Abstract
Prostatitis is a common condition in andrology and urology that significantly impacts the quality of life of affected individuals. Current treatments often fail to provide lasting benefits. To identify novel therapeutic targets, we conducted a drug-targeted Mendelian randomization (MR) study. Using cis-expression quantitative trait loci (cis-eQTL) data from the eQTLGen Consortium combined with Genome-Wide Association Studies (GWAS) data on prostatitis from FinnGen, we performed a two-sample MR analysis. This analysis identified nine potential causal genes: ANXA1, CRY2, DSTYK, FKBP1A, LAMA5, NENF, PTGIR, STK39, and TGFA. Following heterogeneity testing, horizontal pleiotropy assessment, and bidirectional MR, CRY2 and PTGIR were validated in the Genotype-Tissue Expression (GTEx) portal replication phase. Bayesian colocalization analysis and genetic correlation analysis investigations provided strong evidence of shared causal variants with prostatitis and negative genetic correlations for these genes. PheWAS indicated negligible horizontal pleiotropy, and drug prediction analysis identified potential targeting agents for CRY2 and PTGIR. This study highlights CRY2 and PTGIR as promising therapeutic targets for prostatitis, providing new insights into its genetic underpinnings and offering potential pathways for developing effective treatments.
Similar content being viewed by others
Introduction
Prostatitis, a common condition in urology, accounts for approximately 8% of all patients seen in the department1. It is particularly prevalent among men under the age of 50, making it the most common reason for urological visits in this age group2. Clinical symptoms in patients include lower urinary tract symptoms, pain in the lower back and perineal region, and possibly sexual dysfunction and psychological abnormalities3. Research indicates that the effect of prostatitis on patients’ quality of life is similar to that experienced by individuals with angina, congestive heart failure, Crohn’s disease, and diabetes4.
Due to the unclear etiology and pathogenesis of prostatitis, a unified and effective treatment protocol has not yet been established. For bacterial prostatitis, culture-guided antibiotic therapy is typically employed3. However, non-bacterial prostatitis, which constitutes a larger proportion of prostatitis cases, has not shown significant treatment efficacy with the traditional approach of combining antibiotics and alpha-blockers, as confirmed by randomized controlled trials5. Thus, investigating the genetic basis of prostatitis and identifying effective drug targets is essential to enhancing treatment outcomes.
MR represents an effective strategy for evaluating the connection between exposures or risk factors and clinical diseases6. Through harnessing gene variations distributed randomly during conception, MR markedly diminishes the influence of environmental and lifestyle elements. Recently, MR analysis has been widely used to advance drug target discovery7. Expression quantitative trait locis (eQTLs), which are closely associated with changes in gene expression, can simulate the long-term effects of drugs on the proteins they encode8,9. By integrating data from GWAS related to prostatitis risk, MR techniques can be employed to thoroughly explore the causal links between gene expression and disease. This method has proven effective in investigating conditions such as aortic aneurysm, hyperparathyroidism, and Alzheimer’s disease10,11,12. Our aim is to employ MR analysis to pinpoint potential drug targets associated with prostatitis, thereby facilitating innovative approaches to its pharmacological treatment.
Method
Study design and data sources
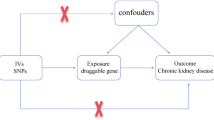
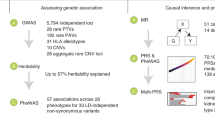
The objective of this study is to discover new therapeutic targets for treating prostatitis. Flow diagram (Fig. 1) and directed acyclic graph (Fig. 2) illustrate the methodological process, and Supplementary Table S1 offers comprehensive details on data sources.
First, a two-sample Mendelian randomization analysis will be conducted, integrating eQTL data sourced from eQTLGen consortium with prostatitis GWAS data retrieved from FinnGen database, to identify potential causal genes. Second, the research findings will undergo validation by heterogeneity testing, assessment of horizontal pleiotropy, and identification of reverse causal relationships. Third, the utilization of GTEx database for replication analysis will provide an external validation. Later on, a complex Mendelian randomization study will explain the combined causal impact of several risk variables. Finally, Bayesian colocalization analysis, genetic correlation analysis, PheWAS investigations, and drug prediction will be employed to ascertain therapeutic targets for forthcoming clinical therapy.
Identifiable drug targets
For our analysis, we identified 4,464 druggable genes annotated by HGNC and located on autosomal chromosomes. This set comprises 2,360 genes categorized within known drug target families, 674 genes connected to proteins targeted by licensed medicines or chemicals, and 1,425 genes encoding targets for protein now undergoing clinical development. Further detailed information on these pharmacologically viable targets can be found in the study by Chris et al.13.
Utilizing eQTL data to identify genetic instrumental variables
Two different eQTL datasets were used to identify genetic instrumental factors. The eQTLGen Consortium provided eQTL data for the discovery cohort. Furthermore, the replication cohort was obtained from the GTEx database, ensuring strong cross-database corroboration of our results.
eQTLGen consortium
Cis-eQTLs for 16,987 genes (false discovery rate < 0.05) were obtained from the eQTLGen consortium, utilizing 31,684 blood samples from healthy individuals of European ancestry. Given their proximity to target genes and direct impact on gene expression, eQTLs play a crucial role in drug development studies. We specifically focused on single nucleotide polymorphisms (SNPs) located within 100 kb upstream or downstream of transcription sites of druggable genes, identifying eQTLs associated with 2447 such genes14.
GTEx
We obtained eQTL data for 670 whole blood samples as independent replication cohorts from version 8 of the GTEx database15.
Outcome data
Prostatitis
We obtained GWAS summary statistics for prostatitis from FinnGen Release10, using samples from the Finnish Biological Bank’s National Network. This study included 4,160 prostatitis cases and 130,139 controls, covering 21,311,942 SNPs. All participants were of European ancestry. Prostatitis diagnoses were based on International Classification of Diseases (ICD) codes, categorized as N41 in ICD-10 and 601 in ICD-916.
Risk factors
Three risk factors associated with prostatitis were identified: body mass index (BMI), smoking, and alcohol consumption17. Summary statistics for these potential confounding factors were obtained from the Integrative Epidemiology Unit Open Genome-Wide Association Study (IEU Open GWAS) project, using the GWAS IDs ieu-b-73, ukb-b-223, and ukb-b-2303, respectively18.
Mendelian randomization analysis
Two-sample MR analysis was conducted using the TwoSampleMR R package (version 0.6.0)19. Prior to MR testing, stringent quality control was applied to the SNP instruments. Initially, variants with inadequate instrument strength (F-statistic < 10, calculated as F = (beta/se)2) were excluded from analysis. Next, we selected variants that were conditionally independent and had low linkage disequilibrium (LD r2 < 0.2 per 1000 Genomes European panel). Lastly, we applied Steiger filtering to eliminate genes where SNPs accounted for more outcome variance than exposure variance (Supplementary Table S2).
For cases with a single SNP, the Wald ratio method was selected as the primary analysis method, whereas the inverse variance weighted (IVW) method was used when multiple SNPs were available. The IVW method, which assumes all genetic instruments are valid, offers high statistical power20. Bonferroni corrections were implemented to establish adjusted significance thresholds. MR-Egger regression, weighted median, and weighted mode methods were employed as complementary sensitivity analyses. When the number of SNPs exceeds two, horizontal pleiotropy is assessed by determining whether the MR-Egger intercept deviates significantly from zero21. Additionally, Cochran’s Q statistic is used to evaluate heterogeneity, with a P-value < 0.05 indicating the presence of heterogeneity and horizontal pleiotropy22.Genes from the GTEx cohort that passed quality control were examined in further detail. The GTEx cohort was analyzed using a stringent Bonferroni correction to reduce false positives and validate the results, thereby complementing the initial findings from the eQTLGen analysis.
Multivariable Mendelian randomization (MVMR) analysis was carried out using the MVMR R package to examine the associations between prostatitis risk and both CRY2 and PTGIR, controlling for additional variables. Multivariable MR, building upon univariate MR, can elucidate the combined causal impacts of multiple risk factors23.
Reverse causality detection
To adhere to the eQTL screening criteria, we rigorously selected genetic instruments for prostatitis from the FinnGen GWAS dataset. After that, a bidirectional MR study was conducted using these tools to investigate possible reverse causality. The statistical significance was determined using a cutoff point of P < 0.05, guaranteeing a rigorous assessment of the reliability and validity of the observed connections.
Colocalization analysis
In order to assess colocalization, we conducted a study using the coloc R package with default priors on genes that had significant relationships in both the eQTLGen and GTEx cohorts using Mendelian randomization24. The purpose of this Bayesian strategy was to determine whether the connections between the expression of genes and prostatitis were caused by common causal variations at a single location, rather than being impacted by linkage disequilibrium. We assessed five distinct hypotheses: (H0) absence of any connection with either trait, (H1) connection solely with gene expression, (H2) connection solely with prostatitis, (H3) connection with both traits but with distinct causal variants, and (H4) connection with both traits influenced by a common causal variant25. The presence of strong colocalization was supported by a posterior probability of 0.8 or higher for H4, whereas probabilities between 0.5 and 0.8 indicated moderate colocalization10.
Genetic correlation analysis
LD score regression (LDSC) is used to assess the genetic correlation between the CRY2 and PTGIR genes and prostatitis26. Its estimated values fall between − 1 and 1, where a full negative genetic correlation is represented by a value of − 1 and a complete positive genetic correlation by a value of 1. In order to measure the inflationary effect resulting from a genuine polygenic signal or bias, LDSC looks at the relationship between test statistics and linkage disequilibrium. Even when there are overlapping samples, the LDSC regression slope offers an objective estimation of genetic connection using GWAS summary data. Statistical significance was determined using a stringent Bonferroni correction. When P values fell between the Bonferroni-adjusted threshold and 0.05, the findings were considered to suggest a potential genetic association.
Phenome-wide association analysis
The AstraZeneca PheWAS Portal and the PheWeb database were utilized to perform PheWAS, providing a thorough evaluation of horizontal pleiotropy and potential adverse effects of the therapeutic targets27,28.
Candidate drug prediction
The target genes found in this work were added to the Drug Signatures Database (DSigDB) in order to evaluate protein-drug interactions. DSigDB is an extensive database including 22,527 gene sets and 17,389 distinct chemicals linked to 19,531 genes. This large database helps to connect different drugs and substances to the genes that they target. To identify possible targeting agents for CRY2 and PTGIR, the DSigDB drug database on Enrichr was used for analysis29.
Ethics statement
The study conducted a secondary analysis utilizing data that was accessible to the public. The analysis focused on collected data from three well-known genome-wide association repositories: the eQTLGen Consortium14, the GTEx dataset30, and FinnGen16. Informed consent was obtained from all subjects and their legal guardians, and ethical approval was obtained from the respective institutional review boards; therefore, no additional ethical approval is required. All methods were carried out in accordance with relevant guidelines and regulations. The STROBE-MR checklist has been submitted as supplementary material31.
Results
Mendelian randomization uncovers 9 viable targets for prostatitis treatment
Based on MR analysis, nine genes showed a causal association with prostatitis risk, meeting the Bonferroni-corrected significance level (P < 2.04e−05), derived from 0.05/2447, where 2447 is the number of druggable genes tested in the eQTLGen dataset, as shown in Figs. 3 and 4. These genes include: annexin A1(ANXA1), cryptochrome circadian regulator 2(CRY2), dual serine/threonine and tyrosine protein kinase (DSTYK), FKBP prolyl isomerase 1A(FKBP1A), laminin subunit alpha 5(LAMA5), neudesin neurotrophic factor (NENF), prostaglandin I2 receptor (PTGIR), serine/threonine kinase 39 (STK39), and transforming growth factor alpha (TGFA). Specifically, increased expression of DSTYK (OR = 1.01; 95% CI 1.04–1.10; P = 1.04e−05), FKBP1A (OR = 1.14; 95% CI 1.08–1.20; P = 5.55e−06), LAMA5 (OR = 1.19; 95% CI 1.11–1.29; P = 6.57e−06), STK39 (OR = 1.25; 95% CI 1.15–1.36; P = 2.10e−07), and TGFA (OR = 1.89; 95% CI 1.47–2.44; P = 7.71e−07) increased the risk of prostatitis, while elevated ANXA1 (OR = 0.86; 95% CI 0.81–0.92; P = 4.10e−06), CRY2 (OR = 0.84; 95% CI 0.78–0.90; P = 1.21e−06), NENF (OR = 0.66; 95% CI 0.56–0.79; P = 2.30e−06), and PTGIR (OR = 0.67; 95% CI 0.57–0.78; P = 5.45e−07) decreased the risk of prostatitis. In the initial analysis, the effect directions for all nine genes were consistent across the three different methods (Supplemental Table S3). Additionally, no heterogeneity was observed (P > 0.05, Supplemental Table S4). The horizontal pleiotropy analysis results (P > 0.05, detailed in Supplemental Table S5) revealed that four genes (ANXA1, DSTYK, NENF, STK39) displayed evidence of horizontal pleiotropy. Consequently, these genes were excluded from subsequent investigations. The other five discovered genes did not exhibit any causal effects of prostatitis on their expression, according to the bidirectional Mendelian randomization study (Supplementary Table S6).
Three genes maintain significance in GTEx eqtl replication phase
Replication analyses that followed did not include TGFA as it was not present in GTEx whole blood samples. The approach used in the replication MR analysis was the same as that used in the first discovery cohort. In the analysis of GTEx whole blood samples, genetically predicted expression levels of CRY2, LAMA5, and PTGIR demonstrated significant causal associations with the risk of prostatitis (P < 1.25e−02). This significance threshold was derived using Bonferroni correction (0.05/4), based on the number of genes (n = 4) that were tested for replication in the GTEx dataset. Although initially associated, FKBP1A was excluded from further investigation due to inconsistent effects direction in the replication set (Fig. 5; Supplementary Table S7). For all three genes, the directionality of impact was the same in both the replication and discovery sets.
Colocalization analysis
In both the training and testing datasets, we saw steady and consistent estimated impacts of PTGIR, LAMA5, and CRY2, both positive and negative. This high level of consistency has revealed a significant correlation: upregulation of CRY2 and PTGIR expression, or downregulation of LAMA5 expression, appears to be closely associated with a reduced risk of prostatitis. This finding suggests that agonists targeting CRY2 and PTGIR, or inhibitors targeting LAMA5, could potentially become key components in the development of novel therapeutic strategies for prostatitis. However, this finding must also be approached with caution. Previous research has suggested that SNPs in linkage disequilibrium may be the cause of some significant Mendelian randomization findings. SNPs are linked to genetic loci related to exposure and outcome through various causal variants, which may lead to false-positive outcomes32. We carried out a colocalization study in order to comprehend this connection better. When SNPs are linked to both qualities, our analysis showed if the exposure and result had shared causal SNPs22. We discovered compelling evidence that PTGIR (coloc. abf-PPH 4 = 0.65) and CRY2 (coloc. abf-PPH 4 = 0.95) shared the same variations linked to prostatitis by Bayesian co-localization analysis (Figs. 6, 7 and Supplementary Table S8).
LDSC regression analysis
LDSC analysis to evaluate the genetic correlations between CRY2, PTGIR, and prostatitis. The Bonferroni-corrected p-value threshold was set at 0.025 (0.05 divided by 2). The results showed a significant genetic correlation between PTGIR and prostatitis (rg = − 0.475, SE = 0.167, P = 0.004) and a potential genetic correlation between CRY2 and prostatitis (rg = − 0.509, SE = 0.238, P = 0.032). The LDSC analysis indicated that these genetic correlations are negative, consistent with the findings from the discovery and replication sets. (Supplemental Table S9).
CRY2 and PTGIR were shown to have independent correlations with prostatitis by multivariable MR analysis
To validate the potential independent relationship between genes and prostatitis, we conducted separate multivariable Mendelian randomization analyses for the genes CRY2 and PTGIR, accounting for their associations with three risk factors. After adjusting for potential confounders, the results of the multivariable MR analysis demonstrated a strong association between CRY2 and PTGIR with prostatitis (CRY2: P = 2.83e−11 [IVW]; PTGIR: P = 1.33e−09 [IVW], Supplemental Table S10 and Supplemental Table S11).
PheWAS
We conducted phenome-wide Mendelian randomization (PheWAS) using the PheWAS Portal and PheWeb databases to investigate potential adverse outcomes associated with these two previously targeted genes25,26. Neither of the two pharmacological targets showed any significant genome-wide connections (genome-wide association P < 5e−08) with additional symptoms in the PheWeb or PheWAS Portal databases (Supplemental Figures S1–S2 and Supplementary Table S12–13).
Prediction of candidate drugs
Determining the viability of using target genes as prospective therapeutic targets requires evaluating protein-drug interactions. In order to identify possible targeted agents, we analyzed CRY2 and PTGIR using the DSigDB drug database on Enrichr. (Table 1). Aminolevulinic acid (CTD 00005375), tesaglitazar (CTD 00004468), and muraglitazar (CTD 00004445) are the most significant drugs linked to CRY2, while ETYA (CTD 00007037), proscillaridin (CTD 00006639), and flunisolide (CTD 00000364) are the most significant drugs linked to PTGIR.
Discussion
Prostatitis is a prevalent condition in the fields of urology and andrology, significantly impacting the quality of life for affected individuals. In 1995, the National Institutes of Health (NIH) in the United States categorized prostatitis into four types: Type I constitutes acute bacterial prostatitis (ABP); Type II represents chronic bacterial prostatitis (CBP); Type III encompasses chronic nonbacterial prostatitis (CNBP) or chronic pelvic pain syndrome (CPPS), with Type IIIA denoting inflammatory chronic pelvic pain syndrome and Type IIIB indicating non-inflammatory chronic pelvic pain syndrome, commonly referred to as prostatodynia; Type IV designates asymptomatic inflammatory prostatitis (AIP)33. This classification system remains widely utilized in current practice. Type I and Type II prostatitis are identified by the presence of urinary pathogens, detectable in semen, expressed prostatic secretions (EPS), or urine collected post-prostatic massage. These types generally respond effectively to antibiotic therapy34,35. In contrast, Type IV prostatitis patients exhibit no clinical symptoms and are usually diagnosed incidentally during routine physical exams. Importantly, more than 90% of clinical prostatitis cases fall under Type III (CPPS), where bacterial cultures of prostatic secretions typically return negative results.
The etiology of CPPS is intricate and multifactorial. Although recurrent infections by pathogens are commonly regarded as a primary cause, these infections might merely act as a triggering factor36,37. Potential disease mechanisms could involve compromised urothelial integrity and function, undetected infections, autoimmune responses, hormonal imbalances, pelvic floor muscle spasms or soreness, urinary issues, increased peripheral and central nervous system sensitivity, altered neural connections, and psychological factors38,39. Current evidence from both patient studies and animal models indicates that chronic prostatitis is likely driven by abnormal immune responses37,40,41. Research has shown that patients with chronic prostatitis exhibit T-cell autoreactivity to prostate-specific antigens like PSA and prostatic acid phosphatase42,43. This autoreactivity is marked by an increase in IFNγ-secreting Th1 lymphocytes, heightened antibody response levels, and T-cell infiltration within prostate tissue44,45. Moreover, analyses of clinical samples reveal an increased presence of inflammatory cells, cytokines (including IL-1β, TNFα, IL-6, and IL-8), and chemokines, suggesting that the prostate’s inflammatory process remains active even without infection37. Experimental autoimmune prostatitis animal models further underscore the pivotal role of immune responses in mediating prostatitis and chronic pelvic pain37,46.
At present, no standardized treatment derived from randomized controlled trials guarantees long-term benefits for patients with chronic prostatitis. Consequently, the development of effective treatment methods is urgently needed. To address this, we have conducted MR analysis by integrating data from genome-wide association studies, pharmacogenomics databases, and gene expression databases. Our objective is to identify potential molecular targets for the treatment of prostatitis.
This study utilized multiple MR methods to pinpoint two key drug targets for treating prostatitis: CRY2 and PTGIR. MR-Egger, Wald ratio/IVW, weighted median method, horizontal pleiotropy test, Cochran’s Q test for heterogeneity, bidirectional MR analysis, colocalization analysis, and multivariable MR analysis were among the techniques used. In order to validate these targets and support their validity as therapeutic targets, parallel research using the GTEx database revealed substantial correlations between prostatitis and CRY2 and PTGIR. To explore their pleiotropic effects and potential off-target impacts comprehensively, a phenome-wide association study (PheWAS) was conducted. Ultimately, by predicting compounds that bind to these targets, the study underscored their promising therapeutic potential.
CRY2 genes for a protein that binds to flavin adenine dinucleotide and is an essential part of the circadian core oscillator complex, which controls the circadian clock. The core oscillator of the circadian clock influences various physiological and pathological processes through direct or indirect effects. This includes modulation of the expression of inflammatory cytokines and chemokines, regulation of immune cell proliferation, differentiation, and migration, thereby shaping rhythmic changes in immune function47. Studies have shown that mice with double knockout of CRY1 and CRY2 develop an autoimmune phenotype48. This includes elevated serum IgG levels, the presence of serum antinuclear antibodies, deposition of IgG, IgM, and complement component 3 in the glomeruli, and significant leukocyte infiltration in the lungs and kidneys49. Our study found that upregulation of the CRY2 gene is associated with a reduced risk of prostatitis(eQTLGen OR = 0.84, P = 1.21e−06, PPH 4 = 0.95; GTEx whole blood sample OR = 0.62, P = 2.25e−48). LDSC analysis indicates a potential genetic correlation between CRY2 and prostatitis (rg = − 0.509, se = 0.238, P = 0.032). Phenome-wide and multivariable Mendelian randomization analyses suggest that this effect may be achieved through direct regulatory mechanisms. Additionally, the knockout of the CRY2 gene leads to immune homeostasis imbalance, resulting in an autoimmune phenotype. Therefore, enhancing CRY2 gene expression could be a potential therapeutic approach for treating prostatitis.
PTGIR, also known as IP, is a member of the G protein-coupled receptor family and has been identified as the receptor for prostaglandin I2 (PGI2). Recent findings have shown that PGI2 regulates both the innate and adaptive immune systems through the IP receptor, and in most cases, it exhibits anti-inflammatory or immunosuppressive effects50. One possible mechanism is that PTGIR mediates the effects of PGI2 and its analogs in a dose-dependent manner, inhibiting the maturation and function of bone marrow-derived dendritic cells (BMDCs) and the production of pro-inflammatory cytokines such as IL-12, TNF-α, IL-1α, and IL-6, while simultaneously increasing the production of the anti-inflammatory cytokine IL-1051. Our analysis revealed a novel genotype–phenotype correlation between PTGIR variants and altered risk of prostatitis (eQTLGen OR = 0.67, P = 5.45e−07; GTEx whole blood sample OR = 0.58, P = 1.87e−18). This result is supported by colocalization analysis (PPH 4 = 0.65) and genetic correlation analysis (rg = − 0.475, se = 0.167, P = 0.004). These findings suggest that inhibiting PTGIR could be a promising therapeutic strategy for prostatitis and warrants further investigation.
This study possesses several strengths. Firstly, the selection of genes already validated as drug targets enhances the reliability of the findings and increases the success rate for future drug development. Secondly, the collection of extensive eQTL data, particularly cis-acting variants, helps uncover gene regulatory mechanisms and signaling pathways. Our results undergo Bayesian colocalization analysis, genetic correlation analysis and cross-validation across different datasets, providing more robust evidence. Lastly, validation through Phe-MR analysis confirms the safety of these potential drug genes, which is crucial guidance for subsequent drug development. These strengths ensure the credibility and potential application of the study findings, offering scientific groundwork for new strategies in treating prostatitis.
To date, the genetic architecture of prostatitis remains largely unexplored, and few GWAS have identified reproducible risk loci for this condition. Unlike well-characterized genetic risk factors in other inflammatory diseases, existing candidate genes in prostatitis—such as those involved in androgen signaling or cytokine production—have shown limited consistency and lack functional validation. In this context, our identification of CRY2 and PTGIR as novel, genetically correlated, and functionally plausible targets is particularly significant. The LDSC results demonstrating negative genetic correlations (CRY2: rg = − 0.509; PTGIR: rg = − 0.475) suggest protective effects, which are biologically supported by their known roles in immune modulation.
It is important to take into account several limitations while considering the findings of our study. Firstly, our investigation specifically targeted individuals of European heritage, which may restrict the applicability of our results to other ethnic or racial populations. Secondly, the lack of subtyping for prostatitis in the FinnGen database restricted our ability to perform subgroup analyses. Additionally, due to limited genetic studies at the protein level, we could not ascertain Protein Quantitative Trait Loci (PQTL) for CRY2 and PTGIR to validate their association with prostatitis risk at the protein level. Furthermore, it is imperative to conduct clinical trials in order to evaluate the efficacy and security of targeting CRY2 and PTGIR as a treatment for prostatitis. Ultimately, the absence of supplementary relevant GWAS datasets limited our ability to confirm findings using independent exposure rather than outcome data.
In summary, Mendelian randomization and colocalization analysis using cis-eQTLs identified CRY2 and PTGIR inhibitors as promising targets for treating prostatitis. However, performing randomized controlled trials is crucial in order to definitively assess the effectiveness and safety of these prospective therapeutic targets.
Data availability
The GWAS summary data for the training set’s cis-eQTL can be accessed from the publicly available eQTLGen Consortium website (https://www.eqtlgen.org/). The validation set, GTEx dataset, is accessible at the GTEx Portal (https://gtexportal.org/home/). The GWAS data related to prostatitis is provided by the FinnGen Biobank (https://www.finngen.fi/en).
References
Collins, M. M., Stafford, R. S., O’Leary, M. P. & Barry, M. J. How common is prostatitis? A national survey of physician visits. J. Urol. 159, 1224–1228 (1998).
Qin, P., He, Y., Shao, H. & Jiang, D. Genetic insights into gut microbiota and risk of prostatitis: A Mendelian randomization study. Front. Microbiol. 15, 1389715 (2024).
Yebes, A. et al. Prostatitis: A review. Curr. Urol. Rep. 24, 241–251 (2023).
McNaughton Collins, M. et al. Quality of life is impaired in men with chronic prostatitis: The chronic prostatitis collaborative research network. J. Gen. Intern. Med. 16, 656–662 (2001).
Qin, Z. et al. Oral pharmacological treatments for chronic prostatitis/chronic pelvic pain syndrome: A systematic review and network meta-analysis of randomised controlled trials. eClinicalMedicine 48, 101457 (2022).
Sekula, P., Del Greco, M. F., Pattaro, C. & Köttgen, A. Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 27, 3253–3265 (2016).
Reay, W. R. & Cairns, M. J. Advancing the use of genome-wide association studies for drug repurposing. Nat. Rev. Genet. 22, 658–671 (2021).
Schmidt, A. F. et al. Genetic drug target validation using Mendelian randomisation. Nat. Commun. 11, 3255 (2020).
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
Chen, B. et al. Unveiling potential drug targets for hyperparathyroidism through genetic insights via Mendelian randomization and colocalization analyses. Sci. Rep. 14, 6435 (2024).
Su, W.-M. et al. Systematic druggable genome-wide Mendelian randomisation identifies therapeutic targets for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 94, 954–961 (2023).
Chen, Y. et al. Genetic insights into therapeutic targets for aortic aneurysms: A Mendelian randomization study. EBioMedicine 83, 104199 (2022).
Finan, C. et al. The druggable genome and support for target identification and validation in drug development. Sci. Transl. Med. 9, eaag1166 (2017).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53, 1300–1310 (2021).
GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518 (2023).
Zhang, Y. et al. Evidence for a causal effect of major depressive disorder, anxiety on prostatitis risk: A univariate and multivariate Mendelian randomization study. Prostate 83, 1387–1392 (2023).
Liu, M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 51, 237–244 (2019).
Hemani, G. et al. The MR-base platform supports systematic causal inference across the human phenome. Elife 7, e34408 (2018).
Guidelines for performing Mendelian randomization investigations: Update for summer 2023—PubMed. https://pubmed.ncbi.nlm.nih.gov/32760811/.
Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator—PubMed. https://pubmed.ncbi.nlm.nih.gov/27061298/.
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings—PubMed. https://pubmed.ncbi.nlm.nih.gov/30535378/.
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Foley, C. N. et al. A fast and efficient colocalization algorithm for identifying shared genetic risk factors across multiple traits. Nat. Commun. 12, 764 (2021).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Wang, Q. et al. Rare variant contribution to human disease in 281,104 UK Biobank exomes. Nature 597, 527–532 (2021).
Gagliano Taliun, S. A. et al. Exploring and visualizing large-scale genetic associations by using PheWeb. Nat. Genet. 52, 550–552 (2020).
Kuleshov, M. V. et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
GTEx Consortium. Human genomics. The genotype-tissue expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 348, 648–660 (2015).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 375, n2233 (2021).
Evaluating the potential role of pleiotropy in Mendelian randomization studies—PubMed. https://pubmed.ncbi.nlm.nih.gov/29771313/.
Krieger, J. N., Nyberg, L. & Nickel, J. C. NIH consensus definition and classification of prostatitis. JAMA 282, 236–237 (1999).
Shoskes, D. A. & Katz, E. Multimodal therapy for chronic prostatitis/chronic pelvic pain syndrome. Curr. Urol. Rep. 6, 296–299 (2005).
Rivero, V. E., Motrich, R. D., Maccioni, M. & Riera, C. M. Autoimmune etiology in chronic prostatitis syndrome: An advance in the understanding of this pathology. Crit. Rev. Immunol. 27, 33–46 (2007).
Khan, F. U. et al. Comprehensive overview of prostatitis. Biomed. Pharmacother. 94, 1064–1076 (2017).
Breser, M. L., Salazar, F. C., Rivero, V. E. & Motrich, R. D. Immunological mechanisms underlying chronic pelvic pain and prostate inflammation in chronic pelvic pain syndrome. Front. Immunol. 8, 898 (2017).
Bharucha, A. E. & Lee, T. H. Anorectal and pelvic pain. Mayo Clin. Proc. 91, 1471–1486 (2016).
Lai, H. et al. Animal models of urologic chronic pelvic pain syndromes: Findings from the multidisciplinary approach to the study of chronic pelvic pain research network. Urology 85, 1454–1465 (2015).
Penna, G. et al. Seminal plasma cytokines and chemokines in prostate inflammation: Interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur. Urol. 51, 524–533 (2007) (discussion 533).
Murphy, S. F., Schaeffer, A. J. & Thumbikat, P. Immune mediators of chronic pelvic pain syndrome. Nat. Rev. Urol. 11, 259–269 (2014).
Salazar, F. C. et al. CD8 T cells are dispensable for experimental autoimmune prostatitis induction and chronic pelvic pain development. Front. Immunol. 15, 1387142 (2024).
T-cell recognition of prostatic peptides in men with chronic prostatitis/chronic pelvic pain syndrome—PubMed. https://pubmed.ncbi.nlm.nih.gov/19765754/.
Identification of antigen-specific IgG in sera from patients with chronic prostatitis—PubMed. https://pubmed.ncbi.nlm.nih.gov/15359108/.
Zhang, M. et al. Targeting CXCL12/CXCR4 Signaling with AMD3100 Might Selectively Suppress CXCR4+ T-cell chemotaxis leading to the alleviation of chronic prostatitis. J. Inflamm. Res. 15, 2551–2566 (2022).
Chen, L., Zhang, M. & Liang, C. chronic prostatitis and pelvic pain syndrome: Another autoimmune disease?. Arch. Immunol. Ther. Exp. 69, 24 (2021).
Geiger, S. S., Fagundes, C. T. & Siegel, R. M. Chrono-immunology: Progress and challenges in understanding links between the circadian and immune systems. Immunology 146, 349–358 (2015).
Narasimamurthy, R. et al. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. U. S. A. 109, 12662–12667 (2012).
Cao, Q. et al. Circadian clock cryptochrome proteins regulate autoimmunity. Proc. Natl. Acad. Sci. U. S. A. 114, 12548–12553 (2017).
PGI 2 as a Regulator of Inflammatory Diseases—Dorris—2012—Mediators of Inflammation—Wiley Online Library. https://doi.org/10.1155/2012/926968.
Zhou, W. et al. Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells1. J. Immunol. 178, 702–710 (2007).
Acknowledgements
For allowing this analysis to be possible, we are grateful to the eQTLGen Consortium, UK Biobank, GTEx, FinnGen, and all of the individual research participants and investigators for making summary statistics publically available.
Author information
Authors and Affiliations
Contributions
P.Z., K.Y., and B.H.C. conceptualized and designed the study. K.Y. and Y.F.T. conducted the statistical analyses. D.Z. drafted the initial version of the manuscript. P.Z. took responsibility for editing the draft. K.Y., Z.H.L., and C.G.L. participated in data curation, analysis, and visualization. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, K., Tao, Y., Chen, B. et al. Identifying causal genes for prostatitis through drug-targeted Mendelian randomization. Sci Rep 15, 19069 (2025). https://doi.org/10.1038/s41598-025-03510-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03510-w