water involved formal or informal commercial suppliers providing 5-gallon jugs with self-contained dispensers with taps, they may inadvertently represent a safer form of stored water than water from other sources that is typically stored in open containers that are exposed to the environment, animals, and household members55. The relationship between ESCrE colonization and water merits further exploration, and it will be important to identify storage and treatment practices to understand the potential impact of source versus household-level water contamination on human colonization with ESCrE and other bacteria.
Self-medication with antibiotics was not predictive of ESCrE colonization, nor did it predict hospital or clinic visits, the former of which is consistent with similar community-based studies conducted outside of Guatemala 6,39. In Guatemala, tetracycline and amoxicillin are the primary antibiotics available in communities 25,56, with the former having the potential to co-select ESCrE strains if they are tetracycline resistant, and the latter potentially selecting directly for ESCrE strains in the event that sufficient antibiotic reaches these bacteria in the lower gastrointestinal tract57,58. Nevertheless, prior work in the same study population showed that a threefold reduction in antibiotic self-administration during the COVID-19 pandemic resulted in no significant change in the community prevalence of ESCrE bacteria (45% vs. 47%, respectively)17. Consequently, the lack of relationship between self-medication with antibiotics and ESCrE colonization can probably be expected in the context of our study community. Importantly, some of the study population may have been taking prescribed antibiotics or other medications that exert selective pressures that favor ESCrE, but these associations did not lead to a parsimonious model.
There are several limitations to this study. One is the uncertainty about inferring causality from statistical associations (see discussion above). Secondly, while the odds ratios in our model are generally large, the confidence intervals are also large, consistent with the need for a larger sample size to increase confidence for our point estimates, and to increase the number of factors that could be included in the model. For example, age was not a significant direct or indirect factor, but we surmise that potential transmission dynamics will be quite different between adults and children and so may require different intervention strategies. Future work would benefit from both a larger sample size and a longitudinal or repeated measures study design to address these shortcomings. The strengths of this study included the use of path modeling that allowed simultaneous consideration of multiple dependent and independent variables in a single model, which is particularly useful when assessing hypothesized causal pathways (e.g., Fig. 1). This analysis was further improved because of the rigorous randomization process used to select participants (reducing potential bias), and incorporation of the sampling design into the analysis (via svyset), which improves the accuracy of standard error estimates and thus increases the robustness of the analysis.
Conclusions
The risk of colonization with ESCrE organisms in the community is associated with several variables that are distinct from hospital risk factors, although at a broader level the potential for transmission via environmental sources and compromised WASH undoubtedly apply in both contexts, particularly in low- and middle-income countries. And while more work is needed to ascertain the causal relationships underlying transmission-related associations identified herein, our findings suggest that monitoring prevalence of ESCrE colonization could serve as an effective measure for evaluating the efficacy of community-level public health interventions aimed at these potential mechanisms of transmission.
Data availability
Materials described in this manuscript, including all relevant raw data, will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality. Data requests can be sent by email to the corresponding author, Brooke M. Ramay.
References
Ikuta, K. S. et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet 400, 2221–2248 (2022).
MacLean, R. C. & San Millan, A. The evolution of antibiotic resistance. Science 365, 1082–1083 (2019).
Bottery, M. J., Pitchford, J. W. & Friman, V.-P. Ecology and evolution of antimicrobial resistance in bacterial communities. ISME J. 15, 939–948 (2021).
Bruinsma, N., Stobberingh, E., De Smet, P. & Van Den Bogaard, A. Antibiotic use and the prevalence of antibiotic resistance in bacteria from healthy volunteers in the Dutch community. Infection 31, 9–14 (2003).
Barbosa, T. M. & Levy, S. B. The impact of antibiotic use on resistance development and persistence. Drug Resist. Updates 3, 303–311 (2000).
Caudell, M. A. et al. Identification of risk factors associated with carriage of resistant Escherichia coli in three culturally diverse ethnic groups in Tanzania: A biological and socioeconomic analysis. Lancet Planet. Health 2, e489–e497 (2018).
Omulo, S. et al. The impact of fecal sample processing on prevalence estimates for antibiotic-resistant Escherichia coli. J. Microbiol. Methods 136, 71–77 (2017).
Ramay, B. M. et al. Antibiotic use and hygiene interact to influence the distribution of antimicrobial-resistant bacteria in low-income communities in Guatemala. Sci. Rep. 10, 13767 (2020).
Collignon, P., Beggs, J. J., Walsh, T. R., Gandra, S. & Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2, e398–e405 (2018).
Allel, K. et al. Global antimicrobial-resistance drivers: An ecological country-level study at the human–animal interface. Lancet Planet. Health 7, e291–e303 (2023).
The Global Sewage Surveillance project consortium et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 10, 1124 (2019).
Macareño-Castro, J., Solano-Salazar, A., Dong, L. T., Mohiuddin, M. & Espinoza, J. L. Fecal microbiota transplantation for Carbapenem-Resistant Enterobacteriaceae: A systematic review. J. Infect. 84, 749–759 (2022).
Fernández-Martínez, N. F. et al. Risk factors for multidrug-resistant gram-negative bacteria carriage upon admission to the intensive care unit. IJERPH 19, 1039 (2022).
Smith, R. M. et al. Human colonization with multidrug-resistant organisms: Getting to the bottom of antibiotic resistance. Open Forum Infect. Dis. 8, ofab531 (2021).
Sharma, A. et al. Multi-country cross-sectional study of colonization with multidrug-resistant organisms: protocol and methods for the Antibiotic Resistance in Communities and Hospitals (ARCH) studies. BMC Public Health 21, 1412 (2021).
Ita, T. et al. Prevalence of colonization with multidrug-resistant bacteria in Kenya. Sci. Rep. 12, 22290 (2022).
Ramay, B. M. et al. Colonization with antibiotic-resistant bacteria in a hospital and associated communities in Guatemala: An antibiotic resistance in communities and hospitals (ARCH) Study. Clin. Infect. Dis. 77, S82–S88 (2023).
Araos, R. et al. High burden of intestinal colonization with antimicrobial-resistant bacteria in Chile: An antibiotic resistance in communities and hospitals (ARCH) study. Clin. Infect. Dis. 77, S75–S81 (2023).
Styczynski, A., Herzig, C., Luvsansharav, U.-O., McDonald, L. C. & Smith, R. M. Using colonization to understand the burden of antimicrobial resistance across low- and middle-income countries. Clin. Infect. Dis. 77, S70–S74 (2023).
Mannathoko, N. et al. Colonization with extended-spectrum cephalosporin-resistant Enterobacterales (ESCrE) and carbapenem-resistant Enterobacterales (CRE) in healthcare and community settings in Botswana: An antibiotic resistance in communities and hospitals (ARCH) study. Int. J. Infect. Dis. 122, 313–320 (2022).
Verani, J. R. et al. Surveillance for hospitalized acute respiratory infection in Guatemala. PLoS ONE 8, e83600 (2013).
Instituto nacional de estadistica, INE. Censo Nacional de Población y VII de Vivienda - 2018 (2018).
CLSI. Performance standards for antimicrobial susceptibility testing.31st ed. CLSI supplement M100. Clinical and Laboratory Standards Institute (2021).
Mashreky, S. R. et al. Health seeking behaviour of parents of burned children in Bangladesh is related to family socioeconomics. Injury 41, 528–532 (2010).
Ramay, B. M. et al. Self-medication and ILI etiologies among individuals presenting at pharmacies with influenza-like illness: Guatemala City, 2018 influenza season. BMC Public Health 22, 1541 (2022).
Ramay, B. M., Lambour, P. & Cerón, A. Comparing antibiotic self-medication in two socio-economic groups in Guatemala City: A descriptive cross-sectional study. BMC Pharmacol. Toxicol. 16, 11 (2015).
Stata Corp. Stata Statistical Software: Release 15. StataCorp LLC (2017).
R Core Team, 2022. R: A language and environment for statistical computing, Vienna, Austria. Available at: https://www.R-project.org/.
Lautenbach, E. et al. Risk factors for community colonization with extended-spectrum cephalosporin-resistant enterobacterales (ESCrE) in Botswana: An antibiotic resistance in communities and hospitals (ARCH) study. Clin. Infect. Dis. 77, S89–S96 (2023).
Caudell, M. A. et al. Risk factors for colonization with multidrug-resistant bacteria in urban and rural communities in Kenya: An antimicrobial resistance in communities and hospitals (ARCH) study. Clin. Infect. Dis. 77, S104–S110 (2023).
Sukhum, K. V. et al. Antibiotic-resistant organisms establish reservoirs in new hospital built environments and are related to patient blood infection isolates. Commun. Med. 2, 62 (2022).
Odoyo, E. et al. Environmental contamination across multiple hospital departments with multidrug-resistant bacteria pose an elevated risk of healthcare-associated infections in Kenyan hospitals. Antimicrob. Resist. Infect. Control 12, 22 (2023).
Hefzy, E. M., Wegdan, A. A. & Abdel Wahed, W. Y. Hospital outpatient clinics as a potential hazard for healthcare associated infections. J. Infect. Public Health 9, 88–97 (2016).
Wilson, A. M. et al. Effects of patient room layout on viral accruement on healthcare professionals’ hands. Indoor Air 31, 1657–1672 (2021).
Li, M. et al. Infection-prevention and control interventions to reduce colonisation and infection of intensive care unit-acquired carbapenem-resistant Klebsiella pneumoniae: A 4-year quasi-experimental before-and-after study. Antimicrob. Resist. Infect. Control 8, 8 (2019).
Huang, J. et al. Impact of multicenter unified enhanced environmental cleaning and disinfection measures on nosocomial infections among patients in intensive care units. J. Int. Med. Res. 48, 030006052094976 (2020).
Schlechte, J. et al. Dysbiosis of a microbiota–immune metasystem in critical illness is associated with nosocomial infections. Nat. Med. https://doi.org/10.1038/s41591-023-02243-5 (2023).
Shealy, N. G., Yoo, W. & Byndloss, M. X. Colonization resistance: metabolic warfare as a strategy against pathogenic Enterobacteriaceae. Curr. Opin. Microbiol. 64, 82–90 (2021).
Omulo, S. et al. Carriage of antimicrobial-resistant bacteria in a high-density informal settlement in Kenya is associated with environmental risk-factors. Antimicrob. Resist. Infect. Control 10, 18 (2021).
Kurowski, K. M. et al. Social and environmental determinants of community-acquired antimicrobial-resistant escherichia coli in children living in semirural communities of Quito, Ecuador. Am. J. Trop. Med. Hyg. 105, 600–610 (2021).
Allel, K. et al. Transmission of gram-negative antibiotic-resistant bacteria following differing exposure to antibiotic-resistance reservoirs in a rural community: A modelling study for bloodstream infections. Sci. Rep. 12, 13488 (2022).
Aiello, A. E., Coulborn, R. M., Perez, V. & Larson, E. L. Effect of hand hygiene on infectious disease risk in the community setting: A meta-analysis. Am. J. Public Health 98, 1372–1381 (2008).
Wolf, J. et al. Effectiveness of interventions to improve drinking water, sanitation, and handwashing with soap on risk of diarrhoeal disease in children in low-income and middle-income settings: A systematic review and meta-analysis. The Lancet 400, 48–59 (2022).
Chard, A. N. et al. The impact of school water, sanitation, and hygiene improvements on infectious disease using serum antibody detection. PLoS Negl. Trop. Dis. 12, e0006418 (2018).
Sharma Waddington, H., Masset, E., Bick, S. & Cairncross, S. Impact on childhood mortality of interventions to improve drinking water, sanitation, and hygiene (WASH) to households: Systematic review and meta-analysis. PLoS Med. 20, e1004215 (2023).
Nadimpalli, M. L. et al. Drinking water chlorination has minor effects on the intestinal flora and resistomes of Bangladeshi children. Nat. Microbiol. 7, 620–629 (2022).
Bekele, T., Rawstorne, P. & Rahman, B. Effect of water, sanitation and hygiene interventions alone and combined with nutrition on child growth in low and middle income countries: A systematic review and meta-analysis. BMJ Open 10, e034812 (2020).
Clasen, T. et al. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: A cluster-randomised trial. Lancet Glob. Health 2, e645–e653 (2014).
Patil, S. R. et al. The effect of India’s total sanitation campaign on defecation behaviors and child health in Rural Madhya Pradesh: A cluster randomized controlled trial. PLoS Med. 11, e1001709 (2014).
Walson, J. L., Marshall, B., Pokhrel, B. M., Kafle, K. K. & Levy, S. B. Carriage of antibiotic-resistant fecal bacteria in Nepal reflects proximity to Kathmandu. J. Infect. Dis. 184, 1163–1169 (2001).
Collignon, P. & McEwen, S. One health—its importance in helping to better control antimicrobial resistance. TropicalMed 4, 22 (2019).
Chandler, C. I. R. & Nayiga, S. Antimicrobial resistance in cities: an overlooked challenge that requires a multidisciplinary approach. The Lancet 401, 627–629 (2023).
Swarthout, J. M., Chan, E. M. G., Garcia, D., Nadimpalli, M. L. & Pickering, A. J. Human colonization with antibiotic-resistant bacteria from nonoccupational exposure to domesticated animals in low- and middle-income countries: A critical review. Environ. Sci. Technol. 56, 14875–14890 (2022).
Hayward, C., Brown, M. H. & Whiley, H. Hospital water as the source of healthcare-associated infection and antimicrobial-resistant organisms. Curr. Opin. Infect. Dis. 35, 339–345 (2022).
Geneva: World Health Organization & United Nations Children’s Fund (UNICEF). Safely managed drinking water thematic report on drinking water 2017. (2017).
Moreno, P. et al. Availability of over-the-counter antibiotics in Guatemalan corner stores. PLoS ONE 15, e0239873 (2020).
Yang, L. et al. The varying effects of antibiotics on gut microbiota. AMB Exp. 11, 116 (2021).
Bhalodi, A. A., Van Engelen, T. S. R., Virk, H. S. & Wiersinga, W. J. Impact of antimicrobial therapy on the gut microbiome. J. Antimicrob. Chemother. 74, i6–i15 (2019).
United Nations Children’s Fund (UNICEF) and World Health Organization (WHO). Progress on Household Drinking Water, Sanitation and Hygiene 2000–2022: Special Focus on Gender. (2022).
Rutstein, S. O. & Johnson, K. The DHS Wealth Index. http://dhsprogram.com/pubs/pdf/CR6/CR6.pdf (2004).
Acknowledgements
The authors thank L. Godoy, A. Real, N. Lopez, V. Sicajau, and J. Guillermo Rivera for their assistance with data collection and sample processing. Staff and leadership from the Ministerio de Salud Pública y Asistencia Social, the Hospital Regional San Juan de Dios de Occidente, the Universidad del Valle de Guatemala, and the Centers for Disease Control and Prevention (CDC; Atlanta, Georgia, and the Central American Regional Office, Guatemala City) provided invaluable assistance for this work.
Author information
Authors and Affiliations
Contributions
BMR- methodology, data curation, formal analysis, writing—original draft preparation, writing—review and editing, visualization, supervision. MAC- methodology, data curation, formal analysis, writing—original draft preparation, writing—review and editing, visualization, supervision. CC- methodology, writing—review and editing, visualization, supervision.
LG- methodology, writing—review and editing, data curation, formal analysis, visualization, supervision. LS- data curation, formal analysis, writing—review and editing, visualization, supervision. JCR- methodology, data curation, formal analysis, writing—review and editing, visualization. MRL- methodology, writing—review and editing, supervision. SO- methodology, writing—review and editing, supervision. MFN- conceptualization, writing—review and editing, supervision, project administration. GHP- conceptualization, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition. RMS- conceptualization, methodology, writing—review and editing, project administration. CTAH- conceptualization, methodology, writing—review and editing, project administration. AS- conceptualization, methodology, writing—review and editing, project administration. CCR- methodology, data curation, formal analysis, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration. DRC- conceptualization, methodology, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ramay, B.M., Caudell, M.A., Castillo, C. et al. Risk factors associated with community colonization of extended-spectrum cephalosporin-resistant Enterobacterales from an antibiotic resistance in communities and hospitals (ARCH) study, Guatemala. Sci Rep 15, 18925 (2025). https://doi.org/10.1038/s41598-025-03379-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03379-9