Abstract
The mandibulate euarthropods are the most speciose animal group, but the evolutionary gaps in origin of mandibulate body plan remain unresolved. Marrellomorphs, a common Paleozoic euarthropod group, had a long evolutionary history from Cambrian to Devonian. With computed microtomography, here we report the fine-scale soft-bodied morphoanatomy of the oldest marrellomorph Primicaris larvaformis, a millimeters-sized euarthropod from the ~ 518-million-year-old Chengjiang biota, China. Primicaris possesses a body plan featuring morphologically similar post-antennular biramous appendages, but also mandibulate diagnostic features including multi-segmented exopodites, a well-developed and differentiated hypostome-labrum complex, and a pancrustacean-like topological configuration of frontalmost three pairs of appendages. Phylogenetic analysis resolves Acercostraca and Marrellida as stem-Mandibulata. The undifferentiated post-antennular appendages in Primicaris suggest a possibility that the head appendages acquired a crown-mandibulate configuration before their morphological specialization in mandibulate origin. The emergence of novel appendage morphotypes in Acercostraca and Marrellida reveals that the complexity of limb tagmatization evolved independently in different Euarthropoda clades.
Similar content being viewed by others
Introduction
The Euarthropoda is the most diverse phylum among the animal kingdom and is divided into two major extant clades, Mandibulata and Chelicerata, but their origin and early evolution remain questionable1,2,3. The Mandibulata, consisting of myriapods and pancrustaceans (crustaceans and hexapods), is characterized by food-processing mandibles and sensory antennules on their fourth and second head segments, respectively4,5. Molecular clock studies calibrated the splitting of Mandibulata and Chelicerata during the early Cambrian6. The oldest crown-mandibulates were known from the phosphatized and carbonaceous microfossils in the Cambrian Epoch 27,8. Almost contemporarily, diverse macroscopic stem-mandibulates, represented by the bivalved-formed hymenocarines from Burgess Shale-type deposits, were recognized from the Cambrian Stage 3 and onward3,9,10,11. Together, the molecular clock calibration and early fossil record indicate a rapid origin of mandibulates during the Cambrian explosion12. This raises the question of how the tagmatization and specialization of mandibulate appendages originated in such a short time interval, which awaits to be resolved by the morphological transformations recorded by early mandibulate fossils.
Primicaris larvaformis Zhang et al.13 is a tiny, shield-shaped euarthropod from the early Cambrian Chengjiang biota (Series 2, Stage 3), Yunnan, China. It was first regarded as the juvenile of trilobitomorph euarthropod Naraoia spinosa14,15, but a later systematic description confirmed Primicaris as a separate taxon13. Primicaris and other Paleozoic euarthropods featuring unsegmented dorsal carapace are grouped into Acercostraca16,17,18,19,20. Acercostraca has close affinities with Marrellida, euarthropods with multiple pairs of elongated cephalic spines represented by Marrella splendens from the Burgess Shale21,22 and related taxa23,24,25,26,27. Acercostraca and Marrellida constitute ‘Marrellomorpha’19,20,25,26,28, but the monophyly of marrellomorphs was challenged by a recent study27. Moreover, the evolutionary affinities of marrellomorphs have been problematic, which have been variably resolved as stem-mandibulates28,29,30,31, stem-euarthropods9, or stem-chelicerates32.
With computed microtomography and other imaging techniques, here we document the high-resolution soft-bodied morphoanatomy of Primicaris after 2 decades from its original description. The results reveal mandibulate features in Primicaris and support a stem-group mandibulate affinity for marrellomorphs. The configuration of appendages in Primicaris illuminates the origin of head appendages in mandibulates and the independent evolution of limb tagmosis in early euarthropods.
Results
Systematic palaeontology
Phylum Euarthropoda Lankaster, 1904.
Order Acercostraca Lehmann, 1955.
Genus Primicaris Zhang, Han, Zhang, Liu and Shu, 200313.
Primicaris larvaformis Zhang, Han, Zhang, Liu and Shu, 200313.
Emended diagnosis
Small euarthropod with a body length less than 6 mm. Undivided dorsal shield with up to twelve pairs of lateral marginal spines and a pair of posterior spines. One pair of uniramous antennules is followed by up to 14 homonomous pairs of biramous appendages (emended from ref. 13).
Description
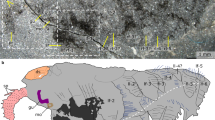
Our specimens exhibit a cordiform dorsal shield, ranging between 0.7 and 5.1 mm in length (posterior spines excluded) and 0.7–4.5 mm in width (Supplementary Fig. S1; Supplementary Table S1). The dorsal shield is undivided, showing no segmental boundaries (Fig. 1; Supplementary Fig. S2). In dorsal view, the dorsal shield is slightly curved and arched dorsally at the axial region. In specimens showing strong positive relief, the anterior, lateral, and posterior margins and axial region of the dorsal shield are thickened and ridged, with the anterior and posterior ridges connected by axial and lateral ridges (‘ax’, ‘rg’ in Figs. 1d, g, 2i, l; Supplementary Figs. S2a, c, S3m, S4g). However, in many other specimens, the ridges are hardly observable due to high dorsoventral compression. The rest of dorsal shield is thinner than the ridged parts, including two ear-shaped pleural regions between the axial and lateral ridges (‘pl’ in Fig. 1j, l; Supplementary Fig. S2e, f). In some specimens, a fusiform anterior border is found in the anteriormost axial region and located in front of the anterior ridge (‘ab’ in Figs. 1g, m, 2h; Supplementary Fig. S2d, g). The anterior border is composed of a thin cuticle, which can also be depression in the anteriormost axial region.
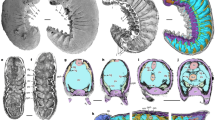
Overall morphology of P. larvaformis. (a) NIGP 200783A, optical photo, ventral view. (b, c) Juvenile specimens. (b) NIGP 200792, optical photo, ventral view. (c) NIGP 200791A, optical photo, dorsal view. (d, e) NIGP 200812 (see also in Fig. 3). (d) Optical photo, dorsal view. (e) Tomographic maximum projection image, dorsal view. (f) NIGP 200798A, optical photo, ventral view. (g) NIGP 200809A, optical photo, dorsal view. (h) NIGP 200810, optical photo, dorsal view. (i) NIGP 200813A, optical photo, ventral view. (j, k) NIGP 200799. (j) Optical photo, dorsal view. (k) SEM-EDS map of carbon. (i, m) NIGP 200817. (l) Optical photo, dorsal view. (m) SEM-EDS map of iron. a1–14, post-antennular appendage 1–14; ab, anterior border; an, antennule; ax, axial region; ba, base of appendage; dv, gut diverticulae; eb, eye bulge; er, eye ridge; gt, gut; ls1–11, lateral spine of dorsal shield 1–11; pl, pleural region; ps, posterior spine of dorsal shield; rg, ridge of dorsal shield; st, setae. Scale bars represent: 0.5 mm (a–m).
Tomographic reconstruction of P. larvaformis. (a–c) NIGP 200817. (d–f) NIGP 200816. (g–i) NIGP 200814. (j–l) NIGP 200808A. (a, d, g, j) Maximum projection images, dorsal view. (b, e, h, k) Volume rendering images, dorsal view. (c, f, i, l) Volume rendering images, ventral view. Colours indicate appendages on different segments. For abbreviations see Fig. 1. Additional abbreviations: db, doublre; vh, ventral hollow. Scale bars represent: 0.5 mm (a–l).
The two lateral margins are fringed by a series of twelve squarish to rhombic lateral spines in close sizes in some specimens, with the last lateral spine located at the base of posterior spine (‘ls’ in Figs. 1a, 2k; Supplementary Figs. S2l, S3t, S4j, l). The two posterior spines are triangular and are three to four times the length of a lateral spine (‘ps’ in Figs. 1a, b, d, f, l, 2h, 3a; Supplementary Figs. S2a, b, d, j, l, S4j). The posterior margin of dorsal shield is arc-shaped in a three-dimensional view, still, it varies in curvature from almost straight to strongly convex when the fossil is compressed at different angles (‘pm’ in Supplementary Figs. S2a, c, d, e, S3a; see also in ref.13).
Tomographic reconstruction of P. larvaformis showing details of appendages, NIGP 200812. (a, b) Whole specimen. (a) Dorsal view. (b) Ventral view. (c) Hypostomal structures covering anterior axial region. (d) Antennules. (e–h) First to fourth left post-antennular appendages showing exopodites with comb-like setae. (i–m) Fourth right post-antennular appendage. (i–l) Whole appendage in different views. (m) Protopodite. (n–r) Fifth right post-antennular appendage. (n–q) Whole appendage in different views. (r) Distal part of endopodite. Colours indicate appendages on different segments. For abbreviations see Fig. 1 and 2. Additional abbreviations: e1–7, endopodite podomere 1–7; ed, endite; gs, gnathobasic spine; hs, hypostomal spine; hy, hypostome; p1–15, antennular podomere 1–15; pt, protopodite; x1–10, exopodite podomere 1–10. Scale bars represent: 0.5 mm (a, b), 0.1 mm (c–r).
The anterior border is hollow on the ventral side, and the ventral hollow corresponds to the dorsal anterior ridge (‘vh’ in Fig. 2i, l; Supplementary Fig. S3t). A pair of teardrop eye bulges situated at the corners of the anterior ridge (‘eb’ in Figs. 1f, 4p; Supplementary Figs. S2e, f–h, S3e–s). Each eye bulge is connected to the anteriormost part of axial region through an eye ridge corresponding to the anterior ventral hollow (‘er’, ‘eb’ in Figs. 1d, g, 3a; Supplementary Fig. S2d). In most specimens, the eye bulges are hardly visible and thus are non-biomineralized. In some specimens, however, the eye bulges are filled with iron-rich minerals and appear to be convex on the dorsal side, but this is much like a preservational artefact (‘eb’ in Fig. 1f; Supplementary Fig. S3e).
Tomographic reconstruction of P. larvaformis showing additional morphological details. (a–f) Oral structures showing hypostome, labrum, and bases of appendages, volume rendering images, ventral view. (a) NIGP 200807A. (b) NIGP 200817. (c) NIGP 200802. The yellow arrows and three cyan arrows indicate the positions of virtual slices in i, k–m, respectively. (d) NIGP 200814. (e) NIGP 200808A. (f) NIGP 200809A. (g, h) Antennules, volume rendering images, ventral view. (g) NIGP 200798A. (h) NIGP 200815. (i–m) Virtual slices of oral structures in c, volume rendering images, NIGP 200802. (i) Sagittal virtual slice indicated by the yellow arrows in c, lateral view. The green arrows indicate the position of virtual slice in j. (j) Horizontal virtual slice indicated by the green arrows in i, dorsoventral view. (k–m) Transverse virtual slices indicated by the cyan arrows in a bottom-up order in c, anteroposterior view. (n) Anterior margin of dorsal shield showing doublure, volume rendering image, ventral view, NIGP 200796. (o) Sternites connected to posterior trunk appendages, volume rendering image, ventral view, NIGP 200807A. (p–r) Details of appendages, maximum projection images, dorsal view. (p) NIGP 200798A, showing anterior appendages and eye bulges. (q) NIGP 200802, showing anterior post-antennular appendages. (r) NIGP 200817, showing comb-like setae, bases of appendages, and sternites. For abbreviations see Figs. 1–3. Additional abbreviations: hb, hypostomal bulge; hr, hypostomal ridge; lb, labrum; m, mouth; oe, oesophagus; pl, pharyngeal lumen; sn, sternite. Scale bars represent: 0.1 mm (a–f), 0.2 mm (g–m).
In the ventral view of dorsal shield, the boundaries between shield margins and pleural regions are associated with an outer ridge and an inner narrow doublure in a concentric arrangement (‘rg’, ‘db’ in Fig. 2l). Narrow doublure also occurs along the anterior edge of anterior margin (‘db’ in Figs. 2l, 4n). The ventral axial region exhibits a prominent and elongated hypostome-labrum complex (Fig. 4a–e), which occupies the anterior third of axial length (Fig. 2c, f, i, l). The hypostome can be subdivided into two parts (Fig. 4a–e). The anterior part of hypostome is a narrow hypostomal ridge covering the anterior axial region ventrally (‘hy’ in Fig. 3c). The hypostomal ridge extends from the fusion with the hypostomal bulge to the anteriormost axial region connected to the anterior border of shield (‘hr’ in Fig. 4a–e; Supplementary Fig. S3d, p). The posterior part of hypostome is composed of a prominent hypostomal bulge (‘hb’ in Fig. 4a–e; Supplementary Fig. S3h) associated with a pair of posterolateral fork-like hypostomal spines (‘hs’ in Fig. 4a–e). A well-differentiated fan-shaped labrum, with a rounded posterior margin, is connected to the hypostomal bulge and situated between the two hypostomal spines (‘lb’ in Fig. 4a–e; Supplementary Fig. S3d). The angle at which the labrum is turned up and down is variable among specimens, indicating its flexibility of rotation to cover the mouth opening (‘lb’ in Fig. 4a–d). With the ventral exoskeleton of hypostome removed, an oral cavity within the hypostomal bulge is observable (Fig. 4f).
The first appendages are a pair of uniramous antennules with at least fifteen podomeres (‘an’, ‘p1–15’ in Figs. 1e, 2c, f, i, 3d, 4g, h, p; Supplementary Figs. S3k, l, s, t, S4b, f). The bases of antennules are located on the anterolateral side of hypostomal bulge (‘an’ in Figs. 2c, f, i, 4a–c; Supplementary Fig. S3g, h, t). The antennular podomeres are subcylindrical, but the shape of podomere changes gradually from robust to slender forms in proximal–distal direction (‘p1–15’ in Figs. 3d, 4g, h). Clusters of spine-like setae are present on the distal podomeres and are absent on proximal ones (‘st’ in Figs. 2c, 3d, 4h).
Following the antennules are 14 pairs of biramous appendages with similar morphology (‘a1–14’ in Fig. 2b–l; Supplementary Figs. S2k, S3). The sizes of post-antennular appendages decrease towards the rear, with the first pair being the longest. The insertion of first pair of post-antennular appendages is located dorsolateral to the junction between hypostome and labrum (‘a1’ in Figs. 2f, i, 4a, c, f; Supplementary Fig. S3h, t). The second pair of post-antennular appendages originate from the posterolateral sides of labrum (‘a2’ in Figs. 2f, i, l, 3b; Supplementary Fig. S3h, t). The third post-antennular appendage pair is posterior to the hypostome-labrum complex (‘a3’ in Figs. S2c, f, i, l, S3b).
In each biramous appendage, the base of appendage, or the protopodite, is connected to its corresponding sternite (‘ba’, ‘sn’ in Figs. 1l, m, 4o, r; Supplementary Fig. S5i). Each sternite is subcylindrical and connects with each other by a horizontal articulation (‘sn’ in Fig. 4r). The sizes of sternites gradually taper towards the rear. The protopodite comprises a single podomere with a robust gnathobase bearing more than ten robust spines projecting inward (‘gs’, ‘pt’ in Fig. 3i–q). The endopodite and exopodite are connected to the distal end of the protopodite.
The endopodite is about 30% shorter than the exopodite (‘en1–6’, ‘a1–14’ in Figs. 1e, 3a, b, 4p, q). The endopodite consists of seven podomeres, including a distal claw (‘e1–7’ in Fig. 3i–l, n–q). The proximal five endopodite podomeres have a prominent endite with a cluster of four to six elongate spines (‘ed’ in Fig. 3k, q). The sixth endopodite podomere is non-enditic and is slenderer than the proximal podomeres (‘e6’ in Fig. 3i–l, n–q). The distal claw comprises four talon-like spines (‘e7’ in Fig. 3e–l, n–r).
The exopodite bears ten podomeres (‘x1–10’ in Fig. 3i–l, n–q). The exopodite podomeres become more elongated towards the distal end. All the exopodite podomeres are enditic except for the subcylindrical tenth podomere. Each of the first to ninth exopodite podomeres has four to six enditic setae, and the tenth podomere has two to four terminal setae (‘st’ in Fig. 3e–h, k, l, p, q). All the setae of exopodite together form a comb-like apparatus (‘st’ in Figs. 3e–h, 4p, q).
The hypostome-labrum complex houses a muscular pharynx and a ventral esophagus, which are part of the J-shaped gut. The posteriorly facing mouth opening, located at the distal end of the muscular pharynx, is positioned above the posterior end of the labrum (‘m’ in Fig. 4i, j). The pharynx is made up by a massive muscle housed in the hypostome (‘ph’ in Fig. 4i). The triangular pharyngeal lumen is found in the cross-section (‘pl’ in Fig. 4k). The oesophagus starts from a narrow posterior end and has a lumen that gradually expands forward (‘oe’ in Fig. 4i, l, m). The transitional zone from ventral oesophagus to a dorsal section of the gut is located at the junction between the axial region and anterior margin (Fig. 1i). A straight gut runs through the axial region (‘gt’ in Fig. 1a, h, i; Supplementary Fig. S2m–p). A pair of curved gut diverticulae originate from the anteriormost part of gut, with each diverticula comprising a crescent-shaped anterior section and an elongated lateral section (‘dv’ in Fig. 1i, j, k). The gut diverticulae are distributed along the outer edge of pleural region, likely associated with the ventral ridge and doublure. Both the gut and gut diverticulae are in dark matter (Fig. 1i, j) and rich in carbon (Fig. 1k).
The smaller and larger individuals are similar in shape (Fig. 1; Supplementary Fig. S2). The length and width of dorsal shield show approximately isometric growth during ontogeny (Supplementary Fig. S1). The relative size of hypostome-labrum to body size is lower in largest individuals than in smaller individuals (Fig. 2c, i, l; Supplementary Fig. S4j, l), indicating negative allometry of the hypostome-labrum complex during growth.
Discussion
Comparisons to other euarthropod groups
The appendages in Primicaris reflect an artiopodan condition, which comprise a pair of uniramous antennae followed by a series of homonomous biramous appendages with their size decreasing towards rear (Fig. 5a). Each post-antennular appendage is composed of a gnathobasic one-segmented protopodite, an endopodite comprising seven podomeres including a terminal claw, and an exopodite consisting of ten setaceous podomeres (Fig. 5d–j). The morphology of the terminal claw closely resembles that of the distal spine observed in Fezouata marrellids33. The combination of homonomous post-antennular appendages, one-segmented gnathobasic protopodite, and seven-segmented endopodite is typical of the Artiopoda15,34,35. These shared characters support the sister relationship between Acercostraca and Artiopoda under parsimony analyses (Fig. 5k, l; Supplementary Fig. S7).
Reconstruction and phylogenetic placement of P. larvaformis. (a, b) Morphological reconstruction. (a) Ventral view. (b) Dorsal view. (c) Artistic reconstruction. (d–j) Reconstruction of a post-antennular appendage. (d–f) Entire appendage in different views. (d) Lateral view. (e) Dorsal view. (f) Ventral view. (g–j) Magnifications of different parts of post-antennular appendage. (g) Proximal parts of appendage in lateral view, from the boxed area in d. (h) Gnathobasic protopodite and enditic endopodites in ventral view, from the boxed area in f. (i) Distal part of endopodite in ventral view, from the boxed area in f. (j) Distal part of exopodite in ventral view, from the boxed area in f. (k–m) Simplified consensus trees from phylogenetic analyses using parsimony analyses. P. larvaformis is placed within Acercostraca, which is depicted in red. (k) Equal weighting of characters with concavity constant k = ∞. (l) Implied weighting of characters with concavity constant k = 3 or 5. (m) Internal relationships within Acercostraca and Marrellida. (n) Diagrams showing the limb tagmatization and differentiation in Acercostraca and Marrellida. Different limb morphotypes are indicated by numbers in bold on segments. Anterior and posterior tagmata are shown in grey and pink colors. Positionally homologous appendages are depicted in the same colors. Limb formula (in brackets) and tagmosis value (Brillouin Index, h) as in ref.65 are shown on the right of each diagram. For abbreviations see Figs. 1–3.
Primicaris also exhibits a number of mandibulate characters. Multi-segmented exopodites are common in numerous stem- or crown-mandibulates36,37. Likewise, the exopodites of Primicaris are multi-segmented and composed of ten podomeres with comb-like non-overlapping bristly setae along the inner side (Fig. 5d–f). Comparable multi-segmented exopodites are also present in anterior appendages of other acercostracans, such as Xylokorys chledophilia17 and Vachonisia rogeri18. Marrellids bear different biramous appendages with apparently annulated exopodites composed of up to ~ 40–50 annuli with slender filamentary setae22,23. Among trilobites, a similar exopodite with an annulated form was found in Triarthrus, which was composed of about 100 annuli38. By contrast, the exopodites of post-antennular appendages in many other Cambrian euarthropod groups are typically lobe-like flaps bearing flattened lamellate setae or non-overlapping setae9,34,35,39,40,41,42. An exception among artiopodans is Sinoburius lunaris, whose first two post-antennular appendages are specialized with exopodites consisting of over 12 podomeres, while its trunk appendages have exopodites composed of two or three lamellate podomeres, but these setal structures are all along the outer side of exopodites43. Segmented exopodites composed of five to seven podomeres are also present in the head appendages of some stem-chelicerates represented by Habelia optata, Offacolus kingi, and Dibasterium durgae, but the exopodite and endopodite are morphologically similar and they are inserted independently to body wall rather than being connected to protopodite32,44,45. Multi-segmented exopodites resembling those of Primicaris, with setae along the inner side and at least five podomeres, are regarded as an important evolutionary novelty in pancrustaceans36,46, including the second antennae and mandibles of extant crustacean nauplii and adult mystacocarids37,47. Superficially similar exopodites appear to be present in the second and third appendages of Orsten-type crustaceanomorphs36, although it is difficult to interpret whether they are annulated or segmented. Segmented exopodites composed of fewer podomeres (three or four) can be found in the trunk appendages of crustaceans such as copepods and remipedes47, stem-pancrustacean Tanazios dokeron48 and agnostids49. As reflected by other acercostracans17,18, the multi-segmented exopodites shared by acercostracans and crustaceans reflect a mandibulate affinity for Primicaris.
The two terms hypostome and labrum, both as pre-oral structures, have been poorly defined and were used interchangeably for the same structures in literature50,51. The hypostome, originally used in trilobites and other artiopodans, has been adopted for describing other euarthropod fossil groups39,52. In recently studies, the hypostome has been hypothesized to be homologous with the epistome and clypeus of mandibulates53,54. The labrum is a common pre-oral protrusion or plate in extant and extinct euarthropods, whose nature has been long problematic50,55. Alternative criteria for distinguishing between hypostome and labrum have been proposed recently9,30,51. Here we recognize the labrum as a mouth-covering structure and hypostome as a pre-labral sclerotized structure30,53. Primicaris possesses a hypostome-labrum complex composed of a well-differentiated rounded fan-shaped labrum and an elongated hypostome bearing a pair of fork-like spines (Fig. 5a). Three-dimensional reconstruction shows that the labrum covers the mouth opening and the hypostome accommodates the oesophagus (Fig. 4i, j). A comparable elongated hypostome-labrum complex has been described in the Silurian acercostracan Xylokorys17. The morphology and position of labrum vary across different arthropod groups. The labrum is prominent during the embryonic stages of chelicerates, but it becomes reduced and stays at the anteriormost position in adult chelicerates32,56. Although Xiphosura possesses a differentiated hypostome-labrum complex, it also appears in a reduced form57. Similar developmental reduction of the labrum is also present in the stem-chelicerate megacheirans40, and an anteriormost labrum seems to be also present in some of the Cambrian stem-mandibulate hymenocarines9,10,30. Well-developed posteroventrally positioned labrum, as present in Primicaris, is characteristic of most mandibulates9,37,47,53. Counterparts of the fan-shaped plate-like labrum with a rounded posterior margin in Primicaris can be found in extant pancrustaceans, especially the Laevicaudata and leptestheriid Spinicaudata among Branchiopoda, where the structure maintains articulated attachment37. The morphology of labrum in other Cambrian euarthropods is quite distinct from that of Primicaris. The labrum in Orsten-type labrophoran pancrustaceans is typically a bulging protrusion, exhibiting a fused attachment to the hypostome, as in Primicaris 58,59. In fuxianhuiids, a partially fused bilobed labrum posteriorly attached to the hypostome was recently proposed53. Among artiopodans, the labrum is thought to be fused to the posterior part of the hypostome in trilobites51, or as an individualized soft lobate structure that is well-differentiated from the hypostome54.
The configuration of the first three pairs of appendages associated with the hypostome-labrum complex in Primicaris is reminiscent of the typical mandibulate condition. The anterior margin of dorsal shield houses the eye bulges and belongs to the ocular domain (Fig. 5a). Following the anterior border, the hypostome-labrum complex thus belongs to a post-ocular and pre-oral region. As the first pair of appendages, the uniramous antennules of Primicaris emerges from the lateral sides of hypostome (Figs. 2c, f, i, 4a–c, 5a), a pre-labral position as in artiopodans60, some hymenocarines11, fuxianhuiids39,61, myriapods62, and pancrustaceans37,52. Although the second and third pairs of appendages in Primicaris have similar morphology to other post-antennular biramous appendages, their positions relative to the labrum are conspicuous. The insertion of second pair of appendages in Primicaris is located laterally to the base of labrum (Figs. 2i, 4a, c, f, 5a), resembling the position of biramous second antennae in Cambrian and extant pancrustaceans11,37,47,52. Meanwhile, the base of the third pair of appendages of Primicaris, also the first post-oral appendages, is situated at the posterolateral sides of labrum (Figs. 2f, i, l, 3b, 5a; Supplementary Fig. S3d, h). Such a topology is most similar to the configuration of mandibles present in total-group mandibulates, including myriapods62, pancrustaceans37,52, hymenocarines11, and probably fuxianhuiids53. Other acercostracans and marrellids, with their second or third appendages specialized as in pancrustaceans, appear to have similarly positioned first three pairs of appendages as in Primicaris, despite their presence of labrum has not been confirmed17,18,20,22,23,27. Overall, the arrangement of anteriormost three pairs of appendages relative to the hypostome-labrum complex in Primicaris, other acercostracans, and marrellids most resembles the condition of antennules (first antennae), second antennae, and mandibles in pancrustaceans. This topological similarity suggests that the first and second pairs of post-antennular appendages in Primicaris are homologous to the second antennae (or intercalary segment in insects) and mandibles in mandibulates, respectively.
In all, Primicaris possesses a body plan featuring undifferentiated post-antennular appendages composed of single-segmented protopodite and seven-segmented endopodite that may be ancestral for Deuteropoda34, but also shows pancrustacean character combination including a well-differentiated labrum, similar topology of anteriormost three pairs of appendages, and multi-segmented exopodites with setae along the inner side. Such a combination of characters in Primicaris supports a stem-mandibulate affinity for Acercostraca.
Phylogenetic relationships of Acercostraca and Marrellida
The phylogenetic results including Primicaris show some variations under different methods and character weighting parameters (Fig. 5k, l; Supplementary Fig. S7). In general, the consensus tree from Bayesian inference has a lower resolution than those of parsimony analysis (Fig. 5k–m; Supplementary Fig. S7). The positions of some taxa, such as hymenocarines and fuxianhuiids, are sensitive to weighting parameters (Fig. 5k, l). The bivalved and tailed hymenocarines have been regarded as a very basal stem group of Euarthropoda28,63. As an increasing number of hymenocarines have been found with mandibles, the mandibulate affinity of hymenocarines is increasingly endorsed and accepted9,10,11. Like hymenocarines, fuxianhuiids have been considered as stem-euarthropods39,61,63, despite a crustacean-like complex central nervous system64. A possible mandibulate affinity of fuxianhuiids was proposed recently, with the specialized post-antennular appendages interpreted as mandibles53. Our phylogenetic analyses recover hymenocarines and fuxianhuiids as stem-mandibulates under Bayesian inference and equal-weighted parsimony (Fig. 5k; Supplementary Fig. S7) or as crown-mandibulates when implied-weighted parsimony is applied (Fig. 5l). Such alternative positions of hymenocarines and fuxianhuiids are also reflected in recent phylogenetic studies using different versions of a dataset9,10,53.
Like previous phylogenetic investigations20,28, the current study recovers Primicaris as a member of Acercostraca. A stable monophyletic group composed of Marrellida, Acercostraca, and Artiopoda is found in the resulting trees (Fig. 5k–m; Supplementary Fig. S7). Acercostraca and Marrellida have been grouped into a monophyletic ‘Marrellomorpha’ in early studies20,28. However, similar to some recent analyses27, a paraphyletic grouping between Acercostraca and Marrellida is favoured here (Fig. 5k–m; Supplementary Fig. S7b–f). Although Bayesian inference reconstructs Marrellida, Acercostraca, and Artiopoda in a polytomy (Supplementary Fig. S7a), a sister-group relationship of Acercostraca and Artiopoda is revealed by all parsimony analyses (Fig. 5k–m), which is supported by the homonomous post-antennular appendages shared by Primicaris and most artiopodans13,15.
The present phylogenetic reconstructions retrieve the Artiopoda-Acercostraca-Marrellida group among the stem lineage of Mandibulata (Fig. 5k–m; Supplementary Fig. S7). Although Artiopoda has been widely accepted as a euarthropod crown group, whether Artiopoda is stem-Mandibulata30,41 or stem-Chelicerata28,53 is still hotly debated and favoured by different recent studies. Our study supports that a mandibulate affinity for Artiopoda is more likely (Fig. 5k, l; Supplementary Fig. S7), but the issue of Artiopoda affinity is far from reaching consensus.
Marrellida has been common in phylogenetic analyses of early euarthropods, but its placement has been highly variable, ranging from stem-mandibulates28,30, stem-chelicerates10, to stem-euarthropods9. In contrast to Marrellida, Acercostraca was only included in a couple of studies using large datasets, where Acercostraca and Marrellida were reconstructed as stem-mandibulates20,28. This placement gains support from our results (Fig. 5k, l; Supplementary Fig. S7).
The interrelationships in Acercostraca and Marrellida are overall compatible with the temporal ranges of genera or species. In results based on parsimony analysis, among Acercostraca, the Cambrian taxa Primicaris and Skania constitute a clade that is sister group to the monophyletic group of the Ordovician Enosiapis, Silurian Xylokorys, and Devonian Vachonisia, with the latter two being sister groups to each other (Fig. 5m). In Marrellida, the Cambrian Marrella is the earliest diverging taxon, and the Ordovician marrellid from the Fezouata biota and the Devonian Mimetaster form a clade (Fig. 5m). Earlier studies also recovered an almost identical or very similar phylogenetic topology20,27.
Evolution of limb tagmatization in Acercostraca and Marrellida
Despite the diagnostic cordiform dorsal shield is unsegmented in acercostracans, a bipartite tagmatization of the dorsal shield has been discussed in Acercostraca, and a comparable tagmatization is suggested in Marrellida19,20. A detailed discussion about the dorsal tagmatization of in marrellomorphs is provided in the Supplementary Discussion.
The ventral structures including hypostome-labrum complex and differentiated anterior appendages also reflect tagmatization in acercostracans and marrellids. The hypostome-labrum complex occupies the posterior half of the anterior tagma in Primicaris (Fig. 2c, f, i, l). The posterior tagma is characterized by the sternites associated with post-antennular appendages (Figs. 2a, 4r). Akin to that observed in Primicaris, an elongated hypostome-labrum complex is also evident in Xylokorys17. An elongated hypostome-labrum complex is most likely present in Enosiaspis and Vachonisia, as observed at their axial region18,20. A hypostome-labrum complex is probably present in Skania, although its detailed morphology is unclear16,19. Among Acercostraca, the rear end of hypostome-labrum complex covers the second pair of post-antennular appendages in Primicaris and Xylokorys, and the third pair in Vachonisia17,18 (Fig. 5n). A hypostome-labrum complex covering most of the ventral side of anterior tagma has also been recognized in Marrellida, and its rear end roughly corresponds to the boundary between anterior and posterior tagmata18,23,33 (Fig. 5n).
Associated with the hypostome-labrum complex are antennules and a set of anterior post-antennular appendages, which constitute the appendages of anterior tagma. Apart from the potentially biramous antennules suggested in Enosiaspis20, the antennules in most acercostracans are uniramous and do not stick out far from the dorsal shield17,18,19. In Primicaris, all the post-antennular appendages, including those in the anterior tagma, show only size variation and no obvious shape differentiation (Fig. 5a). Among them, only two pairs of undifferentiated post-antennular appendages are associated with the hypostome-labrum complex. The rest of the post-antennular appendages are distanced from the hypostome-labrum complex and are more reasonably assigned to the posterior tagma (Figs. 2c, f, i, l, 5a, n). However, in other acercostracans, the anterior post-antennular appendages associated with the hypostome-labrum complex are morphologically differentiated from the more posterior appendages. In Skania and Enosiaspis, the detailed morphology of anterior post-antennular appendages is obscure, although potential differentiation has been interpreted19,20. Two sets of post-antennular appendages are suggested in Skania16,19. The anterior set includes at least four pairs of elongated and sigmoidal appendages, while the posterior appendages of the trunk seem to be shorter and less curved. The two sets of post-antennular appendages in Enosiaspis are more conspicuous20. The anterior cluster of at least three pairs of appendages is stouter, while the trunk appendages are filamentous. Xylokorys and Vachonisia share a similar pattern of differentiation in their anterior post-antennular appendages17,18. The first three pairs are biramous and have exopodites longer than endopodites, while the fourth pair are uniramous and very slender. The first two pairs generally resemble each other, but the third pair have very reduced endopodites. In Xylokorys and Vachonisia, four pairs of morphologically variable and differentiated post-antennular appendages are assigned to the anterior tagma, and the morphologically distinct trunk appendages with enditic endopodites and filamentous exopodites are included in the posterior tagma17,18. Although the third and fourth pairs of post-antennular appendages can be posterior to the hypostome-labrum complex (Fig. 5n), they are situated at a very close position to the labrum and in a compact arrangement, which are more reasonably considered as functional components of the anterior tagma17,18.
To summarize, during the evolution of Acercostraca, there was a trend that more appendages were incorporated into the anterior tagma (Fig. 5n). The anterior appendages then became differentiated from the homonomous appendages of the posterior tagma, resulting into the origination of new limb morphotypes. Among Marrellida, a similar evolutionary history of increasing number and types of appendages is found in the anterior tagma. A single pair of post-antennular appendages is associated with the hypostome-labrum complex in the Cambrian Marrella22, whereas two pairs of such appendages with different morphotypes are present in the Ordovician marrellids27,33 and the Devonian Mimetaster23.
By quantifying the degree of limb tagmatization with the limb formula and the Brillouin Index65, the Cambrian Primicaris and Marrella had a lower limb tagmosis value than their post-Cambrian counterparts (Fig. 5n), suggesting an increase of limb tagmatization in the early Paleozoic phase of Acercostraca and Marrellida. Such a general tendency has been reported in crown-group crustaceans65. However, without prominent origination of novel appendage morphotypes, a larger number of homonomous appendages appeared in the posterior tagma of the late Paleozoic (Early Devonian) taxa Vachonisia and Mimetaster, resulting in a decline of tagmosis value during the early to late Paleozoic transition (Fig. 5n). In all, the presence of generally homonomous post-antennular appendages in the Cambrian taxa and the emergence of specialized appendages in the post-Cambrian forms indicate that limb tagmatization and specialization evolved independently during the history of Acercostraca and Marrellida. This suggests that the origination of specialized appendages and the increase of limb complexity were not unique for the evolution of crown-group euarthropod lineages, which also happened in some extinct euarthropods clades with a long evolutionary history.
Implications for the origin of Mandibulata
The presence of deutocerebral uniramous antennules, as in Primicaris, is a ground plan of mandibulates36,52,55,66, despite whether antennules are a mandibulate synapomorphy or a euarthropod plesiomorphy depends on the phylogenetic scenarios3,28,41,61. Among crown-group mandibulates, the pre-oral tritocerebral second antennae are present in crustaceans but are absent in myriapods and hexapods, as a modification of terrestrialization48,55. Like most crustacean second antennae67, the positionally homologous second pair of appendages are biramous in acercostracans18 and artiopodans34, but this is not the case in marrellids22,23, fuxianhuiids39,53,61 and hymenocarines9,11, suggesting a complicated morphological transformation.
Primicaris provides new insights into the origin of head appendages in mandibulates. Crown-mandibulates represented by crustaceans typically possess morphologically specialized second antennae and mandibles37,47. As discussed above, their topological homologs are the first and second pairs of post-antennular appendages in acercostracans and probably marrellids, supporting the scenario that second antennae and mandibles originated from undifferentiated post-antennular biramous appendages52,67. The pancrustacean-like configuration of anterior appendages in Primicaris also suggests that undifferentiated biramous appendages were the ground plan for the origins of post-antennular head appendages in mandibulates. Consequently, we propose a hypothesis that the first two post-antennular appendages were incorporated by the head at a crown-group mandibulate position before the morphological differentiation of second antennae and mandibles occurred in the head tagmatization of mandibulate origin.
Several evolutionary novelties have been proposed in the evolutionary origin of mandibulates from stem-group euarthropods9,36,52. Among these, the most critical novelties are the incorporation of trunk segments by the head and the specialization of anterior appendages36. The combination of character states in Primicaris and the evolution of limb tagmatization and specialization in Acercostraca provide an important reference for the origin of mandibulate body plan. The mandibulate-like arrangement of anterior three appendages in Primicaris confirmed the idea that the inclusion of unmodified appendages into the anterior/head tagma preceded the differentiation and specialization of these appendages36. The Cambrian to Devonian history of Acercostraca (and probably Marrellida) witnessed the incorporation of more appendages into the anterior/head tagma, the differentiation of anterior appendages from more posterior ones, and the emergence of new limb types, showing a parallel evolutionary trend as in the evolution of crustaceans36,52,65, although the other diagnostic characters of crown-group Mandibulata, including the mandibles, never appeared in Acercostraca. The presence of this general evolutionary trend in stem- and crown-mandibulates suggests that the evolutionary potential and developmental mechanism of segmental and limb patterning along the body axis already existed in the early-diverging stem groups of Mandibulata.
Material and methods
Fossil material
A total of 835 specimens of Primicaris were examined, which were collected from the fossil localities of the early Cambrian Chengjiang biota at Jianshan (n = 140), Mafang (n = 119), Ercai (n = 147), and Shankou (n = 429) around Kunming at Yunnan, China (Supplementary Table S1). The fossils are preserved in yellowish to greenish mudstone of the Maotianshan Shale Member, Yu’anshan Formation (Cambrian Series 2, Stage 3)68. All the specimens are housed at the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (NIGP).
Imaging
The optical photos were taken using a Leica M205C stereomicroscope with a Leica DFC450 camera. Computed tomographic data of 19 specimens were acquired with a Zeiss Xradia 520 Versa X-ray microscope. A 0.4X or 4X object with a LE2, LE3, or LE4 filter was used to capture 2001–3001 projections under a voltage of 50 or 60 kV and a power of 4 or 5 W. The exposure time of each projection was 0.5–3 s. Tomographic data were reconstructed in the VGStudio Max 3.0 software. Electron images and energy dispersive spectroscopy were captured for six specimens by using a Tescan Maia 3 scanning electron microscope with an Ultim Max 170 detector. X-ray fluorescence spectroscopy was conducted on a Horiba XGT-7200 analytical microscope for 13 specimens.
Phylogenetic analyses
The phylogenetic dataset in this study covers 98 panarthropod taxa and 297 characters. It is adapted from the original dataset in ref. 41, with five acercostracans and three marrellids added. Several characters in ref. 41 have been revised or broken into two characters to avoid logistic problems, and 16 new characters are added to the dataset (see supplementary material for details).
Maximum parsimony analyses were conducted in TNT v.1.569. All characters were treated as unordered. Each analysis, with equal or implied weighting settings applied (concavity constant k = 2, 3, 5, 10, ∞), consisted of a traditional search comprising 1,000 replicates of tree bisection and reconnection with 1,000 trees saved per replicate. Nodal supports are calculated as bootstrap supports, jackknife supports and group present/contradicted (GC) frequency differences by resampling using 1000 replicates of traditional search with change probability of 50%, 33% and 33%, respectively.
Bayesian phylogenetic inference was performed in MrBayes v.3.2.7a with default prior distributions and Markov chain Monte Carlo (MCMC) settings70. Each Bayesian analysis had two runs of 50,000,000 MCMC generations containing four chains under the Mkv + gamma model. Trees were sampled every 1,000 generations with the first 25% discarded as burn-in. Convergence was verified by an average standard deviation of split frequencies falling below 0.01 and effective sample size values over 2,600.
Data availability
All data analysed in this paper, including the phylogenetic dataset, are available as part of the article and its electronic supplementary material.
References
Daley, A. C., Antcliffe, J. B., Drage, H. B. & Pates, S. Early fossil record of Euarthropoda and the Cambrian explosion. Proc. Natl Acad. Sci., 115, 5323–5331 (2018).
Edgecombe, G. D. Arthropod origins: integrating paleontological and molecular evidence. Annu. Rev. Ecol. Evol. Syst. 51, 1–25 (2020).
Aria, C. The origin and early evolution of arthropods. Biol. Rev. 97, 1786–1809 (2022).
Rota-Stabelli, O. et al. A congruent solution to arthropod phylogeny: Phylogenomics, microRNAs and morphology support monophyletic Mandibulata. Proc. R. Soc. B 278, 298–306 (2010).
Edgecombe, G. D. Palaeontology: The cause of jaws and claws. Curr. Biol. 27, R807–R810 (2017).
Carlisle, E., Yin, Z. J., Pisani, D. & Donoghue, P. C. Ediacaran origin and Ediacaran-Cambrian diversification of Metazoa. Sci. Adv. 10, eadp7161 (2024).
Zhang, X. G., Siveter, D. J., Waloszek, D. & Maas, A. An epipodite-bearing crown-group crustacean from the lower Cambrian. Nature 449, 595–598 (2007).
Harvey, T. H. & Butterfield, N. J. Sophisticated particle-feeding in a large Early Cambrian crustacean. Nature 452, 868–871 (2008).
Aria, C. & Caron, J.-B. Burgess Shale fossils illustrate the origin of the mandibulate body plan. Nature 545, 89–92 (2017).
Vannier, J., Aria, C., Taylor, R. S. & Caron, J.-B. Waptia fieldensis walcott, a mandibulate arthropod from the middle Cambrian Burgess Shale. R. Soc. Open Sci. 5, 172206 (2018).
Zhai, D. Y. et al. Three-dimensionally preserved appendages in an early Cambrian stem-group pancrustacean. Curr. Biol. 29, 171–177 (2019).
Lee, M. S. Y., Soubrier, J. & Edgecombe, G. D. Rates of phenotypic and genomic evolution during the Cambrian explosion. Curr. Biol. 23, 1889–1895 (2013).
Zhang, X. L., Han, J., Zhang, Z. F., Liu, H. Q. & Shu, D. G. Reconsideration of the supposed naraoiid larva from the Early Cambrian Chengjiang Lagerstätte, South China. Palaeontology 46, 447–465 (2003).
Hou, X. G., Ramsköld, L. & Bergström, J. Composition and preservation of the Chengjiang Fauna: A lower Cambrian soft-bodied biota. Zool. Scr. 20, 295–411 (1991).
Hou, X. G. & Bergström, J. Arthropods of the lower Cambrian Chengjiang fauna, southwest China. Foss. Strat. 45, 1–116 (1997).
Lin, J. P. et al. A Parvancorina-like arthropod from the Cambrian of South China. Hist. Biol. 18, 33–45 (2006).
Siveter, D. J., Fortey, R. A., Sutton, M. D., Briggs, D. E. G. & Siveter, D. J. A Silurian marrellomorph arthropod. Proc. R. Soc. B 274, 2223–2229 (2007).
Kühl, G., Bergström, J. & Rust, J. Morphology, palaeobiology and phylogenetic position of Vachonisia rogeri (Arthropoda) from the Lower Devonian Hunsrück Slate (Germany). Palaeontogr. Abt. A 286, 123–157 (2008).
Legg, D. A. The morphology and affinities of Skania fragilis (Arthropoda) from the middle Cambrian Burgess Shale. Bull. Geosci. 90, 509–518 (2015).
Legg, D. A. An acercostracan marrellomorph (Euarthropoda) from the Lower Ordovician of Morocco. Sci. Nat. 103, 1–7 (2016).
Whittington, H. B. Redescription of Marrella splendens (Trilobitoidea) from the Burgess Shale, Middle Cambrian, British Columbia. Geol. Surv. Can. 209, 1–24 (1971).
García-Bellido, D. C. & Collins, D. H. A new study of Marrella splendens (Arthropoda, Marrellomorpha) from the Middle Cambrian Burgess Shale, British Columbia, Canada. Can. J. Earth Sci. 43, 721–742 (2006).
Kühl, G. & Rust, J. Re-investigation of Mimetaster hexagonalis: A marrellomorph arthropod from the lower Devonian Hunsrück Slate (Germany). Paläont. Z. 84, 397–411 (2010).
Van Roy, P. et al. Ordovician faunas of burgess shale type. Nature 465, 215–218 (2010).
Rak, Š, Ortega-Hernández, J. & Legg, D. A. A revision of the late ordovician marrellomorph arthropod Furca bohemica from Czech Republic. Acta Palaeontol. Pol. 58, 615–628 (2013).
Aris, M. J., Corronca, J. A., Quinteros, S. & Pardo, P. L. A new marrellomorph euarthropod from the early Ordovician of Argentina. Acta Palaeontol. Pol. 62, 1–8 (2017).
Moysiuk, J., Izquierdo-López, A., Kampouris, G. E. & Caron, J.-B. A new marrellomorph arthropod from southern Ontario: A rare case of soft-tissue preservation on a late Ordovician open marine shelf. J. Paleontol. 96, 1–16 (2022).
Legg, D. A., Sutton, M. D. & Edgecombe, G. D. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nat. Commun. 4, 2485 (2013).
Briggs, D. E. G., Siveter, D. J., Siveter, D. J., Sutton, M. D. & Legg, D. Tiny individuals attached to a new Silurian arthropod suggest a unique mode of brood care. Proc. Natl Acad. Sci., USA 113, 4410–4415 (2016).
Aria, C. & Caron, J.-B. A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573, 586–589 (2019).
Zhai, D. Y. et al. Variation in appendages in early Cambrian bradoriids reveals a wide range of body plans in stem-euarthropods. Commun. Biol. 2, 1–6 (2019).
Aria, C. & Caron, J.-B. Mandibulate convergence in an armoured Cambrian stem chelicerate. BMC Evol. Biol. 17, 261 (2017).
Laibl, L. et al. Early developmental stages of a Lower Ordovician marrellid from Morocco suggest simple ontogenetic niche differentiation in early euarthropods. Front. Ecol. Evol. 11, 1232612 (2023).
Ortega-Hernández, J., Legg, D. A. & Braddy, S. J. The phylogeny of aglaspidid arthropods and the internal relationships within Artiopoda. Cladistics 29, 15–45 (2013).
Zeng, H., Zhao, F. C., Yin, Z. J. & Zhu, M. Y. Appendages of an early Cambrian metadoxidid trilobite from Yunnan, SW China support mandibulate affinities of trilobites and artiopods. Geol. Mag. 154, 1306–1328 (2017).
Haug, J. T., Maas, A., Haug, C. & Waloszek, D. Evolution of crustacean appendages. In The Natural History of the Crustacea, Volume 1, Functional Morphology and Diversity (eds Watling, L. & Thiel, M.) 34–73 (Oxford University Press, 2013).
Martin, J. W., Olesen, J. & Høeg, J. T. Atlas of Crustacean Larvae (Johns Hopkins University Press, 2014).
Cisne, J. L. Anatomy of Triarthrus and the relationships of the Trilobita. In Evolution and Morphology of the Trilobita, Trilobitoidea and Merostomata: Proceedings of the Oslo Meeting (ed. Martinsson, A.) 45–63 (Universitetsforlaget, 1973).
Yang, J. et al. Early Cambrian fuxianhuiids from China reveal origin of the gnathobasic protopodite in euarthropods. Nat. Commun. 9, 1–9 (2018).
Liu, Y., Ortega-Hernández, J., Zhai, D. Y. & Hou, X. G. A reduced labrum in a Cambrian great-appendage euarthropod. Curr. Biol. 30, 3057-3061e2 (2020).
Zeng, H., Zhao, F. C., Niu, K. C., Zhu, M. Y. & Huang, D. Y. An early Cambrian euarthropod with radiodont-like raptorial appendages. Nature 588, 101–105 (2020).
Losso, S. R. & Ortega-Hernández, J. Claspers in the mid-Cambrian Olenoides serratus indicate horseshoe crab–like mating in trilobites. Geology 50, 897–901 (2022).
Chen, X. H. et al. The appendicular morphology of Sinoburius lunaris and the evolution of the artiopodan clade Xandarellida (Euarthropoda, early Cambrian) from South China. BMC Evol. Biol. 19, 1–20 (2019).
Sutton, M. D., Briggs, D. E., Siveter, D. J., Siveter, D. J. & Orr, P. J. The arthropod Offacolus kingi (Chelicerata) from the Silurian of Herefordshire, England: computer based morphological reconstructions and phylogenetic affinities. Proc. R. Soc. B 269, 1195–1203 (2002).
Briggs, D. E. et al. Silurian horseshoe crab illuminates the evolution of arthropod limbs. Proc. Natl Acad. Sci., USA 109, 15702–15705 (2012).
Boxshall, G. A. Arthropod limbs and their development. In Arthropod Biology and Evolution (eds Minelli, A. et al.) 241–268 (Springer, 2013).
Schram, F. R. & Koenemann, S. Evolution and Phylogeny of Pancrustacea (Oxford University Press, 2021).
Siveter, D. J., Sutton, M. D., Briggs, D. E. & Siveter, D. J. A new probable stem lineage crustacean with three-dimensionally preserved soft parts from the Herefordshire (Silurian) Lagerstätte. UK. Proc. R. Soc. B 274, 2099–2108 (2007).
Müller, K. J. & Walossek, D. Morphology, ontogeny, and life habit of Agnostus pisiformis from the Upper Cambrian of Sweden. Foss. Strat. 19, 1–124 (1987).
Budd, G. E. The origin and evolution of the Euarthropod labrum. Arthropod Struct. Dev. 62, 101048 (2021).
Park, T. Y. S. Trilobite hypostome as a fusion of anterior sclerite and labrum. Arthropod. Struct. Dev. 77, 101308 (2023).
Waloszek, D., Maas, A., Chen, J. Y. & Stein, M. Evolution of cephalic feeding structures and the phylogeny of Arthropoda. Palaeogeogr. Palaeoclimatol. Palaeoecol. 254, 273–287 (2007).
Aria, C., Zhao, F. C. & Zhu, M. Y. Fuxianhuiids are mandibulates and share affinities with total-group Myriapoda. J. Geol. Soc. 178, jgs2020-246 (2021).
El Albani, A. et al. Rapid volcanic ash entombment reveals the 3D anatomy of Cambrian trilobites. Science 384, 1429–1435 (2024).
Scholtz, G. & Edgecombe, G. D. The evolution of arthropod heads: Reconciling morphological, developmental and palaeontological evidence. Dev. Genes. Evol. 216, 395–415 (2006).
Dunlop, J. A. The epistomo-labral plate and lateral lips in solifuges, pseudoscorpions and mites. Ekológia (Bratislava) 19, 67–78 (2000).
Snodgrass, R. E. Textbook of Arthropod Anatomy (Cornell University Press, 2019).
Walossek, D. The upper Cambrian Rehbachiella and the phylogeny of Branchiopoda and Crustacea. Foss. Strat. 32, 1–202 (1993).
Siveter, D. J., Williams, M. & Waloszek, D. A phosphatocopid crustacean with appendages from the Lower Cambrian. Science 293, 479–481 (2001).
Schmidt, M. et al. Before trilobite legs: Pygmaclypeatus daziensis reconsidered and the ancestral appendicular organization of Cambrian artiopods. Phil. Trans. R. Soc. B 377, 20210030 (2022).
Yang, J., Ortega-Hernández, J., Butterfield, N. J. & Zhang, X. G. Specialized appendages in fuxianhuiids and the head organization of early euarthropods. Nature 494, 468–471 (2013).
Edgecombe, G. D. Morphological data, extant Myriapoda, and the myriapod stem-group. Contrib. Zool. 73, 207–252 (2004).
Legg, D. A., Sutton, M. D., Edgecombe, G. D. & Caron, J.-B. Cambrian bivalved arthropod reveals origin of arthrodization. Proc. R. Soc. B 279, 4699–4704 (2012).
Ma, X. Y., Hou, X. G., Edgecombe, G. D. & Strausfeld, N. J. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–261 (2012).
Cisne, J. L. Evolution of the world fauna of aquatic free-living arthropods. Evolution 28, 337–366 (1974).
Boxshall, G. A. The evolution of arthropod limbs. Biol. Rev. 79, 253–300 (2004).
Haug, J. T., Maas, A. & Waloszek, D. Ontogeny of two Cambrian stem crustaceans, †Goticaris longispinosa and †Cambropachycope clarkson. Palaeontograph. Abt. A 289, 1–43 (2009).
Zhao, F. C. et al. Spatial variation in the diversity and composition of the Lower Cambrian (Series 2, Stage 3) Chengjiang biota, Southwest China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 346, 54–65 (2012).
Goloboff, P. A. & Catalano, S. A. TNT, version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238 (2016).
Ronquist, F. et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (42330209, 42272019), the Natural Science Foundation of Jiangsu Province (BK20230110), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (2023322). We thank Suping Wu for assistance in micro-CT scanning, Yan Fang for help in SEM-EDS analysis, Qian Chen for facilitating XRF test, and Dinghua Yang for drawing artistic reconstruction. We are indebted to Prof. Junyuan Chen for examining his Chengjiang fossil collection.
Author information
Authors and Affiliations
Contributions
H.Z. and F.C.Z. conceptualized and designed the research. H.Z., F.C.Z. and M.Y.Z. secured the funding. F.C.Z. and H.Z. collected and curated the material. H.Z. and Y.L. prepared the specimens and acquired the computed tomographic data. Y.L. documented all the specimens with macrophotography and made the 3D reconstructions. H.Z., Y.L. and Y.M.L. quantified the limb formulae and tagmosis values. Y.L. and H.Z. wrote the original draft and conducted the phylogenetic analysis. H.Z. and Y.L. revised the original draft with inputs from F.C.Z., M.Y.Z, Y.M.L. and Y.Y.Z.. All authors participated in the interpretation of the material and the discussions.
Corresponding author
Ethics declarations
Declarations
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Zeng, H., Zhao, F. et al. A tiny Cambrian stem-mandibulate reveals independent evolution of limb tagmatization and specialization in early euarthropods. Sci Rep 15, 19115 (2025). https://doi.org/10.1038/s41598-025-03544-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03544-0