Abstract
Cognitive decline in older adults, often manifested as reduced processing speed and memory, represents a significant challenge to daily functioning and overall quality of life (QoL). Physical inactivity compounds these issues, whereas increased physical activity can lead to healthier cognitive aging. This study examines the impact of wearable sensor-based interactive cognitive-motor training (ICMT) on cognitive and physical performance in older adults. In a single-blind, randomized, parallel-group trial, 36 community-dwelling older adults aged 65 and older were divided into an ICMT and a cognitive training (CT) group. For six weeks, the ICMT group participated in cognitive training using the developed equipment, while the CT group participated in cognitive training using a seated tablet. The ICMT group demonstrated a significant improvement in cognitive function, increasing 1.94 ± 2.98 score (8.60% increase, p < 0.05), and enhanced balance and strength (p < 0.05). Hemodynamic responses of the prefrontal cortex showed a decreasing trend between groups. The ICMT group also demonstrated improved endurance in the 6-minute walk test, covering 18.00 ± 31.0 m more (4.65% farther) than the CT group (p < 0.05). These findings suggest that wearable sensor-based ICMT may offer a practical and effective method to improve cognitive and physical function in older adults, enhancing daily living activities and QoL. Moreover, the wearable sensor-based ICMT offers ease of use, portability, and the ability to conduct training in various settings without requiring specialized facilities, making it a promising method for older adults.
Similar content being viewed by others
Introduction
Cognitive declines in older adults, affecting daily activities and quality of life, can be mitigated by non-pharmacological interventions like increased physical activity1,2,3. During the preclinical stages of dementia, cognitive training has emerged as a preventive activity. Previous studies have focused primarily on single cognitive tasks or integrating them with physical activities in dual-task training, which has proven effective in improving cognition. Cognitive-motor training have improved cognitive and physical performance in older adults4,5, mild cognitive impairment (MCI)6,7, and individuals with neurological diseases various studies have demonstrated the effectiveness of cognitive and motor training for cognitive improvement. And it has been effective in improving gait, balance, muscle strength, and cognitive performance by incorporating the latest technologies6,8,9,10. Additionally, Kuo, et al.11 found that in older adults with mild cognitive impairment, dual cognitive tasks positively influenced the relationship between walking improvements and brain activation, particularly increasing activity in frontal and motor-related areas of the brain. Cognitive-motor training was associated with decreased activation in the prefrontal cortex (PFC), which Erickson, et al.12 interpreted as a cortical response to processing multiple tasks simultaneously. This decrease in PFC hemodynamic response, along with changes in neural networks, suggests increased neural efficiency even after learning is complete13,14, confirming the positive effects of motor dual-task training. Herold, et al.15 suggest that applying exercise and cognition simultaneously is a more promising and time-efficient approach. Both simultaneous and sequential applications of cognitive-motor training have been shown to produce reversible changes in the brain, resulting in improved cognitive function16,17.
Most previous studies have applied motor tasks at moderate or high intensities using a stationary bike18, treadmill19, or combined exercise training (stepping, walking) with cognitive tasks4. The use of stationary exercise equipment5 or the necessity for virtual reality setups9 can impose spatial and economic constraints that limit the accessibility of cognitive-motor training for the older adult population. Moreover, conventional cognitive-motor training methods typically require supervision or support, which increases the need for resources and personnel.
O’Neil-Pirozzi, et al.20 suggested that identifying motivational facilitators and barriers is critical for prescribing personalized exercise regimens that are maximally motivating, thereby optimizing adherence and efficacy. Interventions to improve cognition in older adults require active and sustained participation in cognitive programs and low dropout rates to achieve long-term effects. Hence, an important factor for adherence is the appropriateness of the exercise method, which should be a combination of motivation, enjoyment, effectiveness, and intensity21,22.
However, despite their many advantages, there are limitations to translating existing research into clinical practice. In the clinical setting, a cognitive-motor training is needed that can maximize physical activity while providing cognitive stimulation to improve cognition in the older adults, and a program that minimizes location constraints and ensures motivation and continuity of training. Given these challenges, wearable technology may offer a simple solution for cognitive-motor training. Wearable sensors can provide interactive feedback to support motor learning23 and are portable, which increases the effectiveness of training by adapting it to individual needs. By leveraging the ubiquity and adaptability of wearable devices, we can not only rival the effectiveness of conventional cognitive-motor training but also offer greater accessibility and user autonomy. To serve an aging population effectively and affordably, a system that is cost-effective, portable, and engaging is essential to promote program adherence among older adults. This study hypothesizes that wearable sensor-based interactive cognitive-motor training (ICMT) will be an effective and affordable intervention to improve cognitive function and enhance muscle strength in community-dwelling older adults. The purpose is to investigate the effects of interactive cognitive and motor training using wearable sensors on cognition, prefrontal cortex activity, muscle performance, and balance in community-dwelling older adults.
Materials and methods
Study design, randomization, and ethical approval
This study was a single-blind (assessor blind), randomized, two-arm, parallel clinical trial. Participants were randomly allocated to two groups between the interactive cognitive-motor training group and cognitive training group. Randomization was performed using randomization software (version 2.0, University of Isfahan, Iran)24. The study was approved by the Institutional Review Board of Samhyook University (SYU 2023-06-020-001) and registered in the Clinical Trials Registry (09/08/2023, NCT05983913). This study was conducted in accordance with the latest revision of the declaration of Helsinki.
Participants and sample size
Participants were the older adults 65 years or older who visited and used a M senior welfare center in Seoul. We recruited program participants through bulletin boards and social networking sites at the M senior welfare center. The inclusion criteria were community-dwelling older adults aged 65 years or older who were using a welfare center and had a score of 18 or higher on the Korean version of the Mini-Mental State Examination (MMSE-K) and were not diagnosed with dementia by a medical professional. Exclusions are (1) hospitalized or institutionalized, (2) diagnosed with Alzheimer’s disease or vascular dementia, (3) with musculoskeletal conditions that make physical activity difficult, (4) with have dizziness or vertigo, and (5) with head wounds or bleeding.
The sample size was calculated using the G-power software (ver. 3.1.9.2, University of Kiel, Kiel, Germany) to verify the efficacy of this study using F-test, repeated measures ANOVA, within factors, significance level 0.05, power 0.80, effect size Cohen’s f was set at 0.25, which is a medium effect size, and 40 subjects were recruited considering a dropout rate of 20% as the target number of at least 34 subjects.
Procedure
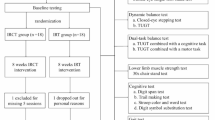
The purpose and procedures of the study were explained to all the participants, and informed consent was obtained from them. After recruiting eligible participants with the help of a physical therapist working at the welfare center, all selected participants were scheduled individually and completed a pretest assessment in a separate room. The study commenced with an assessment of 44 potential participants, from which 4 were excluded for the following reasons: one did not meet the MMSE-K criteria, two declined to participate, and one was excluded for other unspecified reasons. The 40 remaining individuals were randomized into two groups of 20 each, allocated to ICMT and CT, using random allocation software (ver. 2.0, Isfahan University, Iran)24. The pretest measured general characteristics and measured PFC hemodynamic response, cognitive function, physical function (balance and muscle strength), and IADLs. All interventions were performed twice a week for 6 weeks, 50 min per session. Before starting the first session, each participant received a brief orientation tailored to their group to familiarize them with the equipment and task procedures. This orientation covered system usage, movement execution, and other relevant aspects to ensure a consistent understanding of the tasks. The researchers monitored the participants’ discomfort, satisfaction, and health status before and after each session. Both groups performed 5 min of aerobic exercise (standing for the ICMT group and sitting for the CT group) during the program. At the end of the whole intervention, PFC hemodynamic response, cognition, physical function (balance and strength) and IADLs were measured as assessed before the experiment. The analysis was conducted on 18 participants from each group. No participant dropped out of the program due to the difficulty of the program: one person stopped the follow-up test for personal reasons (ICMT group), and one person was hospitalized after a fall in daily life (CT group). One person in each group was excluded from the statistical analysis due to measurement error and missing data (Fig. 1).
Interactive Cognitive-motor training (ICMT)
The wearable sensor-based ICMT device used in this study was developed using Arduino and radio-frequency identification (RFID) reader and RFID tag (Fig. 2). The ICMT consists of five cognitive tasks related to concentration, reaction time, memory, and executive function, combined with physical exercises using an interactive system. The five cognitive tasks include: (1) number sequence, (2) number-word sequence, (3) card matching, (4) number memorization, and (5) route-finding (Fig. 3). The motor task in the ICMT is designed to allow free physical movements, such as transitioning from sitting to standing in a chair, walking, performing pivot turns, and reaching with their arms, during the ICMT.
Prior to the ICMT, all participants performed 5 min of aerobic exercise. The five minutes of aerobic exercise was performed stepping in a regular pattern of forward, backward, left, and right, while to a song at 126 bpm for 5 min without interruption. Once the aerobic exercise had been completed, the participants were instructed to utilize their dominant upper extremity to attach the ICMT wearable device (Fig. 2). The program was conducted after confirming that there was no discomfort when wearing and moving around the ICMT device, which is based on wearable sensors and has been verified by usability testing25. All participants wearing the device were able to perform various activities such as reaching out, sitting, standing, and walking throughout the session. First, participants were asked to sit in a chair to view the monitor and perform the tasks, with the distance between the chair and the cognitive task being at least 3 m. The cognitive tasks, designed to be detachable, could be set up on a wall or table in various ways, depending on the participants’ height and posture. As participants progressed through the 6 weeks, we gradually changed the level of difficulty to easy, medium and hard as they showed learning gains on the cognitive tasks or improved their cognitive function. The results of each cognitive task can be checked by the time taken and the score, and a decrease in the time taken indicates a level of success. The difficulty of the cognitive tasks was adjusted according to the decrease in time. To minimize learning effects, participants performed cognitive tasks in a randomly ordered sequence at each session. No adverse events were observed during the six-week ICMT intervention.
Cognition training (CT)
The CT group performed similar cognitive tasks (memory, attention, spatial and temporal perception) to the ICMT group using a tablet (iPad 9th, Apple Inc., California, USA) while seated. The cognitive task application used brainilis© (version 1.69, appilis Sarl). Before performing the cognitive task, participants in the CT group performed five minutes of seated upper and lower extremity and trunk exercises to a song at 124–136 bpm. All participants then performed the cognitive task, which was selected to be of a similar type to those used in the ICMT group, according to their cognitive abilities, and these tasks could be upgraded to easy, moderate, or hard during the intervention. Participants performed three cognitive tasks, with 5-minute seated exercises in between each task.
Data collection
Primary outcome
Montreal cognitive assessment (MoCA)
The MoCA is a widely used cognitive screening test that helps assess various cognitive functions and detect MCI and early signs of dementia, evaluating domains such as visuospatial abilities, executive function, memory, attention, language, and orientation26. The total possible score on the MoCA is 30 points, with a score of 25 or below generally suggesting MCI. Depending on the number of years of education (less than 6 years), an additional 1 point may be added. The internal consistency of MoCA is excellent level (Cronbach’s alpha: 0.83)26, and its test-retest reliability was found to be 0.81 27. It had a good specificity of 84% and a great sensitivity of 89% for screening MCI28.
Prefrontal cortex (PFC) hemodynamic response
PFC hemodynamic response was evaluated by measuring the change in oxyhemoglobin (HbO) levels during rest and task performance as participants performed the Timed-Up and Go (TUG) and Timed-Up and Go cognitive (TUG-cog). The TUG-cog is a modified version of the TUG, where the examiner announces a random number between 20 and 100, and the participant must subtract 3 consecutively from that number and provide the correct answers29.
The hemodynamic response of the PFC was measured with a functional near-infrared spectroscopy (fNIRS) (NIRSIT Lite, OBELAB, Seoul, Korea)30. It is a portable, wireless, wearable device that measures perfusion status in real-time and uses the difference in absorption of near-infrared light to measure HbO and deoxyhemoglobin (HbR). The fNIRS detects signals from the prefrontal cortex using 15 channels, comprising 5 light sources and 13 detectors spaced 30 mm apart, with channels 1 to 7 positioned on the right side, channel 8 in the center, and channels 9 to 15 on the left forehead31. They were arranged symmetrically and divided into four regions: the anterior cingulate, the medial cingulate, the posterior cingulate, and the parietal forehead. The system utilizes near-infrared light at wavelengths of 780 nm and 850 nm, which, with a source-detector distance of 3 cm, can penetrate approximately 8 mm into the brain’s outer shell. The signals were measured at a sampling rate of 8.138 Hz. Hierarchical clustering analysis, utilizing the agglomerative WARD method32, has delineated channel groupings based on functional connectivity33.
PFC hemodynamic response data processing was performed using NIRSIT Quest software (version 0.1.2, OBELAB, Seoul, Korea). All data were processed in accordance with the recommendations of a review of fNIRS as much as possible34,35. The preprocessing of the measured PFC hemodynamic response dataset consisted of 11 steps: first, channels with negative intensity values were removed using invalid values processing, second, channels with median intensity lower than 30 were removed35. The third step was to remove channels with a coefficient of variation greater than 7.5% 35, and the fourth step was to remove channels with continuous values greater than 5% of the total time series, indicating saturation. Fifth, we converted light intensity to optical density35; sixth, we used temporal derivative distribution repair (TDDR) to remove motion artifacts36; seventh, we used short channel regression to mitigate skin surface noise37; and eighth, we converted optical density data to hemoglobin concentration data38,39. Ninth, we applied digital filtering using discrete cosine transform (DCT) to remove frequency noise35, and tenth, we removed the extreme channels of negatively correlated HbO and HbR40. Finally, padding was removed to replace the average value of nearby channels. This initial processing of the data improved the results of the study.
Secondary outcome
To assess dynamic balance, we used the TUG, four-square step test (FSST), functional reach test (FRT), and single leg stance (SLS). The TUG is a simple, reliable test for assessing mobility, balance, gait, and fall risk, as well as functional independence in older adults41,42. At the same time as the “Go” command, the subject gets up, walks a distance of 3 m, comes back and sits down in a chair43. The TUG-cog were asked to complete the test while counting backward by threes from a randomly selected number between 20 and 100 44,45. The FRT assesses a dynamic balance by measuring the farthest distance they can reach forward while standing, without taking a step. The test requires simple equipment such as a yardstick and is administered in approximately 5 min46,47, and its results correlate well with measures of balance and mobility. The FSST is a dynamic balance and coordination assessment that measures the time (in seconds) required to step over canes obstacles arranged in a plus sign (+) pattern, assessing dynamic balance and coordination48. The SLS test, often referred to as the “one-legged stance test,” is a simple, quick, and effective way to assess static postural and balance control49. The SLS measures the time (sec) to stand on one leg with both hands on the hips and eyes open.
Upper extremity muscle strength was assessed using the arm curl test (ACT) and grip strength (GS). The ACT, typically used to measure upper extremity strength in older adults, is performed by counting the number of repetitions completed in 30 s while seated without armrests or back support, using dumbbells (female: 2.3 kg, male: 3.6 kg)50,51. The GS was measured using a hand-held digital dynamometer (TKK 5401, GRIP D, Takei, Japan)52 with participants seated, arms extended, elbows flexed at 90 degree, and wrists in extension. The dynamometer was grasped for 3 s, and the average value (kg) over 3 trials was recorded, testing each hand alternately with a 1-minute rest between trials. Lower extremity muscle strength was assessed using the five times sit to stand test (5xSTS). The 5xSTS is a test of the time taken to sit down and stand up from a chair five times, with the instruction to complete the task as quickly as possible. The total time taken to complete the test is recorded in seconds.
Endurance was assessed with the 6-minute walk test (6MWT), where participants walked as far as possible in six minutes. The total distance walked, the number of rest breaks, and their duration were recorded, with a chair available if the participant needed to rest during the test. The IADL was assessed using the Korean Instrumental Activities of Daily Living (K-IADL) questionnaire. The K-IADL is a self-administered questionnaire that uses a re-standardized version of the scoring system to improve the accuracy of dementia diagnosis53,54, with a cutoff score of around 0.43 or higher often indicating significant impairment consistent with a diagnosis of dementia55.
Statistical analysis
All statistical analyses were performed using SPSS software (ver. 25.0, SPSS Inc., Chicago, IL, USA). Normality of data was assessed using the Shapiro-Wilk test. Chi-square analysis and independent samples t-tests were used to assess homogeneity between groups. The mean and standard deviation of the general characteristics of the subjects were obtained using independent t-tests. To compare the effect of ICMT and CT groups on all dependent variables over time, repeated measures analysis of variance was applied to obtain means (standard deviations). In addition, for cognitive function and prefrontal cortex (PFC) hemodynamic responses, ANCOVA was used to control for baseline values as covariates to adjust for any initial group differences. The dependent variables (TUG-cog, TUG, FSST, SLS) that did not meet the homogeneity in the statistical analysis due to dropouts were analyzed using the baseline data as covariates. The effect size (partial eta-square, ηp2 was calculated as the ratio of the between-group sum of squares to the sum of the between-group sum of squares and the error sum of squares. Partial eta-square of 0.01, 0.06, and 0.14 were interpreted as small, moderate, and large, respectively. Statistical interpretation was adjusted for multiple comparisons by applying the Bonferroni correction for 95% confidence intervals. The statistical significance level was set at 0.05.
Results
Of the 40 participants recruited, 36 were included in the statistical analysis, excluding two who dropped out during the program and two who were excluded from the final analysis. The general characteristics of the participants are described in Table 1.
Comparison of cognitive function among the ICMT and CT
Table 2 presents the results of analyses comparing cognitive performance between the ICMT and CT groups after six weeks, with baseline scores entered as covariates. For the MoCA, although both groups showed improvements, the ICMT group demonstrated a larger adjusted increase (mean difference = − 1.561, 95% CI [–3.267, 0.145]), with a medium effect size (ηp2 = 0.095). However, the group × time interaction did not reach statistical significance (F(1,33) = 3.467, p = 0.072). Regarding the TUG-cog, both groups showed reductions in completion time, but there was no significant interaction between group and time (F(1,33) = 0.072, p = 0.791), and the effect size was small (ηp2 = 0.002). The adjusted mean difference was 0.300 s (95% CI [–1.977, 2.577]).
Comparison of hemodynamic response (ΔHbO) in crusted PFC
Table 3 presents the results of ANCOVA analyses comparing ΔHbO responses between the ICMT and CT groups across seven PFC subregions, using baseline values as covariates. No statistically significant group × time interaction was observed (p > 0.05). However, small-to-moderate effect sizes were observed in the upper and lower frontal areas. In particular, the upper right PFC showed the medium effect size (ηp2 = 0.052, F(1,33) = 1.813, p = 0.187), followed by the lower left (ηp2 = 0.053, p = 0.183) and upper left (ηp2 = 0.045, p = 0.222) regions. In contrast, lateral and central PFC areas, including lateral right (ηp2 = 0.001) and center (ηp2 = 0.003), exhibited small effect sizes and wide confidence intervals crossing zero, indicating minimal differences between groups.
Comparison of balance, endurance, and IADLs among the ICMT and CT
Table 4 presents the adjusted comparisons between the ICMT and CT groups after the 6-week intervention. Among balance measures, a statistically significant group × time interaction was found in the FSST, where the ICMT group demonstrated greater improvement compared to the CT group (F(1,33) = 4.468, p = 0.042, ηp2 = 0.116). Other balance indicators, such as TUG, FRT, and SLS, showed non-significant differences between groups, but some measures (e.g., FRT, SLS) indicated within-group changes. In terms of muscle strength, a significant interaction was observed in left arm curl (F(1,33) = 4.200, p = 0.048, ηp2 = 0.110), favoring the ICMT group. Additionally, moderate effect sizes were noted in right grip strength (ηp2 = 0.093, p = 0.070) and right arm curl (ηp2 = 0.064), despite the lack of statistical significance. For endurance, the 6MWT showed a statistically significant group × time interaction (F(1,33) = 9.082, p = 0.005, ηp2 = 0.211), indicating a large effect size and suggesting that ICMT may have a stronger influence on cardiorespiratory endurance compared to CT. Lastly, the K-IADL score demonstrated a near-significant difference (F(1,33) = 3.619, p = 0.066), with a moderate effect size (ηp2 = 0.096) in the ICMT group.
Discussion
The study aimed to investigate the impact of wearable sensor-based ICMT on the cognitive and physical function of community-dwelling older adults. The ICMT group engaged in cognitive tasks with wearable sensor-based devices and the CT group performed cognitive tasks using a tablet in sitting. The ICMT participants were encouraged to perform free physical movements include sitting, standing, walking, arm reaching and trunk bending, which improved their problem-solving skills in a large space. The CT group was required to complete similar cognitive tasks to those undertaken by the ICMT group, in a seated position. Key findings included significant improvements in cognitive abilities in the ICMT group, with MoCA scores increasing by 8.60%, compared to a 3.63% increase in the CT group. Both groups showed improvements in dynamic and static balance ability, as well as enhancements in upper and lower extremity strength. Notably, the ICMT group exhibited a 4.65% increase in distance in the 6MWT.
In this study, when comparing the MoCA of both groups at baseline and 6 weeks, the number of participants predicted to have MCI (score < 23) decreased from 8 to 3 in the ICMT group and from 11 to 8 in the CT. The ICMT group showed a mean increase of 1.94 points in MoCA scores across all age groups, while the CT group exhibited a smaller increase of 0.77 points. Although these improvements did not reach the minimum detectable change (MDC) value (4 points) for the MoCA in the older adults27 and the minimal clinically important difference (MCID) of 2.15 in adults with stroke patients56, the results of this study clearly showed an improvement in cognition in the ICMT group. Improvements in cognitive abilities were also observed in the TUG-cog and TUG assessments. Pre-test, both groups had TUG-cog scores higher than the normative data45 but post-test, the ICMT group showed 2.25% reduction in time, while the CT group showed 26.21% reduction. However, despite these improvements, neither group reached the MDC threshold for TUG (2.3 s)57. In a previous study reported that TUG-cog (rho = -0.712) correlated more strongly with MoCA scores than the standard TUG (rho= -0.682), suggesting its higher sensitivity to cognitive function29, and another studies applying VR-based cognitive-motor training also reported significant reductions in TUG and TUG-cog58. A reduction in TUG-cog time may indicate improvements in cognitive function and dual-task performance and may enhanced problem-solving ability in daily life activities59. Cognitive-motor training likely led to decreased task performance speed due to increased demands on attention, motor planning, and problem-solving58,60. While previous studies have reported significant improvements in both TUG and TUG-cog following cognitive-motor training, the failure to meet the MDC for both measures in the present study may be due to differences in training protocols, participant characteristics, or intervention duration.
Although the improvements in MoCA and TUG-cog alone indicate the effectiveness of ICMT in enhancing cognitive abilities, this study aimed to confirm the role of cognitive-motor training in neuro-plasticity and neural activity by measuring hemodynamic response in PFC ΔHbO16,17. The results showed an initial increase in PFC hemodynamic response during TUG-cog, but a post-intervention decrease, aligning with previous studies where cognitive-motor training significantly reduced PFC ΔHbO changes6,18. In particular, moderate effect sizes were observed in the upper right (ηp2 = 0.052), upper left (ηp2 = 0.045), and lower left PFC (ηp2 = 0.053), indicating region-specific trends in neural response to ICMT. Research on the relationship between brain function and blood supply61,62 has shown increased brain blood flow and oxygen use during cognitive activity in the older adults63 with increased brain activation proportional to the complexity of cognitive tasks64. An increase in ΔHbO in PFC during cognitive-motor tasks reflects heightened neural activity and metabolic demand. Repeated activation through such training can promote neuroplastic adaptations, such as synaptic strengthening and cortical reorganization, ultimately supporting cognitive improvements in older adults65. The reduction in PFC ΔHbO at TUG-cog in ICMT is consistent with the findings of Voelcker-Rehage, et al.66 that 12 months of aerobic and coordination training reduced cortical activity during specific cognitive tasks. Similarly, Park6 found that 16 sessions over 8 weeks of simultaneous cognitive-motor tasks resulted in decreased ΔHbO and improved cognition. According to Chang, et al.67 the most significant positive effect on cognitive abilities occurs 10–20 min post-exercise, diminishing after 20 min. Based on these previous findings, this study hypothesized that pre-programmed aerobic exercise would positively affect cognitive performance while maintaining hemodynamic responses, resulting in improved cognitive performance and reduced PFC ΔHbO. It is also thought that cognitive improvement is due to the effects of ICMT on task focus, problem solving, and ignoring unnecessary stimulation68. Because the PFC is a critical role in higher-order cognitive processes, changes in blood flow and oxygenation in this region may indicate changes in cognitive abilities69. In this study, we observed a trend of decreased hemodynamic response in the PFC with cognitive changes, though statistically significant results were not obtained. This could be due to the shorter intervention period compared to other studies observing blood oxygen concentration in the PFC through cognitive-motor training. Our study conducted ICMT twice weekly for six weeks. In contrast, Park6 applied dual cognitive-physical tasks over eight weeks, observing significant hemodynamic response reduction in MCI executive functions (trail making test-B). Studies using fNIRS to monitor the PFC during cognitive tasks (digit span backward) in older adults have shown that variations in hemodynamic responses correlate with differences in cognitive performance70. Additionally, Pellegrini-Laplagne, et al.5 found an immediate increase in HbO during cognitive tasks (Stroop test) in a cross-sectional study. A meta-analysis found that the left prefrontal cortex is activated during low-demand verbal working memory tasks, whereas the right prefrontal cortex is involved in spatial working memory tasks71. In this study, channel 9 (upper Lt.), corresponding to Brodmann areas 6, 8, and 9 within the superior frontal cortex, showed a notable group × time difference. These regions are associated with working memory updating and sequential information processing. The lack of significant hemodynamic response reduction in our study might be attributed to the insufficient duration for observable changes and the monitoring changes during the TUG-cog task, different from previous studies. In addition, because we used simultaneous or sequential cognitive-motor training, both groups performed cognitive training, which may have contributed to the lack of differences between groups. Future research should consider applying tasks related to executive function, decision-making, and planning to observe PFC hemodynamic responses. These findings are significant because they suggest that monitoring PFC hemodynamic responses may provide insight into cognitive status and potential impairments. Overall, although the hemodynamic changes did not reach statistical significance, the pattern of regional PFC responses and moderate effect sizes support the potential of ICMT to elicit meaningful neural adaptations in older adults.
Furthermore, the ICMT group increased grip strength by 6.12% on the left hand and 6.63% on the right hand, while the CT group showed improvements of 4.20% on the left and 12.22% on the right. However, despite these improvements, neither group reached the MCID threshold for grip strength (19.5%)72. In this study, the pre grip strength was lower in the ICMT group (male: 25 kg, female: 17.56 kg) and the CT group (male: 25.14 kg, female: 18.34 kg), indicating lower values compared to the average for Asian older adult (male: 33.8 kg, female: 21.3 kg)73. Previous research suggests that a decrease in grip strength is associated with an increased risk of MCI and has a strong correlation with cognitive ability74,75. Furthermore, lower grip strength is linked to a higher risk of cognitive decline and dementia, making it a potential early indicator of cognitive dysfunction76. The study found cognitive skills improved, grip strength increased, confirming the correlation. Although grip strength improved in this study, it did not meet the MCID. This highlights the need for further studies with longer intervention periods or higher training intensities to achieve significant functional improvements.
Balance ability is a major predictor of cognitive decline in older adults, along with grip strength and cognitive function77, and this study used tests such as the TUG, FRT, FSST, and OLS to assess balance. The study found that FSST times exhibited significant decreased by 26.72% (2.40 s) for ICMT and 38.60% (4.56 s) for CT. These improvements exceeded the MDC, which ranges from 1.8 to 2 s, indicating that both interventions effectively enhanced dynamic balance abilities. This substantial improvement suggests the efficacy of both cognitive-motor and cognitive training in enhancing balance-related functions in older adults.
In the study, both groups surpassed the 18.5 cm FRT threshold, indicating a low fall risk78, with the ICMT group increasing by 21.5% and the CT group by 18.20%. Neither group reached the MDC for FRT, which is 6.35 cm79, despite these improvements. Although the SLS test did not show statistically significant differences, both groups exhibited improvements, suggesting enhanced balance abilities. Nascimento, et al.80 found that 12 weeks of cognitive-motor training effectively improved body balance, gait, and lower extremity strength, including significant reductions in TUG (28.35%) and TUG-cog (30.37%) times. However, this study did not observe significant clinical changes in TUG and TUG-cog, likely due to the already low baseline values. The primary focus of this study was to enhance cognitive abilities through aerobic exercise, which might account for the less pronounced differences in balance-related variables. The pre-intervention TUG was already within the cutoff range for older adults, which could explain the minimal changes observed post-intervention. While aerobic exercise contributed to cognitive improvements, its impact on certain balance metrics was not as significant, possibly due to the initial baseline levels of the participants. Previous research primarily focused on balance training, resulting in improvements in balance and strength80. In this study, the ICMT used aerobic exercise and free physical movement as the motor task, which may explain the lack of significant differences in balance and strength variables. However, endurance showed a significant increase in the ICMT, as it consisted of 50 min of mostly standing, moving, or walking, with less time spent sitting. Therefore, future studies should design programs with more challenging cognitive-motor tasks to improve both physical and cognitive functions.
Previous studies have shown that tai chi exercises have a positive effect on cognitive function (MoCA) and balance (TUG), suggesting that they may be influenced by lower extremity function81. In addition, significant changes in static balance movements were observed in older adults following a cognitive-motor training, and there were also positive effects on dynamic balance82,83,84. Meta-analytic research has demonstrated the effectiveness of cognitive-motor training in improving dynamic balance and mobility functions84. Previous studies and the positive correlation between balance and lower extremity strength observed in this study (r = 0.607, p = 0.000) support the relationship between cognitive and motor function in older adults.
Finally, the study found that 5xSTS significantly decreased the ICMT by 1.289 s (14.90%) and CT by 2.23 s (20.70%), resulting in improved lower extremity strength, which is close to the MDC (2.3 s)85. In addition, 6MWT significantly increased the ICMT by 18.009 m, but was less than the MCID (50 m)86. Xiao, et al.81 found an association among MoCA score, chair to stand, and 6MWT. In addition, Wollesen, et al.87 found that dual-task training consisting of balance exercises and various cognitive tasks resulted in an average improvement of 58 m in 6MWT and 12 cm/s in walking speed. The results of the cognitive-motor training were similar in terms of distance gains in the 6MWT, but the magnitude of change was significantly different. Similarly, Wollesen, et al.87 used progressive balance training as exercise training for 12 weeks. This study did not focus on balance training but applied aerobic exercise and performed cognitive tasks with free body movement, so it is thought that the difference may be due to the fact that only light movements (sitting, standing, walking, turning, stretching, etc.) were performed.
In this study, unlike traditional cognitive-motor dual-task training, an interactive system was used to adjust the difficulty level according to participants’ cognitive abilities (easy, moderate, hard). Over the six-week period, tasks progressively increased in difficulty, from easy to challenging. Motivation for task completion was provided through on-screen feedback about the time taken to solve cognitive tasks, encouraging quicker problem-solving. Positive and negative audio cues (beep sound) and verbal cue (good job, excellent, cheer up) were used to further motivate participants. Consequently, the ICMT group showed more active participation and lower dropout rates than the CT group. The importance of exercise self-efficacy in such programs is supported by Wollesen, et al.87, who found a positive relationship between self-efficacy and frequency, challenge, and enjoyment of cognitive-motor exercises in older adults.
The cognitive and physical improvements observed in the ICMT group have important clinical implications, particularly for older adults with MCI. Given the improvements in cognitive, balance, and endurance, ICMT may help maintain functional independence in older adults. Additionally, the increase in grip strength, though not reaching MCID, suggests potential benefits for upper extremity function, which is essential for daily activities. These findings support the integration of cognitive-motor training into preventive and rehabilitative programs for older adults. The findings suggest that ICMT could be feasibly implemented in community and clinical rehabilitation settings for older adults at risk of cognitive and physical decline. The use of wearable sensor-based interactive technology allows for flexible, engaging, and progressively challenging cognitive-motor training, which may enhance adherence compared to traditional cognitive or physical training methods.
Limitations of this study include its location in a specific senior center, which may not represent broader demographic variations, including gender distribution. In addition, it did not account for lifestyle factors of community-dwelling older adults, such as living family members, that may influence cognitive decline. And the cognitive tasks used to observe PFC hemodynamic responses may not have been ideal. We avoided cognitive tasks from previous studies in our cognitive-motor training to avoid learning effects. Future studies should consider using a longer intervention period to observe effects more comprehensively. In addition, for cognitive tasks, it would be beneficial to integrate tasks specifically designed to enhance executive function or target different cognitive abilities appropriate for older adults to examine their effects on PFC hemodynamic responses. In addition, a broader range of variables affecting cognition in the older adults should be considered. Furthermore, we believe that the ICMT device we have developed can be freely modified to suit the environment and can be easily used and accessed by the local community. Therefore, in the next study, we will conduct further advancement and research on a device that can be applied to objects related to the actual living environment of the older adults.
Conclusion
This study is designed to demonstrate the effects of the wearable sensor-based interactive cognitive-motor training program on community-dwelling older adults, and the results of the study have demonstrated that a wearable sensor-based interactive cognitive-motor training. The wearable sensor based ICMT program represents a novel approach to address the challenges of cognitive decline and physical limitations often associated with aging. Wearable sensor based ICMT, by integrating technology with physical and cognitive exercises, offers the advantage of enhancing cognitive functions while improving physical abilities. And the use of wearable technology in this context is particularly promising. It not only provides a personalized and adaptive training experience, but also ensures that the exercises are performed accurately and safely. This could lead to increased participation and adherence among older adults, as training can be tailored to their individual needs and abilities. This approach also aligns with current trends in healthcare that emphasize preventive care and the use of technology to enable more personalized treatment plans. Ultimately, this is a forward-thinking solution that fits into the evolving healthcare landscape where technology and personalized care play an increasingly important role.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Harada, C. N., Love, N., Triebel, K. L. & M. C. & Normal cognitive aging. Clin. Geriatr. Med. 29, 737–752. https://doi.org/10.1016/j.cger.2013.07.002 (2013).
Makino, K. et al. Relationship between instrumental activities of daily living performance and incidence of mild cognitive impairment among older adults: A 48-month follow-up study. Arch. Gerontol. Geriatr. 88, 104034. https://doi.org/10.1016/j.archger.2020.104034 (2020).
Law, C. K., Lam, F. M. H., Chung, R. C. K. & Pang, M. Y. C. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: a systematic review. J. Physiotherapy. 66, 9–18. https://doi.org/10.1016/j.jphys.2019.11.014 (2020).
Embon-Magal, S. et al. The effect of co-dependent (thinking in motion [TIM]) versus single-modality (CogniFit) interventions on cognition and gait among community-dwelling older adults with cognitive impairment: a randomized controlled study. BMC Geriatr. 22 https://doi.org/10.1186/s12877-022-03403-x (2022).
Pellegrini-Laplagne, M., Dupuy, O., Sosner, P. & Bosquet, L. Acute effect of a simultaneous exercise and cognitive task on executive functions and prefrontal cortex oxygenation in healthy older adults. Brain Sci. 12, 455. https://doi.org/10.3390/brainsci12040455 (2022).
Park, J. H. Effects of cognitive-Physical Dual-Task training on executive function and activity in the prefrontal cortex of older adults with mild cognitive impairment. Brain Neurorehabilitation. 14 https://doi.org/10.12786/bn.2021.14.e23 (2021).
Thaiyanto, J., Sittichoke, C., Phirom, K. & Sungkarat, S. Effects of multicomponent exercise on cognitive performance and fall risk in older women with mild cognitive impairment. J. Nutr. Health Aging. 25, 160–164. https://doi.org/10.1007/s12603-020-1458-5 (2021).
Delbroek, T., Vermeylen, W. & Spildooren, J. The effect of cognitive-motor dual task training with the biorescue force platform on cognition, balance and dual task performance in institutionalized older adults: a randomized controlled trial. J. Phys. Ther. Sci. 29, 1137–1143. https://doi.org/10.1589/jpts.29.1137 (2017).
Liao, Y. Y., Chen, I. H., Lin, Y. J., Chen, Y. & Hsu, W. C. Effects of virtual Reality-Based physical and cognitive training on executive function and Dual-Task gait performance in older adults with mild cognitive impairment: A randomized control trial. Front. Aging Neurosci. 11, 162. https://doi.org/10.3389/fnagi.2019.00162 (2019).
Schoene, D. et al. Interactive cognitive-Motor step training improves cognitive risk factors of falling in older Adults - A randomized controlled trial. PLoS One. 10, e0145161. https://doi.org/10.1371/journal.pone.0145161 (2015).
Kuo, H. T. et al. Effects of different dual task training on dual task walking and responding brain activation in older adults with mild cognitive impairment. Sci. Rep. 12, 8490. https://doi.org/10.1038/s41598-022-11489-x (2022).
Erickson, K. I. et al. Training-induced functional activation changes in dual-task processing: an FMRI study. Cereb. Cortex. 17, 192–204. https://doi.org/10.1093/cercor/bhj137 (2007).
Sakai, K., Hikosaka, O. & Nakamura, K. Emergence of rhythm during motor learning. Trends Cogn. Sci. 8, 547–553. https://doi.org/10.1016/j.tics.2004.10.005 (2004). https://doi.org/https://doi.org/
VanSwearingen, J. M. & Studenski, S. A. Aging, motor skill, and the energy cost of walking: implications for the prevention and treatment of mobility decline in older persons. J. Gerontol. Biol. Sci. Med. Sci. 69, 1429–1436. https://doi.org/10.1093/gerona/glu153 (2014).
Herold, F., Hamacher, D., Schega, L. & Müller, N. G. Thinking while moving or moving while Thinking – Concepts of Motor-Cognitive training for cognitive performance enhancement. Front. Aging Neurosci. 10 https://doi.org/10.3389/fnagi.2018.00228 (2018).
Bliss, E. S., Wong, R. H., Howe, P. R. & Mills, D. E. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow. Metab. 41, 447–470. https://doi.org/10.1177/0271678x20957807 (2021).
Tarassova, O., Ekblom, M. M., Moberg, M., Lövdén, M. & Nilsson, J. Peripheral BDNF response to physical and cognitive exercise and its association with cardiorespiratory fitness in healthy older adults. Front. Physiol. 11, 1080. https://doi.org/10.3389/fphys.2020.01080 (2020).
Pellegrini-Laplagne, M., Dupuy, O., Sosner, P. & Bosquet, L. Effect of simultaneous exercise and cognitive training on executive functions, baroreflex sensitivity, and pre-frontal cortex oxygenation in healthy older adults: a pilot study. Geroscience 45, 119–140. https://doi.org/10.1007/s11357-022-00595-3 (2023).
Park, S. Y. & Schott, N. The immediate and sustained effects of Exercise-Induced hemodynamic response on executive function during fine Motor-Cognitive tasks using functional Near-Infrared spectroscopy. J. Integr. Neurosci. 21, 98. https://doi.org/10.31083/j.jin2103098 (2022).
O’Neil-Pirozzi, T. M., Cattaneo, G., Solana-Sánchez, J., Gomes-Osman, J. & Pascual-Leone, A. The importance of motivation to older adult physical and cognitive exercise program development, initiation, and adherence. Front. Aging. 3 https://doi.org/10.3389/fragi.2022.773944 (2022).
Tak, E. C., van Uffelen, J. G., Paw, M. J. C. A., van Mechelen, W. & Hopman-Rock, M. Adherence to exercise programs and determinants of maintenance in older adults with mild cognitive impairment. J. Aging Phys. Act. 20, 32–46 (2012).
van Alphen, H. J., Hortobágyi, T. & van Heuvelen, M. J. Barriers, motivators, and facilitators of physical activity in dementia patients: A systematic review. Arch. Gerontol. Geriatr. 66, 109–118. https://doi.org/10.1016/j.archger.2016.05.008 (2016).
Gordt, K., Gerhardy, T., Najafi, B. & Schwenk, M. Effects of wearable Sensor-Based balance and gait training on balance, gait, and functional performance in healthy and patient populations: A systematic review and Meta-Analysis of randomized controlled trials. Gerontology 64, 74–89. https://doi.org/10.1159/000481454 (2018).
Saghaei, M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 4, 26. https://doi.org/10.1186/1471-2288-4-26 (2004).
Jung, J. & Lee, S. Comparison of usability and prefrontal cortex activity of Cognitive-Motor training programs using Sensor-Based interactive systems. Phys. Therapy Rehabilitation Sci. 11, 571–578. https://doi.org/10.14474/ptrs.2022.11.4.571 (2022).
Nasreddine, Z. S. et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x (2005).
Feeney, J. et al. Measurement error, reliability, and minimum detectable change in the Mini-Mental state examination, Montreal cognitive assessment, and color trails test among community living Middle-Aged and older adults. J. Alzheimers Dis. 53, 1107–1114. https://doi.org/10.3233/jad-160248 (2016).
Lee, J. Y. et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal cognitive assessment. J. Geriatr. Psychiatry Neurol. 21, 104–110. https://doi.org/10.1177/0891988708316855 (2008).
Çekok, K. et al. Timed up and go test with a cognitive task: correlations with neuropsychological measures in people with Parkinson’s disease. Cureus 12, e10604. https://doi.org/10.7759/cureus.10604 (2020).
Choi, J. K., Choi, M. G., Kim, J. M. & Bae, H. M. Efficient data extraction method for near-infrared spectroscopy (NIRS) systems with high Spatial and Temporal resolution. IEEE Trans. Biomed. Circuits Syst. 7, 169–177. https://doi.org/10.1109/tbcas.2013.2255052 (2013).
Kim, T. J. et al. Prognostication of neurological outcome after cardiac arrest using wavelet phase coherence analysis of cerebral oxygen. Resuscitation 150, 41–49. https://doi.org/10.1016/j.resuscitation.2020.02.031 (2020).
Ward, J. H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58, 236–244. https://doi.org/10.1080/01621459.1963.10500845 (1963).
Kim, T., Nam, H., Choi, J., Kim, J. & Cha, J. in Society for fNIRS Conference 2022.Boston, MA, United States., (2022).
Baek, C. Y., Kim, H. D., Yoo, D. Y., Kang, K. Y. & Lee, J. W. Change in activity patterns in the prefrontal cortex in different phases during the dual-task walking in older adults. J. Neuroeng. Rehabil. 20, 86. https://doi.org/10.1186/s12984-023-01211-x (2023).
Yücel, M. et al. Best practices for fNIRS publications. Neurophotonics 8, 012101 (2021).
Fishburn, F. A., Ludlum, R. S., Vaidya, C. J. & Medvedev, A. V. Temporal derivative distribution repair (TDDR): A motion correction method for fNIRS. Neuroimage 184, 171–179. https://doi.org/10.1016/j.neuroimage.2018.09.025 (2019).
Gagnon, L. et al. Improved recovery of the hemodynamic response in diffuse optical imaging using short optode separations and state-space modeling. Neuroimage 56, 1362–1371. https://doi.org/10.1016/j.neuroimage.2011.03.001 (2011).
Delpy, D. T. et al. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 33, 1433–1442. https://doi.org/10.1088/0031-9155/33/12/008 (1988).
Zhao, Y., Qiu, L., Sun, Y., Huang, C. & Li, T. Optimal hemoglobin extinction coefficient data set for near-infrared spectroscopy. Biomed. Opt. Express. 8, 5151–5159. https://doi.org/10.1364/boe.8.005151 (2017).
Takizawa, R. et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage 85 Pt. 1, 498–507. https://doi.org/10.1016/j.neuroimage.2013.05.126 (2014).
Steffen, T. M., Hacker, T. A. & Mollinger, L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute walk test, Berg balance scale, timed up & go test, and gait speeds. Phys. Ther. 82, 128–137. https://doi.org/10.1093/ptj/82.2.128 (2002).
Siggeirsdóttir, K., Jónsson, B. Y., Jónsson, H. Jr. & Iwarsson, S. The timed ‘up & go’ is dependent on chair type. Clin. Rehabil. 16, 609–616. https://doi.org/10.1191/0269215502cr529oa (2002).
Podsiadlo, D. & Richardson, S. The timed up & go: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 39, 142–148. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x (1991).
Maranhão-Filho, P. A., Maranhão, E. T., Lima, M. A. & Silva, M. M. Rethinking the neurological examination II: dynamic balance assessment. Arq. Neuropsiquiatr. 69, 959–963. https://doi.org/10.1590/s0004-282x2011000700022 (2011).
Hofheinz, M. & Schusterschitz, C. Dual task interference in estimating the risk of falls and measuring change: a comparative, psychometric study of four measurements. Clin. Rehabil. 24, 831–842. https://doi.org/10.1177/0269215510367993 (2010).
Weiner, D. K., Duncan, P. W., Chandler, J. & Studenski, S. A. Functional reach: a marker of physical frailty. J. Am. Geriatr. Soc. 40, 203–207. https://doi.org/10.1111/j.1532-5415.1992.tb02068.x (1992).
Duncan, P. W., Weiner, D. K., Chandler, J. & Studenski, S. Functional reach: a new clinical measure of balance. J. Gerontol. 45, M192–197. https://doi.org/10.1093/geronj/45.6.m192 (1990).
Dite, W. & Temple, V. A. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch. Phys. Med. Rehabil. 83, 1566–1571. https://doi.org/10.1053/apmr.2002.35469 (2002).
Mak, M. K. & Pang, M. Y. Parkinsonian single fallers versus recurrent fallers: different fall characteristics and clinical features. J. Neurol. 257, 1543–1551. https://doi.org/10.1007/s00415-010-5573-9 (2010).
Singh, D. K. et al. Correlation between nutritional status and comprehensive physical performance measures among older adults with undernourishment in residential institutions. Clin. Interv Aging. 9, 1415–1423. https://doi.org/10.2147/cia.S64997 (2014).
Miotto, J. M., Chodzko-Zajko, W. J., Reich, J. L. & Supler, M. M. Reliability and validity of the Fullerton functional fitness test: an independent replication study. J. Aging Phys. Act. 7, 339–353. https://doi.org/10.1123/japa.7.4.339 (1999).
Bohannon, R. W. Grip Strength: An Indispensable Biomarker For Older AdultsClinical Interventions in AgingVolume 14, 1681–1691 (2019). https://doi.org/10.2147/cia.s194543<\/bib>
Chin, J. et al. Re-standardization of the Korean-Instrumental activities of daily living (K-IADL): clinical usefulness for various neurodegenerative diseases. Dement. Neurocogn Disord. 17, 11–22. https://doi.org/10.12779/dnd.2018.17.1.11 (2018).
KANG, S. J. et al. The reliability and validity of the Korean instrumental activities of daily living (K-IADL). J. Korean Neurol. Assoc., 8–14 (2002).
Chin, J. et al. Re-standardization of the Korean-Instrumental activities of daily living (K-IADL): clinical usefulness for various neurogenerative diseases. Dement. Neurocognitive Disorders. 17, 11. https://doi.org/10.12779/dnd.2018.17.1.11 (2018).
Wu, C. Y. et al. Responsiveness, Minimal Clinically Important Difference, and Validity of the MoCA in Stroke Rehabilitation. Occup. Ther. Int. 2517658 (2019). https://doi.org/10.1155/2019/2517658 (2019).
Beauchamp, M. K. et al. Reliability and minimal detectable change values for Performance-Based measures of physical functioning in the Canadian longitudinal study on aging. J. Gerontol. Biol. Sci. Med. Sci. 76, 2030–2038. https://doi.org/10.1093/gerona/glab175 (2021).
Molhemi, F. et al. Effects of virtual reality vs conventional balance training on balance and falls in people with multiple sclerosis: A randomized controlled trial. Arch. Phys. Med. Rehabil. 102, 290–299. https://doi.org/10.1016/j.apmr.2020.09.395 (2021).
Jardim, N. Y. V. et al. Dual-Task exercise to improve cognition and functional capacity of healthy older adults. Front. Aging Neurosci. 13, 589299. https://doi.org/10.3389/fnagi.2021.589299 (2021).
Mirelman, A. et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial. Lancet 388, 1170–1182. https://doi.org/10.1016/s0140-6736(16)31325-3 (2016).
Davenport, M. H., Hogan, D. B., Eskes, G. A., Longman, R. S. & Poulin, M. J. Cerebrovascular reserve: the link between fitness and cognitive function?? Exerc. Sport Sci. Rev. 40, 153–158. https://doi.org/10.1097/JES.0b013e3182553430 (2012).
Voelcker-Rehage, C. & Niemann, C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci. Biobehav Rev. 37, 2268–2295. https://doi.org/10.1016/j.neubiorev.2013.01.028 (2013).
Mehagnoul-Schipper, D. J. et al. Simultaneous measurements of cerebral oxygenation changes during brain activation by near-infrared spectroscopy and functional magnetic resonance imaging in healthy young and elderly subjects. Hum. Brain. Mapp. 16, 14–23. https://doi.org/10.1002/hbm.10026 (2002).
Agbangla, N. F., Audiffren, M., Pylouster, J. & Albinet, C. T. Working memory, cognitive load andcardiorespiratory fitness: testing the crunchmodel with Near-Infrared spectroscopy. Brain Sci. 9 https://doi.org/10.3390/brainsci9020038 (2019).
Pellegrini-Laplagne, M., Dupuy, O., Sosner, P. & Bosquet, L. Effect of simultaneous exercise and cognitive training on executive functions, baroreflex sensitivity, and pre-frontal cortex oxygenation in healthy older adults: a pilot study. GeroScience https://doi.org/10.1007/s11357-022-00595-3 (2022).
Voelcker-Rehage, C., Godde, B. & Staudinger, U. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front. Hum. Neurosci. 5 https://doi.org/10.3389/fnhum.2011.00026 (2011).
Chang, Y. K., Labban, J. D., Gapin, J. I. & Etnier, J. L. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 1453, 87–101. https://doi.org/10.1016/j.brainres.2012.02.068 (2012).
Schoene, D., Valenzuela, T., Lord, S. R. & De Bruin, E. D. The effect of interactive cognitive-motor training in reducing fall risk in older people: a systematic review. BMC Geriatr. 14, 107. https://doi.org/10.1186/1471-2318-14-107 (2014).
Bak, S., Jeong, Y., Yeu, M. & Jeong, J. Brain–computer interface to predict impulse buying behavior using functional near-infrared spectroscopy. Sci. Rep. 12 https://doi.org/10.1038/s41598-022-22653-8 (2022).
Kang, M. J., Cho, S. Y., Choi, J. K. & Yang, Y. S. fNIRS assessment during cognitive tasks in elderly patients with depressive symptoms. Brain Sci. 13 https://doi.org/10.3390/brainsci13071054 (2023).
Wager, T. D. & Smith, E. E. Neuroimaging studies of working memory. Cogn. Affect. Behav. Neurosci. 3, 255–274. https://doi.org/10.3758/cabn.3.4.255 (2003).
Lee, J. K., Jung, M., Lee, H. B., Chung, H. J. & Lee, S. H. Reliability and validity of the Martin Vigorimeter for grip strength measurement in Korean adults. Clin. Orthop. Surg. 16, 610–619. https://doi.org/10.4055/cios23383 (2024).
Auyeung, T. W., Arai, H., Chen, L. K. & Woo, J. Letter to the editor: normative data of handgrip strength in 26344 older adults - a pooled dataset from eight cohorts in Asia. J. Nutr. Health Aging. 24, 125–126. https://doi.org/10.1007/s12603-019-1287-6 (2020).
Vancampfort, D. et al. Associations between handgrip strength and mild cognitive impairment in middle-aged and older adults in six low- and middle-income countries. Int. J. Geriatr. Psychiatry. 34, 609–616. https://doi.org/10.1002/gps.5061 (2019).
Jacob, L. et al. Sarcopenia and mild cognitive impairment in older adults from six Low- and Middle-Income countries. J. Alzheimers Dis. 82, 1745–1754. https://doi.org/10.3233/jad-210321 (2021).
Cui, M., Zhang, S., Liu, Y., Gang, X. & Wang, G. Grip strength and the risk of cognitive decline and dementia: A systematic review and Meta-Analysis of longitudinal cohort studies. Front. Aging Neurosci. 13, 625551. https://doi.org/10.3389/fnagi.2021.625551 (2021).
Blackwood, J. et al. Balance performance and grip strength as predictors of cognitive function among community-dwelling older adults in the USA. J. Frailty Sarcopenia Falls. 8, 23–31. https://doi.org/10.22540/jfsf-08-023 (2023).
Thomas, J. I. & Lane, J. V. A pilot study to explore the predictive validity of 4 measures of falls risk in frail elderly patients. Arch. Phys. Med. Rehabil. 86, 1636–1640. https://doi.org/10.1016/j.apmr.2005.03.004 (2005).
Ferreira, S., Raimundo, A. & Marmeleira, J. Test-retest reliability of the functional reach test and the hand grip strength test in older adults using nursing home services. Ir. J. Med. Sci. 190, 1625–1632. https://doi.org/10.1007/s11845-020-02492-0 (2021).
Nascimento, M. M. et al. The effects of 12-Week Dual-Task Physical-Cognitive training on gait, balance, lower extremity muscle strength, and cognition in older adult women: A randomized study. Int. J. Environ. Res. Public. Health. 20 https://doi.org/10.3390/ijerph20085498 (2023).
Xiao, T. et al. Correlation between cognition and balance among Middle-Aged and older adults observed through a Tai Chi intervention program. Front. Psychol. 11, 668. https://doi.org/10.3389/fpsyg.2020.00668 (2020).
Kao, C. C. et al. Effect of interactive cognitive motor training on gait and balance among older adults: A randomized controlled trial. Int. J. Nurs. Stud. 82, 121–128. https://doi.org/10.1016/j.ijnurstu.2018.03.015 (2018).
Norouzi, E., Vaezmosavi, M., Gerber, M., Pühse, U. & Brand, S. Dual-task training on cognition and resistance training improved both balance and working memory in older people. Physician Sportsmed. 47, 471–478. https://doi.org/10.1080/00913847.2019.1623996 (2019).
Teraz, K., Šlosar, L., Paravlić, A. H., De Bruin, E. D. & Marusic, U. Impact of Motor-Cognitive interventions on selected gait and balance outcomes in older adults: A systematic review and Meta-Analysis of randomized controlled trials. Front. Psychol. 13 https://doi.org/10.3389/fpsyg.2022.837710 (2022).
Meretta, B. M., Whitney, S. L., Marchetti, G. F., Sparto, P. J. & Muirhead, R. J. The five times sit to stand test: responsiveness to change and concurrent validity in adults undergoing vestibular rehabilitation. J. Vestib. Res. 16, 233–243 (2006).
Perera, S., Mody, S. H., Woodman, R. C. & Studenski, S. A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 54, 743–749. https://doi.org/10.1111/j.1532-5415.2006.00701.x (2006).
Wollesen, B. et al. Multitask training to improve walking performance in older adults with hearing impairment: A feasibility study. Aging Health Res. 1, 100028. https://doi.org/10.1016/j.ahr.2021.100028 (2021).
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2021R1F1A1060798). The funding source had no role in the study.
Author information
Authors and Affiliations
Contributions
J. Jung wrote the main manuscript text and prepared Figs. 1, 2 and 3. S. Lee and H. Ryu conceived conception or design of the work. S. Lee and J. Jung performed the acquisition, analysis, or interpretation of data. H. Ryu created a new software used in this study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jung, J., Ryu, HC. & Lee, S. Enhancing cognitive and physical performance in older adults through wearable sensor-based interactive cognitive-motor training: a randomized clinical trial. Sci Rep 15, 18604 (2025). https://doi.org/10.1038/s41598-025-03725-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03725-x