Abstract
Colorectal cancer (CRC) is a leading cause of cancer-related deaths worldwide, necessitating the development of novel therapeutic strategies. We explore the expression characteristics of SLC39A6 in CRC by combining multiple cohorts and multi-omics. The therapeutic effect and potential mechanism of BRY812 on CRC were explored through in vitro experiments. Our research results show that the expression of SLC39A6 in CRC tissues is higher than that in normal tissues, and it is closely related to tumor pathways, making it a good therapeutic target. BRY812 has an inhibitory effect on the growth, migration and stemness of CRC cells, and may exert its killing effect by downregulating the AKT pathway. This study has identified SLC39A6 as a potential therapeutic target in CRC. BRY812 is expected to become a highly promising therapeutic drug, bringing new hope to patients with CRC.
Similar content being viewed by others
Introduction
CRC is the third most prevalent cancer globally, accounting for nearly 10% of all cancer diagnoses1. Currently, antibody-drug conjugates (ADCs) are becoming an increasingly potent tool in the fight against cancer2, offering new therapeutic options especially for patients who no longer respond to traditional chemotherapy and targeted treatments3. With an improved understanding of the molecular mechanisms underlying various cancers, scientists are continually identifying new potential therapeutic targets4. In the treatment of CRC, ADCs have become an important modality, particularly for patients for whom conventional therapies have failed5. The development of ADCs is advancing, providing more treatment options and hope for patients with CRC6.
SLC39A6, also known as ZIP6 or LIV-1, is a transmembrane zinc transporter that plays an essential role in maintaining intracellular zinc homeostasis, cellular proliferation, differentiation, and metabolism7,8,9,10. Studies have shown that SLC39A6 is upregulated in various cancers, including breast, liver, and esophageal cancers, with higher expression levels correlating with enhanced tumor cell proliferation, migration, and invasiveness8,10,11,12,13. Moreover, in triple-negative breast cancer, esophageal cancer, and gastric cancer, SLC39A6 serves not only as a potential diagnostic marker but also as a therapeutic target.
ADCs represent a novel class of drugs designed for targeted delivery of chemotherapeutic agents to solid tumors. Comprising three key components—monoclonal antibodies (mAb), linkers, and payloads14—ADCs enhance the delivery of cytotoxic drugs to tumor cells. The payload, which can be any type of antitumor agent, is predominantly cytotoxic in current ADC formulations15. Monoclonal antibodies, when not conjugated to toxic drugs, typically do not cause unacceptable toxicity. The clinical application of ADCs in CRC remains limited, with trastuzumab deruxtecan (T-DXd) and SHR-A1811 being among the few agents currently under clinical investigation. Therefore, developing ADCs targeting novel antigens is critical for advancing precision therapy in CRC. BRY812, a first-in-class ADC targeting SLC39A6, was independently developed by Zhejiang Bioray Biopharmaceutical Co., Ltd. (China). This ADC employs an anti-SLC39A6 monoclonal antibody conjugated to the cytotoxic payload monomethyl auristatin E (MMAE) via the CysLink™ irreversible conjugation platform, forming a stable drug-antibody complex. Mechanistically, BRY812 binds to SLC39A6 overexpressed on tumor cell surfaces, undergoes receptor-mediated internalization, and releases MMAE intracellularly to inhibit microtubule polymerization, thereby inducing apoptosis. The linker is designed with a highly stable chemical structure, minimizing premature payload release in circulation to reduce off-target toxicity while enhancing tumor-specific payload delivery. To date, several SLC39A6-targeting ADCs have entered clinical development. Ladiratuzumab vedotin, the first SLC39A6-directed ADC, has been evaluated in breast cancer16,17, with clinical trial results demonstrating preliminary efficacy. For instance, SGNLVA-001, an ongoing phase 1 trial, assesses the safety and tolerability of ladiratuzumab vedotin alone or combined with trastuzumab in metastatic breast cancer17. BRY812 represents the second SLC39A6-targeting ADC to enter clinical studies and is currently in a phase 1 trial (NCT number pending) for advanced malignancies, including CRC, breast cancer, and non-small cell lung cancer. However, no preclinical or clinical studies have specifically explored BRY812 in CRC, highlighting the unmet need and translational potential of this research.
In this study, we first investigated the role of SLC39A6 in CRC by employing multi-omics approaches to study its expression and biological characteristics. We discovered that SLC39A6 is highly expressed in tumor tissues across multiple bulk transcriptomic cohorts, single-cell transcriptomic cohorts, and spatial transcriptomic cohorts. Through enrichment analysis of genes and ssGSEA scoring, we found that SLC39A6 is correlated with tumor-associated pathways. Furthermore, using the SPATA algorithm in spatial transcriptomics, we identified spatial co-localization of SLC39A6 with multiple tumor pathways. These findings suggest that SLC39A6 has potential as a target in CRC. We then proceeded to investigate the cytotoxic effects of the SLC39A6-targeted ADC drug BRY812 in vitro. The experimental results showed that BRY812 significantly inhibited the growth, stemness, and metastasis of CRC cells. Additionally, we explored the mechanism of action of BRY812 using RNA-seq, which revealed a significant downregulation of the AKT pathway, and this was further validated using western blotting. This comprehensive analysis of the expression profile of SLC39A6 in CRC not only highlights its potential as a therapeutic target but also provides insights into the cytotoxic effects and mechanisms of action of BRY812 in CRC cells, offering a new therapeutic strategy for the precision treatment of CRC.
Result
Result 1 SLC39A6 is highly expressed in tumor tissues of CRC
To investigate the expression of SLC39A6 in CRC, we initially downloaded three bulk transcriptomic datasets from the TCGA and GEO databases (TCGA-COAD, GSE33113, GSE39582). The results indicated that SLC39A6 is expressed at higher levels in tumor tissues (Fig. 1A). Furthermore, we validated these findings in single-cell and spatial transcriptomic datasets. Single-cell data revealed that SLC39A6 expression is highest in the tumor center compared to normal and peritumor tissues (Fig. 1B). The spatial transcriptomic data were obtained from the GEO database and the 10X Genomics website, including slides of both normal and tumor tissues. After integration using the harmony method, we observed that the expression level of SLC39A6 is higher in tumor tissues than in normal tissues (Fig. 1C–F). These results suggest that SLC39A6 is overexpressed in CRC tissues compared to normal tissues, indicating its potential as a prognostic target.
SLC39 A6 is highly expressed in CRC tissues. (A) Barplot illustrates the expression levels of SLC39A6 in tumor and normal tissues within the TCGA-COAD, GSE33113 and GSE39582 cohort. (B) tSNE plot showing the transcriptome landscape, which consists of 21 cell clusters. Dot plot and violin plot depicting the expression of SLC39A6 in normal, border and tumor tissues in CRC. (C) Spatial transcriptomics (ST) map of normal and tumor tissues in CRC. (D) umap plot showing all the spots of three slides. (E) The dot plot depictes expression of SLC39A6 on slides. (F) Barplot illustrates the expression levels of SLC39A6 in tumor and normal tissues within the slides. The statistical difference was compared through the Wilcox test. *P < 0.05; **P < 0.01; ***P < 0.001.
Result 2 biological function of SLC39A6 within tumor tissues of CRC
To explore the biological function of SLC39A6, we conducted enrichment analyses on the TCGA-COAD, GSE39582, GSE33113, and GSE17536 cohorts, focusing on pathways associated with SLC39A6 in CRC. The results indicated that the high-SLC39A6 group showed increased activation in pathways such as P53, TGFB, EMT and MAPK (Fig. 2A, Supplementary Fig. 1). To decipher the molecular functions and signaling pathways regulated by SLC39A6, we conducted GO enrichment analysis and KEGG enrichment analysis. The results indicated significant enrichment in various biological pathways, including extracellular matrix remodeling, integrin binding, and collagen binding (Supplementary Fig. 2 A-C). KEGG analysis revealed that ECM-receptor interaction, IL-17 signaling pathway, and Toll-like receptor signaling pathway were significantly enriched (Fig. 2B). GSVA analysis demonstrated that high-SLC39A6 was notably enriched in pathways such as JAK-STAT signaling pathway, Toll-Like Receptor signaling pathway, Positive regulation of extracellular matrix disassembly, Regulation of Complement Activation Alternative Pathway and Neutrophil Apoptotic Process (Fig. 2C, Supplementary Fig. 2D). GSEA analysis further showed significant enrichment in pathways like ECM receptor interaction, pathways in cancer, glycosaminoglycan biosynthesis chondroitin sulfate, and toll-like receptor signaling pathway (Fig. 2D). These findings suggest that the group with high SLC39A6 expression is significantly enriched in gene sets associated with tumor adhesion, and the tumor metastasis pathway. Based on these comprehensive enrichment analyses, high-SLC39A6, compared to low-SLC39A6, appears to have a more substantial impact on tumor progression, particularly in functions related to extracellular matrix remodeling.
Biological function of SLC39 A6 within tumor tissues of CRC. (A) Box plots for 2 groups show the comparisons of the expression levels of tumor-associated signaling pathways. (B) KEGG pathway analysis. (C) The Gene Set Variation Analysis (GSVA) was conducted to assess the signaling pathways associated with high-SLC39A6 compared to low-SLC39A6. (D) Representative Gene Set Enrichment Analysis (GSEA) plots.
Result 3 SLC39A6 exhibits spatial co-localization with tumor-associated pathways
Spatial transcriptomics not only provides higher resolution than bulk transcriptomics but also allows for the observation of spatial positional information. From bulk and single-cell transcriptomic data, we can only understand the expression intensity of genes and tumor pathways, but we are unclear about the location of gene expression and whether the expression positions of genes and pathways are consistent. Therefore, we used the SPATA package for spatial trajectory analysis to observe the spatial co-localization of SLC39A6 with tumor pathways. First, we used ssGSEA to score four tumor slides with the gene sets of 10 common tumor pathways. We divided all spots into SLC39A6 + spots and SLC39A6-spots based on the expression of SLC39A6. The results showed that the expression of all tumor pathways was higher in the SLC39A6 + spot group than in the SLC39A6-spot group, and correlation analysis indicated that SLC39A6 is positively correlated with tumor pathways (Fig. 3A-B), which is consistent with the previous findings in bulk transcriptomics. Next, we performed trajectory analysis to investigate whether SLC39 A6 exhibits spatial co-localization with tumor-associated signaling pathways. To quantitatively assess the spatial expression trends of these pathways relative to SLC39A6, we defined a directional vector (arrow) representing the gradient from high to low SLC39A6 expression regions. The plotted curves illustrate expression changes of pathway-associated genes along this vector, with spots closer to the arrowhead indicating progressive expression shifts. Results demonstrated that, across four tumor sections, all pathways except EMT displayed spatial distribution patterns concordant with SLC39A6 (Fig. 3C, Supplementary Fig. 3). These findings suggest that tumor regions with elevated SLC39A6 expression exhibit enhanced malignant features. Consequently, targeting SLC39A6 to eliminate tumor cells within these high-expression zones may achieve superior therapeutic efficacy by preferentially eradicating subpopulations with aggressive phenotypes.
SLC39A6 exhibits spatial co-localization with tumor-associated pathways. (A) Comparison of various pathways between two clusters in tumor slides. (B) Correlation analysis between SLC39A6 and tumor pathways. (C) Spatial plots show the expression of SLC39A6 and the ssGSEA scores of tumor pathways. The spatial scatter plot above illustrates their spatial distribution: deeper colors indicate higher expression levels. The line graph below displays expression changes of SLC39A6 and pathway scores along the direction of the arrow.*P < 0.05; **P < 0.01; ***P < 0.001.
Result 4 BRY812 suppresses the proliferation, migration and stemness of CRC in vitro
As SLC39A6 is a zinc ion transporter, BRY812 exerts its therapeutic effect by binding to SLC39A6 and releasing a small molecule drug. To eliminate the potential that the binding of SLC39A6 could impact zinc - ion transport and thereby modify the functions of tumor cells, we established a panel of monoclonal antibodies (mAbs). These mAbs are capable of binding to SLC39A6 yet do not carry any small - molecule cytotoxic agents. We assessed the IC50 values of BRY812 and mAb-SLC39A6 after 48 h of incubation (Fig. 4A-B). The results showed that the IC50 values of BRY812 for Caco-2 and HCT116 cells were 1733 nM and 3039 nM, respectively. The monoclonal antibody SLC39A6 had no significant effect on the proliferation of CRC cell lines (Fig. 4A-B). To further investigate the impact of BRY812 on the proliferation of CRC cells, we conducted CCK8 experiments, colony formation experiments, and EdU experiments. In the CCK8 experiment, based on the previous IC50 results, we set a concentration gradient (0µM, 0.5µM, 0.75µM, 1µM). The results showed that all three concentrations of BRY812 significantly inhibited the growth of CRC cells compared to the control group (Fig. 4C). Using the CCK8 concentration gradient for a preliminary colony formation experiment, we found that the colony formation rate was already very low at 0.5µM, and at 0.75µM and 1µM, colony formation was hardly observable. Therefore, we adjusted the concentration gradient and ultimately used 0.1µM, 0.2µM, and 0.5µM for the experiment. The results showed that as the concentration of BRY812 increased, the colony formation rate gradually decreased and there was a statistically significant difference compared to the control group. In contrast, no significant difference in colony formation rate was observed in the mAb group at the three different concentrations compared to the control group (Fig. 4D). In the EdU experiment, as the concentration of BRY812 increased, the proportion of EdU-positive cells in the two CRC cell lines gradually decreased, showing a statistically significant difference compared to the control group. However, in the mAb group, no significant difference in the proportion of EdU-positive cells was observed at the three concentrations compared to the control group (Fig. 4E, Supplementary Fig. 4). These results indicate that BRY812 inhibits the proliferative capacity of CRC cells, and the binding of SLC39A6 to mAb does not affect the proliferation of cancer cells.
BRY812 suppresses proliferation in CRC cell lines in vitro. (A) Cytotoxicity of BRY812 on CRC cell lines. (B) Cytotoxicity of mAb (SLC39A6 monoclonal antibody) on CRC cell lines. (C) Proliferation of Caco-2 and HCT116 cells, as measured by CCK-8 assays, upon exposure to a range of concentrations of BRY812 and SLC39A6 monoclonal antibody. (D) The effects of various concentrations of BRY812 and mAb on the colony-forming capability of Caco-2 and HCT116 cells were assessed through colony formation assays, followed by quantification. (E) EdU incorporation assay and quantification in Caco-2 and HCT116 cells treated with varying concentrations of BRY812. The results are derived from a minimum of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Result 5 BRY812 suppresses the migration and stemness of CRC in vitro
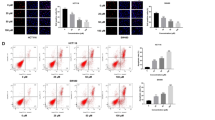
To investigate whether BRY812 would inhibit the stemness of CRC cells, we conducted limiting dilution assay and spheroid formation assay. The results indicated that BRY812 significantly inhibited the stemness of CRC cells. In the BRY812 group, no sphere formation was observed, whereas in the mAb and control groups, cancer cell sphere formation was visible, and there was no significant difference between these two groups (Fig. 5A-B). Furthermore, to explore whether BRY812 would inhibit the metastasis of tumor cells, we performed Transwell assays. The results showed that the number of Caco2 and HCT116 cells that migrated through the Transwell chambers was significantly different from the control group after the addition of BRY812 (Fig. 5C). In contrast, the addition of mAb did not affect the number of cells that migrated through the chambers. These findings suggest that BRY812 can impact the stemness and migration of CRC cells, while mAb does not exert an inhibitory effect.
SLC39A6-Targeting ADC drug BRY812 suppresses the Migration and Stemness of CRC in vitro. (A-B) The stemness of Caco-2 and HCT116 cells was evaluated through sphere formation assays (A) and in vitro limiting dilution assays (B) after treatment with different concentrations of BRY812 and SLC39 A6 monoclonal antibody. (C) Caco-2 and HCT116 cells were subjected to transwell migration assays upon treatment with BRY812 and SLC39A6 monoclonal antibody, with subsequent quantification of migratory activity. The results are derived from a minimum of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Result 6 mechanism of BRY812 killing CRC cells
To explore the potential killing mechanisms of BRY812, we performed RNA-seq on CRC cells from the BRY812 group and the control group. Specifically, for HCT116 and Caco2 cells, we sequenced a total of 12 samples, with three BRY812 samples and three control samples for each cell line. We conducted a differential analysis between the BRY812 group and the control group, identifying differentially expressed genes (P-value < 0.05). The analysis revealed 1,077 upregulated genes and 869 downregulated genes in the BRY812 group (Fig. 6A). Among the downregulated genes in the BRY812 treatment group, several were closely related to the progression of CRC (Fig. 6B). For instance, ADGRG6 has been reported to regulate CRC cell proliferation by mediating the expression of HDAC2 and GLI218. MC1R can reprogram metabolism to regulate the differentiation of Tregs, and its overexpression is associated with poor prognosis in CRC patients19. GRB7 is closely related to the resistance to MEK inhibitors in KRAS-mutant colon cancer and shows potential as a biomarker for predicting outcomes in various cancers20,21. This suggests that BRY812 may exert its killing effects by suppressing these genes. To explore the signaling pathways that may be downregulated by BRY812, we performed an enrichment analysis of the differentially expressed genes. We downloaded the gene set related to the cell death pathway (GO:0008219) for Gene Set Enrichment Analysis (GSEA). Among these, the upregulated pathways included the intrinsic apoptotic signaling pathway in response to DNA damage, apoptosis and its signaling pathways, and negative regulation of cell population proliferation (Supplementary Fig. 5 A). Conversely, the downregulated pathways involved biological processes related to cell projection organization and regulation of cell morphogenesis (Supplementary Fig. 5B). Subsequent KEGG enrichment analysis of differentially expressed genes demonstrated that the PI3 K-AKT pathway was the most significantly downregulated in the BRY812 group (Fig. 6C). We further validated this finding using Western blot assay, which showed a significant downregulation of the PI3 K/AKT pathway in both Caco2 and HCT116 cell lines in the BRY812 group (Fig. 6D). These results suggest that BRY812 may exert its killing function by downregulating the PI3 K/Akt pathway.
Furthermore, we conducted additional mechanistic and clinical explorations on the downregulated gene set in the BRY812-treated group. To investigate other potential regulatory mechanisms, we analyzed the correlation between the BRY812-downregulated gene set and common oncogenic signaling pathways. Single-cell and spatial transcriptomic analyses revealed positive associations between these downregulated genes and multiple pathways beyond AKT, like MAPK and EMT signaling (Supplementary Fig. 6). Using ssGSEA on the TCGA-COAD dataset, we scored the top 50 most significantly downregulated genes in the BRY812 group and found that higher scores correlated with poorer patient prognosis (Fig. 6E), suggesting that reduced expression of these genes is linked to adverse clinical outcomes. Additionally, pan-cancer analysis demonstrated elevated expression scores of these genes in tumor tissues across CRC, gastric cancer, hepatocellular carcinoma, breast cancer, and 17 other TCGA cancer types (Fig. 6F, Supplementary Fig. 7). These results indicate that BRY812-downregulated genes are broadly implicated in tumor progression, highlighting their potential as pan-cancer therapeutic targets.
Mechanism of BRY812 killing CRC cells. (A) The volcano plot shows the differential genes. (B) Heatmap shows differentially expressed genes. (C) KEGG pathway analysis of down-regulated genes in BRY812 group. (D) Expression of pAKT and AKT in BRY812 group and control group at protein level. (E) Kaplan–Meier curve result in TCGA-COAD. (F) Geneset score expression differences in pan-cancer.
Discussion
This study is the first to investigate the cytotoxic effects and potential mechanisms of the ADC drug BRY812, which targets SLC39A6, in CRC. We initially examined the expression characteristics of SLC39A6 in CRC using bulk transcriptomics, single-cell transcriptomics, and spatial transcriptomics data, and found that it is highly expressed in tumor tissues. Through various enrichment analysis methods, we discovered that multiple tumor-associated pathways are more active in patients with high SLC39A6 expression. Furthermore, in spatial transcriptomics, we identified spatial co-localization of SLC39A6 with tumor-associated pathways through trajectory analysis. After confirming SLC39 A6 as a promising therapeutic target, we explored the in vitro cytotoxic effects of BRY812 in two CRC cell lines, Caco2 and HCT116. We further delved into the in vitro cytotoxic mechanisms of BRY812 through RNA-Seq and Western blot experiments. This research provides valuable references for the treatment of CRC.
SLC39A6, also known as LIV-1, a member of the solute carrier family 39 (SLC39A), plays a pivotal role in maintaining intracellular zinc ion homeostasis, which is essential for cell growth and differentiation22,23,24. Studies have indicated that the expression level of SLC39A6 is upregulated in various types of cancer23, including breast, liver, and esophageal cancer, with high expression levels closely associated with enhanced tumor cell proliferation, migration, and invasion capabilities8,25. Unno J et al.26 demonstrated the involvement of LIV-1 in the epithelial-mesenchymal transition (EMT) process in human pancreatic cancer cells, revealing its potential role in promoting the invasive phenotype of pancreatic cancer cells. Additionally, the expression variation of LIV-1 may affect the sensitivity of tumor cells to mitotic toxic drugs27, thereby influencing tumor progression and deterioration. In CRC, the expression level of SLC39A6 is closely related to the immune activity and metabolic characteristics in the tumor microenvironment, potentially modulating the tumor immune microenvironment by affecting the metabolic status of immune cells. Barresi and colleagues28, through transcriptome sequencing, showed that the upregulation of several specific ZIP transporters, including SLC39A6, in CRC may help meet the increased demand for zinc in cancer cells.
Through multiple public databases, we found that SLC39A6 is overexpressed in tumor tissues of CRC. Using various enrichment analysis methods, we observed the activation of tumor proliferation and migration-related pathways in patients with high SLC39A6 expression. Spatial transcriptomics (ST) provides a comprehensive molecular map of transcriptional activity in whole tissue sections. By combining quantitative sequencing data with histological features, it allows for precise analysis of mRNA expression at the cellular level. This technology enables us to understand the expression patterns of different genes in specific regions of tissues or organs, identify the spatial distribution of gene expression in tumor tissues, and thus gain insights into the spatial heterogeneity of tumor development and progression. Additionally, it helps to determine the specific types of genes, proteins, or cells and accurately locate them within the lesion tissue29,30,31. Therefore, through spatial SPATA trajectory analysis, we observed spatial co-localization of SLC39A6 with tumor-associated pathways in four tumor tissue spatial transcriptomic sections. These results indicate that the spatial location of SLC39A6 expression is associated with high activity of tumor-related pathways. In summary, we found that SLC39A6 is overexpressed in CRC tumor tissues and is closely related to tumor-associated pathways, making it a suitable therapeutic target. Consequently, we focused on the ADC drug BRY812 targeting SLC39A6 to explore its potential as a treatment for CRC.
In the therapeutic landscape of CRC, a multifaceted approach is typically adopted, encompassing surgery, chemotherapy, radiation therapy, targeted treatments, and immunotherapy. Within the realm of targeted immunotherapy32,33, monoclonal antibodies such as anti-EGFR agents cetuximab and panitumumab33,34,35, along with the anti-VEGF bevacizumab36, have become mainstays in clinical practice. Notably, the advent of ADCs has heralded a new era in the treatment of CRC, demonstrating significant potential. These precision medicine tools are designed to deliver cytotoxic payloads directly to cancer cells, thereby minimizing damage to healthy cells and enhancing the therapy’s selectivity while reducing side effects. The efficacy of ADCs lies in their ability to harness the targeting capability of antibodies to specifically reach and impact tumor cells. Notably, T-DXd37, ADC targeting HER2, has emerged as a promising therapeutic agent. Results from the DESTINY-CRC01 trial demonstrated satisfactory antitumor activity in HER2-positive metastatic colorectal cancer, with an objective response rate (ORR) of 45.3% in T-DXd-treated patients, underscoring its robust efficacy38. These findings provide a novel therapeutic option for this molecular subtype of CRC39.Currently, several ADCs targeting alternative antigens are under clinical development for CRC, including those directed at TROP2 and CEACAM5. TROP2, highly expressed in CRC, is targeted by sacituzumab govitecan (IMMU-132), an ADC approved for triple-negative breast cancer that has shown potential in CRC. Early-phase trials indicate modest antitumor activity in refractory CRC patients40. Similarly, CEACAM5, a cell surface antigen overexpressed in CRC, is the target of SAR408701. Phase I/II trials revealed favorable tolerability and preliminary efficacy of SAR408701 in metastatic CRC patients with CEACAM5-high tumors41. Despite these advances, further research is needed to optimize efficacy and mitigate toxicity, including the exploration of novel targets, improved linker technologies, and combinatorial therapeutic strategies. In this study, we identified SLC39 A6 as a potential therapeutic target for CRC. Patients with SLC39A6-high tumors represent a candidate population for BRY812 therapy. Preclinical in vitro experiments demonstrated significant BRY812-mediated cytotoxicity against CRC cells, providing robust rationale for its clinical evaluation in CRC. Although direct efficacy comparisons between BRY812 and existing ADCs are currently precluded by the absence of published clinical trial data for BRY812, its innovative design—featuring a stable linker and optimized conjugation platform (CysLink™)—suggests promising therapeutic potential. The enhanced tumor-selective payload delivery and reduced off-target toxicity observed in preclinical models further support its clinical translatability.
Upon binding to colorectal cancer cells, BRY812 releases monomethyl auristatin E, which induces irreversible microtubule disruption and cell cycle arrest, driving cells into an apoptosis-dominant state. Our results show that significantly upregulated genes in the BRY812-treated group were enriched in apoptotic pathways. According to published reports, mitochondrial dysfunction during apoptosis triggers a reactive oxygen species (ROS) burst42, and ROS accumulation has been shown to inhibit the activity of the PI3 K/AKT pathway43. Thus, we hypothesize that BRY812-induced apoptosis leads to ROS accumulation, which suppresses PI3 K/AKT pathway activity. Our findings also demonstrate that the AKT signaling pathway was significantly suppressed in the BRY812 treatment group. Interestingly, studies indicate that the PI3 K/AKT pathway dampens the apoptotic process by inhibiting the expression and function of pro-apoptotic proteins. AKT phosphorylates pro-apoptotic factors Bad and procaspase-9, blocking their pro-apoptotic activity, and simultaneously inhibits the expression of the pro-apoptotic factor Fas ligand, further reducing apoptotic signals44. Therefore, we propose that BRY812-mediated inhibition of the AKT pathway may enhance antitumor effects through a dual mechanism: directly relieving AKT-mediated suppression of apoptosis-related proteins, and leveraging ROS generated during apoptosis to amplify PI3 K/AKT pathway inhibition. This establishes a “positive feedback loop” where activated apoptotic signals and enhanced AKT inhibition reinforce each other, culminating in the killing of tumor cells.
This study has several limitations. The number of publicly available CRC datasets is limited, and the sample size in some dataset is relatively small, which may not fully represent the entire CRC patient population. Although we confirmed the high expression of SLC39A6 in colorectal tissues across multiple databases, the absence of large-scale clinical validation via immunohistochemistry (IHC) or quantitative real-time PCR (qPCR) in clinical tissue samples hinders precise analysis of SLC39A6 expression differences across molecular subtypes of CRC. This information is critical for guiding the application of BRY812 in precision cancer therapy. In vitro experiments demonstrated that the SLC39 A6-targeting ADC drug BRY812 inhibits the growth of CRC cells, indicating its potential therapeutic efficacy. However, in vitro models inherently fail to recapitulate the complexity of the in vivo tumor microenvironment. Thus, in vivo studies are necessary to further investigate its therapeutic effects. Safety is a key consideration in therapy, and animal studies are required to assess the toxicity of BRY812 in vivo. Future research will utilize humanized patient-derived xenograft (PDX) models or organoids—models that closely mimic the native tumor microenvironment—to systematically evaluate drug efficacy and safety, providing a more robust experimental basis for the clinical translation of BRY812 in CRC precision medicine. Additionally, given the limitations of current ADC monotherapy, we plan to explore combination strategies of BRY812 with other targeted agents and evaluate its therapeutic potential in drug-resistant models, aiming to fully exploit its clinical utility.
Conclusion
In summary, this study has identified SLC39A6 as a therapeutic target for CRC, and its ADC drug BRY812 has shown significant in vitro cytotoxic effects on CRC cells. The findings of this study provide new strategies and potential targets for precision medicine in CRC, offering hope for improved treatment outcomes for patients with CRC.
Methods
Data source
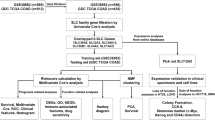
To explore the role of SLC39A6 in CRC, this study conducted a multi-omics analysis leveraging multiple public CRC databases. For scRNA-seq analysis, the GSE144735 database and GSE132257 database were utilized. Bulk RNA-seq data was sourced from GSE39582, GSE33113, and GSE17536. TCGA data was sourced from UCSC Xena (https://xenabrowser.net/datapages/). Spatial transcriptomic analysis was conducted on three slides obtained from GSE236697 and the 10x Genomics official website: Slide 1 (https://www.10xgenomics.com/datasets/human-colorectal-cancer-11-mm-capture-area-ffpe-2-standard), Slide 2 (https://www.10xgenomics.com/datasets/human-colorectal-cancer-whole-transcriptome-analysis-1-standard-1–2-0), and Slide 3 (https://www.10xgenomics.com/datasets/human-intestine-cancer-1-standard.). In order to explore the killing mechanism of BRY812 in vitro, we collected RNA from two cell lines (Caco-2 and HCT116) treated and untreated with BRY812 for eukaryotic reference transcriptome sequencing.
Functional enrichment analysis
To elucidate disparities in gene expression profiles among the characterized subgroups, we employed the DESeq2 R package for differentially expressed genes (DEGs) analysis, applying rigorous thresholds of an adjusted p-value below 0.01 and an absolute log2 fold change exceeding 245[,46. Following the DEGs’ identification, a thorough functional analysis was conducted, including Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Set Enrichment Analysis (GSEA).
Gene set enrichment analysis (GSEA)
To identify the hallmark effect gene sets associated with SLC39A6 mRNA expression in the TCGA-COAD. GSEA was performed using GSEA software (v. 4.1.0; http://www.gsea-msigdb.org/gsea/login.jsp). For enriched genomes, pathways with false discovery rate values < 0.25 and P < 0.05 were considered after 1,000 permutations as significantly enriched pathways.
Spatial trajectories
Employing the SPATA2 software package, version 2.0.4, we conducted a spatial trajectory analysis on tissue slides47,48. Utilizing the addSpatialTrajectory function, we delineated a custom spatial trajectory that encapsulates the spatial variation in gene expression. The visualization of this trajectory was achieved through the plotTrajectoryLineplot and plotTrajectoryRidgeplot functions, which provided graphical representations of gene expression patterns along the defined path. For a detailed methodology, tutorial code is accessible at the provided URL: https://themilolab.github.io/SPATA2/articles/spata-v2-spatial-trajectories.html.
Cell lines culture
Caco-2 cells and HCT116 cells were provided by ATCC. Caco-2 cells and HCT116 cells were maintained in high-glucose DMEM supplemented with fetal bovine serum (FBS, 10%, PAN; ST30-3302), penicillin–streptomycin–gentamicin (1%, Solarbio; P1410), and cultured in 95% air and 5% CO2 at 37 °C.
Preparation of mAb solution
A monoclonal antibody specific for SLC39A6 was generated using hybridoma technology. This mAb specifically binds to SLC39A6 and enters cells via endocytosis but does not release toxins or elicit antibody-dependent cell-mediated cytotoxicity (ADCC) effects. Since binding to SLC39A6 may interfere with zinc ion transport and potentially impact tumor cell growth and metastasis, this mAb was used as a control in this study to evaluate its biological effects on tumor cells.
IC50 assay
Cells were digested, counted, and seeded into 96-well plates at 5,000 cells/well (6 replicates per group), then incubated at 37 °C with 5% CO₂ until adherence. BRY812 and mAb solutions at concentrations of 0, 50, 100, 200, 300, 500, 1,000, 2,000, 3,000, and 5,000 nM were added to corresponding wells, followed by 24/48-hour incubation. After discarding the medium, 100 µL of CCK8 working solution (CCK8 reagent: medium = 1:9) was added to each well. Following a 1-hour incubation, OD values were measured using a microplate reader. Cell proliferation curves were plotted, and IC50 values were calculated to assess drug cytotoxicity.
Cell counting kit-8 (CCK-8) assay
According to the previous IC50 results, the concentration gradients of BRY812 were set at 0, 0.5, 0.75, and 1 µM, and added to the 96-well plates (including blank and cell controls). The absorbance at 450 nm was immediately measured after drug addition (Day 0). The CCK8 working solution was prepared by mixing 1 mL of CCK8 stock solution with 9 mL of serum-free medium (protected from light). The original medium was discarded every 24 h, 100 µL of the working solution was added, and the OD value was measured after incubation for 1 h. The background value of the blank well was subtracted. Continuous measurements were taken for 5 days (Day 0 to Day 4). The growth curve was plotted using statistical software, the proliferation inhibition rate was calculated, and the inhibitory effect of BRY812 on Caco2/HCT116 cells was evaluated.
Plate colony
We seeded Caco-2 and HCT116 cells into 6-well plates at a density of 700 cells per well, and then added BRY812 and mAb solutions at concentrations of 0.1, 0.2, and 0.5 µM, respectively. The culture medium was replenished every three days to support cell growth, which was continued for a period of 8 to 10 days until the colonies reached an appropriate size for assessment. Afterward, the cells were washed with precooled phosphate-buffered saline (PBS) to remove any residual culture medium. They were then fixed using 1 mL of 4% paraformaldehyde for 30 min to preserve their morphology. Following fixation, the cells were stained with 1 mL of crystal violet for 30 min to visualize the colonies. Excess stain was removed with a final rinse using PBS. Subsequently, the stained cell colonies were photographed using a camera, and the images were analyzed to enumerate the colonies using ImageJ software version 1.52v, a widely utilized program for scientific image analysis.
Transwell assay
The concentrations of both BRY812 and mAb groups were set at 2 µM. Post-centrifugation of the digested cell suspension, the supernatant was carefully aspirated, leaving behind Caco-2 and HCT116 cells. These cells were subsequently resuspended in a serum-free medium. A volume of 200 µl, containing approximately 1 × 10^5 cells, was then aliquoted into the upper compartment of a transwell chamber. Subsequently, the lower compartment was filled with 500 µl of culture medium supplemented with 20% Fetal Bovine Serum (FBS). After a 24-hour incubation period under standard culture conditions, the migratory activity of the cells was assessed. The number of cells that had migrated to the selected area was enumerated using ImageJ software, a powerful tool for image analysis.
EdU assay
The concentration gradients for both the BRY812 and mAb groups were set at 0.1, 0.2, and 0.5 µM. An EdU assay, facilitated by a 5-ethynyl-20-deoxyuridine kit from Beyotime, was utilized to evaluate the impact of BRY812 on the proliferative capabilities of hepatocellular carcinoma cells. Post a 48-hour incubation of BRY812, the monoclonal antibody of SLC39A6, cells were subjected to the EdU protocol, strictly adhering to the manufacturer’s guidelines.
Limiting dilution assay
The concentrations of both BRY812 and mAb groups were set at 0.2 µM. Serial dilutions of Caco-2 and HCT116 cells, ranging from 64 to 2 cells per well, were meticulously seeded in eight replicates within 96-well ultra-low-attachment culture plates. The formation of spheres in each well was meticulously observed to estimate the frequency of cancer stem cells.
Spheroid formation assay
The concentrations of both BRY812 and mAb groups were set at 0.2 µM. Cells were inoculated into 96-well ultra-low attachment culture plates provided by Corning Incorporated Life Sciences, at a density of 300 cells per well. The culture medium consisted of DMEM/F12 from Gibco, enriched with 1% Fetal Bovine Serum (FBS), along with 20 ng/mL basic Fibroblast Growth Factor (bFGF) and 20 ng/mL Epidermal Growth Factor (EGF). This medium formulation supported the cells for a period of seven days, during which the formation of spheroids was induced and subsequently quantified. Images of the spheroids were captured to provide a visual representation of the culture outcomes.
Statistical analyses
This study used R software version 4.3.0 for statistical analysis. When dealing with quantitative data, we used the Student’s t test to evaluate the statistical significance of variables that were normally distributed; for variables that were not normally distributed, the Wilcoxon rank sum test was used for analysis. When it comes to comparisons between two or more groups, we selected the non-parametric Kruskal-Wallis test and the parametric one-way analysis of variance (ANOVA) as statistical methods based on the distribution of the data. For the analysis of contingency table data, we selected the chi-square test and Fisher’s exact test as appropriate statistical tools based on the specific grouping conditions and sample size. All statistical comparisons were performed as two-sided tests, and the significance level (alpha) was set at 0.05.
Data availability
To explore the role of SLC39 A6 in CRC, this study conducted a multi-omics analysis leveraging multiple public CRC databases. For scRNA-seq analysis, the GSE144735 database and GSE132257 database were utilized. Bulk RNA-seq data was sourced from GSE39582, GSE33113, and GSE17536. TCGA data was sourced from UCSC Xena (https://xenabrowser.net/datapages/). Spatial transcriptomic analysis was conducted on three slides obtained from GSE236697 and the 10x Genomics official website: Slide 1 (https://www.10xgenomics.com/datasets/human-colorectal-cancer-11-mm-capture-area-ffpe-2-standard), Slide 2 (https://www.10xgenomics.com/datasets/human-colorectal-cancer-whole-transcriptome-analysis-1-standard-1-2-0), and Slide 3 (https://www.10xgenomics.com/datasets/human-intestine-cancer-1-standard). In order to explore the killing mechanism of BRY812 in vitro, we collected RNA from two cell lines (caco2 and HCT116) treated and untreated with BRY812 for eukaryotic reference transcriptome sequencing.
Abbreviations
- ANOVA:
-
Analysis of variance
- CRC:
-
Colorectal cancer
- CPI:
-
Clustering prediction index
- DEGs:
-
Differentially expressed genes
- TCGA:
-
The cancer genome atlas
- OS:
-
Overall survival
- IC50 :
-
Half-maximal inhibitory concentration
- GEO:
-
Gene expression omnibus
- PCA:
-
Principal component analysis
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- HR:
-
Hazard ratio
- KM:
-
Kaplan-meier
- GSEA:
-
Gene set enrichment analysis
- GSVA:
-
Gene set variation analysis
- scRNA-seq:
-
Single-cell RNA sequencing
- ST:
-
Spatial transcriptomics
- UMAP:
-
Uniform manifold approximation and projection
- TKI:
-
Tyrosine kinase inhibitor
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Shastry, M. et al. Rise of Antibody-Drug conjugates: the present and future. Am. Soc. Clin. Oncol. Educ. Book. 43, e390094. https://doi.org/10.1200/EDBK_390094 (2023).
Dumontet, C., Reichert, J. M., Senter, P. D., Lambert, J. M. & Beck, A. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 22, 641–661. https://doi.org/10.1038/s41573-023-00709-2 (2023).
Phuna, Z. X., Kumar, P. A., Haroun, E., Dutta, D. & Lim, S. H. Antibody-drug conjugates: principles and opportunities. Life Sci. 347, 122676. https://doi.org/10.1016/j.lfs.2024.122676 (2024).
Xi, M. et al. Antibody-drug conjugates for targeted cancer therapy: recent advances in potential payloads. Eur. J. Med. Chem. 276, 116709. https://doi.org/10.1016/j.ejmech.2024.116709 (2024).
Liu, H. et al. Synergistic antitumor activity between HER2 antibody-drug conjugate and chemotherapy for treating advanced colorectal cancer. Cell. Death Dis. 15, 187. https://doi.org/10.1038/s41419-024-06572-2 (2024).
Zhang, T., Sui, D. & Hu, J. Structural insights of ZIP4 extracellular domain critical for optimal zinc transport. Nat. Commun. 7, 11979. https://doi.org/10.1038/ncomms11979 (2016).
Cheng, X. et al. Solute carrier family 39 member 6 gene promotes aggressiveness of esophageal carcinoma cells by increasing intracellular levels of zinc, activating phosphatidylinositol 3-Kinase signaling, and Up-regulating genes that regulate metastasis. Gastroenterology 152, 1985–1997. https://doi.org/10.1053/j.gastro.2017.02.006 (2017). e1912.
Taylor, K. M., Hiscox, S. & Nicholson, R. I. Zinc transporter LIV-1: a link between cellular development and cancer progression. Trends Endocrinol. Metab. 15, 461–463. https://doi.org/10.1016/j.tem.2004.10.003 (2004).
Cui, X. B. et al. SLC39A6: a potential target for diagnosis and therapy of esophageal carcinoma. J. Transl Med. 13, 321. https://doi.org/10.1186/s12967-015-0681-z (2015).
Taylor, K. M. et al. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol. Med. 13, 396–406. https://doi.org/10.2119/2007-00040.Taylor (2007).
Cigliano, A., Strain, A. J. & Cadamuro, M. Signaling and molecular networks related to development and inflammation involved in CCA initiation and progression. Hepatoma Res. 9, 15 (2023).
Cadamuro, M., Fabris, L., Zhang, X. & Strazzabosco, M. Tumor microenvironment and immunology of cholangiocarcinoma. Hepatoma Res. 8, 11 (2022).
Beck, A., Goetsch, L., Dumontet, C. & Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 16, 315–337. https://doi.org/10.1038/nrd.2016.268 (2017).
Tarantino, P. et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. Cancer J. Clin. 72, 165–182. https://doi.org/10.3322/caac.21705 (2022).
Sussman, D. et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol. Cancer Ther. 13, 2991–3000. https://doi.org/10.1158/1535-7163.Mct-13-0896 (2014).
Rizzo, A., Cusmai, A., Acquafredda, S., Rinaldi, L. & Palmiotti, G. Ladiratuzumab Vedotin for metastatic triple negative cancer: preliminary results, key challenges, and clinical potential. Expert Opin. Investig. Drugs. 31, 495–498. https://doi.org/10.1080/13543784.2022.2042252 (2022).
Cui, H. et al. GPR126 regulates colorectal cancer cell proliferation by mediating HDAC2 and GLI2 expression. Cancer Sci. 112, 1798–1810. https://doi.org/10.1111/cas.14868 (2021).
Zhu, S. et al. MC1R regulates T regulatory cell differentiation through metabolic reprogramming to promote colon cancer. Int. Immunopharmacol. 138, 112546. https://doi.org/10.1016/j.intimp.2024.112546 (2024).
Yu, C. et al. Genome-wide CRISPR-cas9 knockout screening identifies GRB7 as a driver for MEK inhibitor resistance in KRAS mutant colon cancer. Oncogene 41, 191–203. https://doi.org/10.1038/s41388-021-02077-w (2022).
Attaelmanan, A. M. et al. A comprehensive Pan-Cancer analysis reveals GRB7 as a potential diagnostic and prognostic biomarker. Cureus 16, e74907. https://doi.org/10.7759/cureus.74907 (2024).
Taylor, K. M. The LIV-1 subfamily of zinc transporters: from origins to present day discoveries. Int. J. Mol. Sci. 24 https://doi.org/10.3390/ijms24021255 (2023).
Braso-Maristany, F. et al. Patritumab Deruxtecan in HER2-negative breast cancer: part B results of the window-of-opportunity SOLTI-1805 TOT-HER3 trial and biological determinants of early response. Nat. Commun. 15, 5826. https://doi.org/10.1038/s41467-024-50056-y (2024).
Wu, C. et al. Genome-wide association study identifies common variants in SLC39A6 associated with length of survival in esophageal squamous-cell carcinoma. Nat. Genet. 45, 632–638. https://doi.org/10.1038/ng.2638 (2013).
Gao, J. et al. Involvement of SLC39A6 in gastric adenocarcinoma and correlation of the SLC39A6 polymorphism rs1050631 with clinical outcomes after resection. BMC Cancer. 19, 1069. https://doi.org/10.1186/s12885-019-6222-z (2019).
Unno, J. et al. LIV-1 enhances the aggressive phenotype through the induction of epithelial to mesenchymal transition in human pancreatic carcinoma cells. Int. J. Oncol. 35, 813–821. https://doi.org/10.3892/ijo_00000394 (2009).
Chen, P. et al. The LIV-1-GRPEL1 axis adjusts cell fate during anti-mitotic agent-damaged mitosis. EBioMedicine 49, 26–39. https://doi.org/10.1016/j.ebiom.2019.09.054 (2019).
Barresi, V. et al. Transcriptome analysis reveals an altered expression profile of zinc transporters in colorectal cancer. J. Cell. Biochem. 119, 9707–9719. https://doi.org/10.1002/jcb.27285 (2018).
Valihrach, L., Zucha, D., Abaffy, P. & Kubista, M. A practical guide to Spatial transcriptomics. Mol. Aspects Med. 97, 101276. https://doi.org/10.1016/j.mam.2024.101276 (2024).
Larsson, L., Bergenstråhle, L., He, M., Andrusivova, Z. & Lundeberg, J. SnapShot: Spatial transcriptomics. Cell 185, 2840–2840e2841. https://doi.org/10.1016/j.cell.2022.06.002 (2022).
Zhang, L. et al. Clinical and translational values of Spatial transcriptomics. Signal. Transduct. Target. Therapy. 7, 111. https://doi.org/10.1038/s41392-022-00960-w (2022).
Underwood, P. W., Ruff, S. M. & Pawlik, T. M. Update on targeted therapy and immunotherapy for metastatic colorectal Cancer. Cells 13 https://doi.org/10.3390/cells13030245 (2024).
Kamrani, A. et al. New immunotherapy approaches for colorectal cancer: focusing on CAR-T cell, bite, and oncolytic viruses. Cell. Commun. Signal. 22, 56. https://doi.org/10.1186/s12964-023-01430-8 (2024).
Desai, J. et al. Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: a phase 1b trial. Nat. Med. 30, 271–278. https://doi.org/10.1038/s41591-023-02696-8 (2024).
Biller, L. H. & Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 325, 669–685. https://doi.org/10.1001/jama.2021.0106 (2021).
Van Cutsem, E. et al. ANCHOR CRC: results from a Single-Arm, phase II study of Encorafenib plus binimetinib and cetuximab in previously untreated BRAF(V600E)-Mutant metastatic colorectal Cancer. J. Clin. Oncol. 41, 2628–2637. https://doi.org/10.1200/JCO.22.01693 (2023).
Wang, C. & Fakih, M. Response to trastuzumab and lapatinib in a metastatic colorectal Cancer harboring HER2 amplification and HER2 S310F mutation. J. Natl. Compr. Canc Netw. 19, 670–674. https://doi.org/10.6004/jnccn.2021.7023 (2021).
Yoshino, T. et al. Final results of DESTINY-CRC01 investigating trastuzumab Deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nat. Commun. 14, 3332. https://doi.org/10.1038/s41467-023-38032-4 (2023).
Sartore-Bianchi, A. et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 17, 738–746. https://doi.org/10.1016/s1470-2045(16)00150-9 (2016).
Morgenstern-Kaplan, D. et al. Genomic, immunologic, and prognostic associations of TROP2 (TACSTD2) expression in solid tumors. Oncologist 29, e1480–e1491. https://doi.org/10.1093/oncolo/oyae168 (2024).
Tabernero, J. et al. Tusamitamab Ravtansine in patients with advanced solid tumors: phase I study of safety, pharmacokinetics, and antitumor activity using alternative dosing regimens. Cancer Res. Commun. 3, 1662–1671. https://doi.org/10.1158/2767-9764.Crc-23-0284 (2023).
Yang, J. et al. Prevention of apoptosis by Bcl-2: release of cytochrome C from mitochondria blocked. Science 275, 1129–1132. https://doi.org/10.1126/science.275.5303.1129 (1997).
Ramani, M. et al. Supraphysiological levels of oxygen exposure during the neonatal period impairs signaling pathways required for learning and memory. Sci. Rep. 8, 9914. https://doi.org/10.1038/s41598-018-28220-4 (2018).
Datta, S. R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241. https://doi.org/10.1016/s0092-8674(00)80405-5 (1997).
Love, M. I., Huber, W. & Anders, S. Moderated Estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. https://doi.org/10.1186/s13059-014-0550-8 (2014).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287. https://doi.org/10.1089/omi.2011.0118 (2012).
Kleino, I., Frolovaite, P., Suomi, T. & Elo, L. L. Computational solutions for Spatial transcriptomics. Comput. Struct. Biotechnol. J. 20, 4870–4884. https://doi.org/10.1016/j.csbj.2022.08.043 (2022).
Ren, Y. et al. Spatial transcriptomics reveals niche-specific enrichment and vulnerabilities of radial glial stem-like cells in malignant gliomas. Nat. Commun. 14, 1028. https://doi.org/10.1038/s41467-023-36707-6 (2023).
Acknowledgements
We gratefully acknowledge Zhejiang Bioray Biopharmaceutical Co., Ltd. for providing the SLC39A6-targeting antibody-drug conjugate BRY812, which was instrumental in investigating novel therapeutic strategies for colorectal cancer.
Funding
This study is supported by Natural Science Foundation of Shanghai Municipality(16ZR1400800) and National Natural Science Foundation of China (82473010).
Author information
Authors and Affiliations
Contributions
Xianglin Liu and Wenqiang Liu completed the experiment, programmed the computer code, and reviewed the published work. Yuting Wu and Yichuan Wang wrote the initial draft. Yangyang Li and Qiangliang Jiang revised the manuscript. Liqiang Hao and Hengyu Li managed the responsibility for the research activity planning and execution and provided the study materials. All authors have read the final manuscript and approved it for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. All authors have read and approved the final manuscript and consent to its submission. There are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Ethics approval and consent to participate
The conducted research is not related to either human or animals use
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, X., Liu, W., Wu, Y. et al. Investigation of the cytotoxic effects and mechanisms of the SLC39A6-targeting ADC drug BRY812 in CRC. Sci Rep 15, 18275 (2025). https://doi.org/10.1038/s41598-025-03713-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03713-1